Differences in Genotype and Antimicrobial Resistance between Campylobacter spp. Isolated from Organic and Conventionally Produced Chickens in Sweden
Abstract
1. Introduction
2. Results
2.1. Antibiotic Resistance
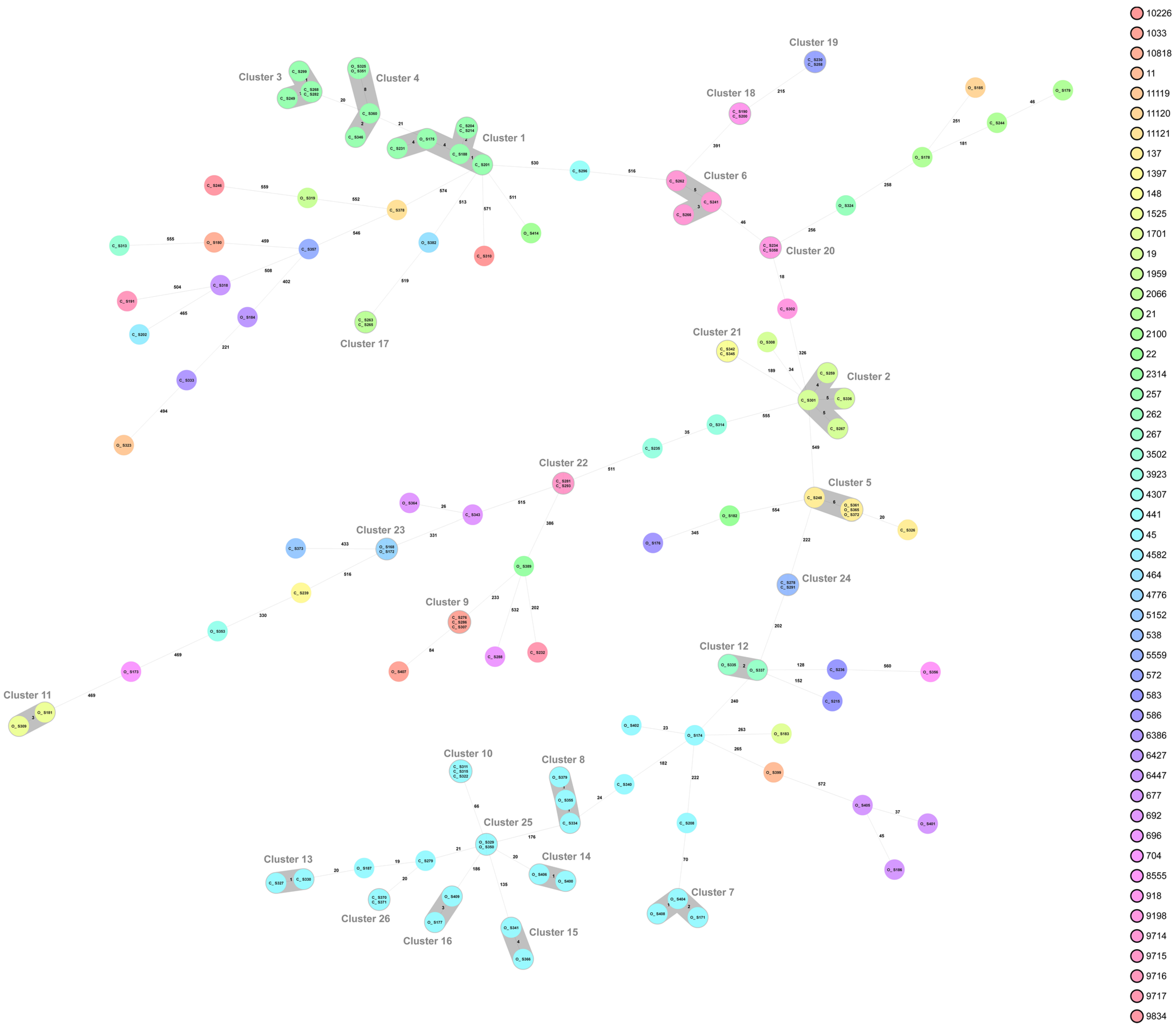
2.2. Campylobacter Genotypes Determined by Whole-Genome Sequencing
3. Discussion
4. Materials and Methods
4.1. Broiler Population
4.2. Sampling and Bacteriological Analysis
4.3. Antibiotic Susceptibility Testing
4.4. Whole-Genome Sequencing
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NNDSS, Annual Report Working Group. Australia’s notifiable disease status, 2014: Annual report of the National Notifiable Diseases Surveillance System. Commun. Dis. Intell. 2016, 40, E48–E145. [Google Scholar]
- Geissler, A.L.; Bustos Carrillo, F.; Swanson, K.; Patrick, M.E.; Fullerton, K.E.; Bennett, C.; Barrett, K.; Mahon, B.E. Increasing Campylobacter Infections, Outbreaks, and Antimicrobial Resistance in the United States, 2004–2012. Clin. Infect. Dis. 2017, 65, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- The Institute of Environmental Science and Research Ltd. (ESR). Notifiable Diseases in New Zealand; Annual Report. 2020. Available online: www.surv.esr.cri.nz (accessed on 28 June 2021).
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 286.
- Center for Disease Control and Prevention (CDC). Campylobacter (Campylobacteriosis). 2020. Available online: https://www.cdc.gov/campylobacter/faq.html (accessed on 28 June 2021).
- Hansson, I.; Gustafsson, P.; Hellquist, B.; Lahti, E.; Pudas, N.; Olsson Engvall, E. Campylobacter in Swedish small scale chicken production. A comparison with findings in conventionally produced Swedish and European broilers. In Proceedings of the CHRO, 17th International Workshop on Campylobacter, Helicobacter and Related Organisms, Aberdeen, Scotland, 15–20 September 2013. [Google Scholar]
- Heuer, O.E.; Pedersen, K.; Andersen, J.S.; Madsen, M. Prevalence and antimicrobial susceptibility of thermophilic Campylobacter in organic and conventional broiler flocks. Lett. Appl. Microbiol. 2001, 33, 269–274. [Google Scholar] [CrossRef]
- Allen, V.M.; Ridley, A.M.; Harris, J.A.; Newell, D.G.; Powell, L. Influence of production system on the rate of onset of Campylobacter colonization in chicken flocks reared extensively in the United Kingdom. Br. Poult. Sci. 2011, 52, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, H.; Boysen, L.; Krogh, A.L.; Jensen, A.N.; Nauta, M. Campylobacter contamination and the relative risk of illness from organic broiler meat in comparison with conventional broiler meat. Int. J. Food Microbiol. 2013, 162, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Golden, C.E.; Mishra, A. Prevalence of Salmonella and Campylobacter spp. in Alternative and Conventionally Produced Chicken in the United States: A Systematic Review and Meta-Analysis. J. Food Prot. 2020, 83, 1181–1197. [Google Scholar] [CrossRef]
- Bouwknegt, M.; van de Giessen, A.W.; Dam-Deisz, W.D.; Havelaar, A.H.; Nagelkerke, N.J.; Henken, A.M. Risk factors for the presence of Campylobacter spp. in Dutch broiler flocks. Prev. Vet. Med. 2004, 62, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Näther, G.; Alter, T.; Martin, A.; Ellerbroek, L. Analysis of risk factors for Campylobacter species infection in broiler flocks. Poult. Sci. 2009, 88, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Sommer, H.M.; Heuer, O.E.; Sørensen, A.I.; Madsen, M. Analysis of factors important for the occurrence of Campylobacter in Danish broiler flocks. Prev. Vet. Med. 2013, 111, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Government Offices of Sweden Homepage. 2018. Available online: https://www.regeringen.se/pressmeddelanden/2018/04/jordbruksverket-ska-jobba-for-okad-ekologisk-produktion (accessed on 1 April 2021).
- World Health Organization (WHO). WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. 2000. Available online: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 28 May 2020).
- Bengtsson, B.; Wierup, M. Antimicrobial resistance in Scandinavia after ban of antimicrobial growth promoters. Anim. Biotechnol. 2006, 17, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet Health 2017, 8, e316–e327. [Google Scholar] [CrossRef]
- Varga, C.; Guerin, M.T.; Brash, M.L.; Slavic, D.; Boerlin, P.; Susta, L. Antimicrobial resistance in Campylobacter jejuni and Campylobacter coli isolated from small poultry flocks in Ontario, Canada: A two-year surveillance study. PLoS ONE 2019, 14, e0221429. [Google Scholar] [CrossRef] [PubMed]
- Wierup, M.; Wahlström, H.; Bengtsson, B. Successful Prevention of Antimicrobial Resistance in Animals-A Retrospective Country Case Study of Sweden. Antibiotics 2021, 10, 129. [Google Scholar] [CrossRef]
- Swedres-Svarm. Sales of Antibiotics and Occurrence of Resistance in Sweden; Public Health Agency and National Veterinary Institute: Solna, Sweden, 2020. [Google Scholar]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union summary report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA J. 2020, 18, e06007. [Google Scholar]
- Hansson, I.; Olsson Engvall, E.; Lahti, E.; Landen, A.; Harbom, B.; Bengtsson, B. Antimicrobial resistance of Campylobacter in Swedish chicken. In Proceedings of the CHRO, 18th International Workshop on Campylobacter, Helicobacter and Related Organisms, Rotorua, New Zealand, 1–5 November 2015. [Google Scholar]
- Frosth, S.; Karlsson-Lindsjö, O.; Niazi, A.; Fernström, L.L.; Hansson, I. Identification of Transmission Routes of Campylobacter and On-Farm Measures to Reduce Campylobacter in Chicken. Pathogens 2020, 9, 363. [Google Scholar] [CrossRef]
- Luangtongkum, T.; Morishita, T.Y.; Ison, A.J.; Huang, S.; McDermott, P.F.; Zhang, Q. Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry. Appl. Environ. Microbiol. 2006, 72, 3600–3607. [Google Scholar] [CrossRef]
- Tenhagen, B.A.; Alt, K.; Käsbohrer, A.; Kollas, C.; Pfefferkorn, B.; Naumann, S.; Wiehle, L.; Thieck, M.; Stingl, K. Comparison of Antimicrobial Resistance of Thermophilic Campylobacter Isolates from Conventional and Organic Turkey Meat in Germany. Foodborne Pathog. Dis. 2020, 17, 750–757. [Google Scholar] [CrossRef]
- Innes, G.K.; Nachman, K.E.; Abraham, A.G.; Casey, J.A.; Patton, A.N.; Price, L.B.; Tartof, S.Y.; Davis, M.F. Contamination of Retail Meat Samples with Multidrug-Resistant Organisms in Relation to Organic and Conventional Production and Processing: A Cross-Sectional Analysis of Data from the United States National Antimicrobial Resistance Monitoring System, 2012–2017. Environ. Health Perspect. 2021, 129, 57004. [Google Scholar] [PubMed]
- Engberg, J.; Neimann, J.; Nielsen, E.M.; Aarestrup, F.M.; Fussing, V. Quinolone-resistant Campylobacter infections: Risk factors and clinical consequences. Emerg. Infect. Dis. 2004, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, M.; Salata, C.; Martini, M.; Montesissa, C.; Piccirillo, A. Antimicrobial resistance of Campylobacter jejuni and Campylobacter coli from poultry in Italy. Microb. Drug Resist. 2014, 20, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Jacobs-Reitsma, W.F.; Koenraad, P.M.; Bolder, N.M.; Mulder, R.W. In vitro susceptibility of Campylobacter and Salmonella isolates from broilers to quinolones, ampicillin, tetracycline, and erythromycin. Vet. Q. 1994, 16, 206–208. [Google Scholar] [CrossRef]
- Velàzquez, J.B.; Jimenez, A.; Chomon, B.; Villa, T.G. Incidence and transmission of antibiotic resistance in Campylobacter jejuni and Campylobacter coli. J. Antimicrob. Chemother. 1995, 35, 173–178. [Google Scholar] [CrossRef]
- Maxwell, A. The molecular basis of quinolone action. J. Antimicrob. Chemother. 1992, 30, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, W.M.; Taylor, D.E. Cloning and nucleotide sequence of the Campylobacter jejuni gyrA gene and characterization of quinolone resistance mutations. Antimicrob. Agents Chemother. 1993, 37, 457–463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Payot, S.; Bolla, J.M.; Corcoran, D.; Fanning, S.; Mégraud, F.; Zhang, Q. Mechanisms of fluoroquinolone and macrolide resistance in Campylobacter spp. Microb. Infect. 2006, 8, 1967–1971. [Google Scholar] [CrossRef]
- Haldenby, S.; Bronowski, C.; Nelson, C.; Kenny, J.; Martinez-Rodriguez, C.; Chaudhuri, R.; Williams, N.J.; Forbes, K.; Strachan, N.J.; Pulman, J.; et al. Increasing prevalence of a fluoroquinolone resistance mutation amongst Campylobacter jejuni isolates from four human infectious intestinal disease studies in the United Kingdom. PLoS ONE 2020, 15, e0227535. [Google Scholar] [CrossRef]
- Hansson, I.; Tamminen, L.M.; Frosth, S.; Fernström, L.L.; Emanuelson, U.; Boqvist, S. Occurrence of Campylobacter spp. in Swedish calves, common sequence types and antibiotic resistance patterns. J. Appl. Microbiol. 2020, 130, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Kovač, J.; Šimunović, K.; Wu, Z.; Klančnik, A.; Bucar, F.; Zhang, Q.; Možina, S.S. Antibiotic resistance modulation and modes of action of (-)-α-pinene in Campylobacter jejuni. PLoS ONE 2015, 1, e0122871. [Google Scholar] [CrossRef]
- Hakkinen, M.; Heiska, H.; Hänninen, M.L. Prevalence of Campylobacter spp. in cattle in Finland and antimicrobial susceptibilities of bovine Campylobacter jejuni strains. Appl. Environ. Microbiol. 2007, 73, 3232–3238. [Google Scholar] [CrossRef]
- Bailey, M.A.; Taylor, R.M.; Brar, J.S.; Corkran, S.C.; Velásquez, C.; Novoa Rama, E.; Oliver, H.F.; Singh, M. Prevalence and antimicrobial resistance of Campylobacter from antibiotic-free broilers during organic and conventional processing. Poult. Sci. 2019, 98, 1447–1454. [Google Scholar] [CrossRef]
- Pedersen, K.; Wedderkopp, A. Resistance to quinolones in Campylobacter jejuni and Campylobacter coli from Danish broilers at farm level. J. Appl. Microbiol. 2003, 94, 111–119. [Google Scholar] [CrossRef]
- Nilsson, O.; Greko, C.; Bengtsson, B.; Englund, S. Genetic diversity among VRE isolates from Swedish broilers with the coincidental finding of transferrable decreased susceptibility to narasin. J. Appl. Microbiol. 2012, 112, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Avrain, L.; Humbert, F.; L’Hospitalier, R.; Sanders, P.; Vernozy-Rozand, C.; Kempf, I. Antimicrobial resistance in Campylobacter from broilers: Association with production type and antimicrobial use. Vet. Microbiol. 2003, 96, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Willer, T.; Pielsticker, C.; Gerzova, L.; Rychlik, I.; Rautenschlein, S. Differences in host breed and diet influence colonization by Campylobacter jejuni and induction of local immune responses in chicken. Gut Pathog. 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- KRAV. Available online: https://www.krav.se/om-krav/krav-markningen/djur/sa-blir-kycklingen-krav-certifierad/ (accessed on 21 March 2020).
- Rezaei, M.; Yngvesson, J.; Gunnarsson, S.; Jönsson, L.; Wallenbeck, A. Feed efficiency, growth performance, and carcass characteristics of a fast-and a slower-growing broiler hybrid fed low-or high-protein organic diets. Org. Agric. 2018, 8, 121–128. [Google Scholar] [CrossRef]
- Hansson, I.; Forshell, L.P.; Gustafsson, P.; Boqvist, S.; Lindblad, J.; Engvall, E.O.; Andersson, Y.; Vågsholm, I. Summary of the Swedish Campylobacter program in broilers, 2001 through 2005. J. Food Prot. 2007, 70, 2008–2014. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; CLSI standard VET01 Ed5; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Souvorov, A.; Agarwala, R.; Lipman, D.J. SKESA: Strategic k-mer extension for scrupulous assemblies. Genome Biol. 2018, 19, 153. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Nennig, M.; Llarena, A.-K.; Herold, M.; Mossong, J.; Penny, C.; Losch, S.; Tresse, O.; Ragimbeau, S. Investigating major recurring Campylobacter jejuni lineages in Luxembourg using four core or whole genome sequencing typing schemes. Front. Cell. Infect. Microbiol. 2021, 10, 608020. [Google Scholar] [CrossRef]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J. Antimicrob. Chemother. 2020, 72, 2764–2768. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]


 |
 |
| Total | Organic | Conventional | ||||
|---|---|---|---|---|---|---|
| CC | MLST | No. of Isolates | No. of Isolates | Resistance | No. of Isolates | Resistance 1 |
| 21 | 19 | 5 | 1 | - | 4 | 4 (cip + nal) |
| 21 | 21 | 3 | 2 | - | 1 | - |
| 21 | 148 | 2 | - | - | 2 | 2 (cip + nal) |
| 21 | 262 | 1 | 1 | - | - | - |
| 21 | 7419 | 3 | - | - | 3 | 3 (cip + nal + tet) |
| 21 | 9198 | 3 | - | - | 3 | - |
| 21 | 11120 | 1 | 1 | - | - | - |
| 22 | 22 | 1 | 1 | - | - | - |
| 45 | 11 | 1 | 1 | - | - | - |
| 45 | 45 | 27 | 16 | - | 11 | - |
| 45 | 137 | 5 | 3 | - | 2 | - |
| 45 | 538 | 3 | - | - | 3 | - |
| 45 | 583 | 2 | - | - | 2 | - |
| 45 | 1701 | 1 | 1 | - | - | - |
| 48 | 918 | 2 | - | - | 2 | - |
| 52 | 2066 | 2 | - | - | 2 | - |
| 52 | 2100 | 1 | 1 | 1 (tet) | - | - |
| 206 | 572 | 2 | - | - | 2 | 2 (cip + nal) |
| 257 | 257 | 14 | 3 | - | 11 | 2 (cip + nal) |
| 283 | 267 | 2 | 2 | - | - | - |
| 464 | 464 | 1 | 1 | 1 (tet) | - | - |
| 677 | 677 | 3 | 3 | - | - | - |
| 692 | 692 | 2 | 1 | - | 1 | - |
| 692 | 4776 | 2 | 2 | - | - | - |
| 702 | 5152 | 1 | - | - | 1 | - |
| 952 | 4582 | 1 | - | - | 1 | - |
| 952 | 6447 | 1 | - | - | 1 | - |
| 952 | 9716 | 1 | - | - | 1 | - |
| 1034 | 1033 | 4 | 1 | - | 3 | - |
| 1034 | 1034 | 1 | - | - | 1 | 1 (cip + nal) |
| 1034 | 2314 | 1 | 1 | - | - | - |
| 1034 | 9715 | 2 | - | - | 2 | - |
| 1332 | 696 | 1 | - | - | 1 | - |
| NA | 441 | 1 | - | - | 1 | 1 (cip + nal + tet) |
| NA | 586 | 1 | 1 | - | - | - |
| NA | 704 | 1 | 1 | - | - | - |
| NA | 1397 | 1 | - | - | 1 | - |
| NA | 1525 | 2 | 2 | - | - | - |
| NA | 1959 | 1 | 1 | - | - | - |
| NA | 3502 | 1 | - | - | 1 | - |
| NA | 3923 | 2 | 1 | - | 1 | - |
| NA | 4307 | 1 | 1 | - | - | - |
| NA | 5559 | 1 | - | - | 1 | - |
| NA | 6386 | 1 | - | - | 1 | - |
| NA | 6427 | 1 | 1 | - | - | - |
| NA | 8555 | 1 | 1 | - | - | - |
| NA | 9834 | 1 | - | -- | 1 | - |
| NA | 10226 | 1 | - | - | 1 | - |
| NA | 10818 | 1 | 1 | - | - | - |
| NA | 11119 | 1 | 1 | - | - | - |
| NA | 11121 | 1 | - | - | 1 | - |
| Total | Organic | Conventional | ||||
|---|---|---|---|---|---|---|
| CC | MLST | No. of Isolates | No. of Isolates | Resistant | No. of Isolates | Resistant |
| 82 | 829 | 19 | 17 | - | 2 | - |
| 82 | 855 | 8 | 8 | - | - | - |
| 82 | 1142 | 1 | - | - | 1 | - |
| 82 | 1544 | 1 | - | - | 1 | 1 (strept) |
| 82 | 2178 | 1 | - | - | 1 | 1 (cip + nal) |
| 82 | 4709 | 3 | - | - | 3 | - |
| Conventional | Organic | |
|---|---|---|
| Chicken producers within the Swedish Campylobacter program | 110 | 15 |
| Chickens produced (2017/2018/2019) | 100/99/103 million | 855,000/660,000/720,000 |
| Chickens in one compartment | Up to 60,000 | Up to 4800 |
| Maximum stocking density (kg/m2) | 36 kg | 20 kg |
| Maximum stocking number (chickens/m2) | 25 | 10 |
| Age of slaughter | 28–35 days * | 60–70 days |
| Outdoor access | Not at all | May to Sep, >4 m2/chicken |
| Breed | Ross and Cobb | Hubbard, Rowan Ranger |
| Coccidiostats | Narasin until 3 d before slaughter | Not at all |
| Campylobacter status (2017/2018/2019) | 11%/9%/5% | 40%/38%/57% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansson, I.; Ellström, P.; Nilsson, O.; Chaba, M.; Skarin, M.; Fernström, L.-L.; Frosth, S. Differences in Genotype and Antimicrobial Resistance between Campylobacter spp. Isolated from Organic and Conventionally Produced Chickens in Sweden. Pathogens 2021, 10, 1630. https://doi.org/10.3390/pathogens10121630
Hansson I, Ellström P, Nilsson O, Chaba M, Skarin M, Fernström L-L, Frosth S. Differences in Genotype and Antimicrobial Resistance between Campylobacter spp. Isolated from Organic and Conventionally Produced Chickens in Sweden. Pathogens. 2021; 10(12):1630. https://doi.org/10.3390/pathogens10121630
Chicago/Turabian StyleHansson, Ingrid, Patrik Ellström, Oskar Nilsson, Matilda Chaba, Moa Skarin, Lise-Lotte Fernström, and Sara Frosth. 2021. "Differences in Genotype and Antimicrobial Resistance between Campylobacter spp. Isolated from Organic and Conventionally Produced Chickens in Sweden" Pathogens 10, no. 12: 1630. https://doi.org/10.3390/pathogens10121630
APA StyleHansson, I., Ellström, P., Nilsson, O., Chaba, M., Skarin, M., Fernström, L.-L., & Frosth, S. (2021). Differences in Genotype and Antimicrobial Resistance between Campylobacter spp. Isolated from Organic and Conventionally Produced Chickens in Sweden. Pathogens, 10(12), 1630. https://doi.org/10.3390/pathogens10121630






