Clinical and Anatomopathological Evaluation of BALB/c Murine Models Infected with Isolates of Seven Pathogenic Sporothrix Species
Abstract
:1. Introduction
2. Results
2.1. In Vivo Assessment of the Sporothrix Species
2.2. Splenic Index, Fungal Burden in the Spleens and Survival Assessment
2.3. Histological Studies
3. Discussion
4. Materials and Methods
4.1. Isolates
4.2. Ethics Statement
4.3. Mice Experimental Inoculation
4.4. Immunosuppressive Treatments
4.5. Fungal Reactivation
4.6. Fungal Inoculation
4.7. Euthanasia, Necropsy, CFU Determination, Splenic Index, and Survival Assessment
4.8. Histopathology
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marimon, R.; Geneé, J.; Cano, J.; Trilles, L.; Dos Santos Lazeéra, M.; Guarro, J. Molecular Phylogeny of Sporothrix schenckii. J. Clin. Microbiol. 2006, 44, 3251–3256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marimon, R.; Cano, J.; Geneé, J.; Sutton, D.A.; Kawasaki, M.; Guarro, J. Sporothrix brasiliensis, S. globosa, and S. mexicana, Three New Sporothrix Species of Clinical Interest. J. Clin. Microbiol. 2007, 45, 3198–3206. [Google Scholar] [CrossRef] [Green Version]
- Marimon, R.; Gené, J.; Cano-Lira, J.F.; Guarro, J. Sporothrix luriei: A rare fungus from clinical origin. Med. Mycol. 2008, 46, 621–625. [Google Scholar] [CrossRef] [Green Version]
- Marimon, R.; Serena, C.; Geneé, J.; Cano, J.; Guarro, J. In Vitro Antifungal Susceptibilities of Five Species of Sporothrix. Antimicrob. Agents Chemother. 2008, 52, 732–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, N.; Oliveira, M.M.E.; Portela, M.A.; Santos, C.; Zancope-Oliveira, R.M.; Lima, N. Sporotrichosis Caused bySporothrix mexicana, Portugal. Emerg. Infect. Dis. 2011, 17, 1975–1976. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.M.E.; Almeida-Paes, R.; Muniz, M.M.; Gutierrez-Galhardo, M.C.; Zancope-Oliveira, R.M. Phenotypic and Molecular Identification of Sporothrix Isolates from an Epidemic Area of Sporotrichosis in Brazil. Mycopathologia 2011, 172, 257–267. [Google Scholar] [CrossRef]
- Morrison, A.S.; Lockhart, S.R.; Bromley, J.G.; Kim, J.Y.; Burd, E.M. An environmental Sporothrix as a cause of corneal ulcer. Med. Mycol. Case Rep. 2013, 2, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Choappa, R.C.; Gaete, S.P.; Badilla, V.R.; Oyarzo, P.V.; Sanchez, H.O. Virulencia de Sporothrix globosa en modelos murinos. Rev. Argent. Microbiol. 2016, 48, 196–199. (In Spanish) [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.M.; Choappa, R.C.; Fernandes, G.F.; de Hoog, G.S.; de Camargo, Z.P. Sporothrix chilensis sp. nov. (Ascomycota: Ophiostomatales), a soil-borne agent of human sporotrichosis with mild-pathogenic potential to mammals. Fungal Biol. 2016, 120, 246–264. [Google Scholar] [CrossRef]
- Valeriano, C.; De Lima-Neto, R.G.; Inácio, C.P.; Rabello, V.B.D.S.; Oliveira, E.P.; Zancopé-Oliveira, R.M.; Almeida-Paes, R.; Neves, R.P.; De Oliveira, M.M.E. Is Sporothrix chilensis circulating outside Chile? PLOS Negl. Trop. Dis. 2020, 14, e0008151. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Bonifaz, A.; Gutierrez-Galhardo, M.C.; Mochizuki, T.; Li, S. Global epidemiology of sporotrichosis. Med. Mycol. 2015, 53, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.A.; Gremião, I.D.F.; Kitada, A.A.B.; Boechat, J.S.; Viana, P.G.; Schubach, T.M.P. The epidemiological scenario of feline sporotrichosis in Rio de Janeiro, State of Rio de Janeiro, Brazil. Rev. Soc. Bras. Med. Trop. 2014, 47, 392–393. [Google Scholar] [CrossRef] [Green Version]
- Gremião, I.D.F.; Oliveira, M.M.E.; De Miranda, L.H.M.; Freitas, D.F.S.; Pereira, S.A. Geographic Expansion of Sporotrichosis, Brazil. Emerg. Infect. Dis. 2020, 26, 621–624. [Google Scholar] [CrossRef] [Green Version]
- Etchecopaz, A.; Toscanini, M.; Gisbert, A.; Mas, J.; Scarpa, M.; Iovannitti, C.; Bendezú, K.; Nusblat, A.; Iachini, R.; Cuestas, M. Sporothrix brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina. J. Fungi 2021, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.M.E.; Veríssimo, C.; Sabino, R.; Aranha, J.; Zancopé-Oliveira, R.M.; Sampaio, P.; Pais, C. First autochthone case of sporotrichosis by Sporothrix globosa in Portugal. Diagn. Microbiol. Infect. Dis. 2014, 78, 388–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordoloi, P.; Nath, R.; Borgohain, M.; Huda, M.M.; Barua, S.; Dutta, D.; Saikia, L. Subcutaneous Mycoses: An Aetiological Study of 15 Cases in a Tertiary Care Hospital at Dibrugarh, Assam, Northeast India. Mycopathologia 2015, 179, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Moussa, T.A.A.; Kadasa, N.M.S.; Al Zahrani, H.S.; Ahmed, S.A.; Feng, P.; Ende, A.H.G.G.V.D.; Zhang, Y.; Kano, R.; Li, F.; Li, S.; et al. Origin and distribution of Sporothrix globosa causing sapronoses in Asia. J. Med. Microbiol. 2017, 66, 560–569. [Google Scholar] [CrossRef]
- Han, H.S.; Kano, R. Feline sporotrichosis in Asia. Braz. J. Microbiol. 2021, 52, 125–134. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; De Hoog, S.; de Carmargo, Z.P. Emergence of pathogenicity in the Sporothrix schenckii complex. Med. Mycol. 2013, 51, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.C.; Lopes, P.G.; Spader, T.B.; Mahl, C.D.; Tronco-Alves, G.R.; Lara, V.M.; Santurio, J.M.; Alves, S.H. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J. Clin. Microbiol. 2011, 49, 3047–3049. [Google Scholar] [CrossRef] [Green Version]
- Thomson, J.; Trott, D.J.; Malik, R.; Galgut, B.; McAllister, M.M.; Nimmo, J.; Renton, D.; Kidd, S.E. An atypical cause of sporotrichosis in a cat. Med. Mycol. Case Rep. 2019, 23, 72–76. [Google Scholar] [CrossRef]
- Corrȴa-Moreira, D.; Borba, C.D.M.; Barreira, T.G.; Menezes, R.C.; Gremião, I.D.; Pereira, S.A.; Oliveira, M.M.E. Molecular epidemiology and experimental sporotrichosis: Challenges and perspective. In Conhecimento, Conservação e uso de Fungos; Oliveira, L.A., Ed.; INPA: Manaus, Brazil, 2020; pp. 67–74. [Google Scholar]
- de Beer, Z.; Duong, T.; Wingfield, M. The divorce of Sporothrix and Ophiostoma: Solution to a problematic relationship. Stud. Mycol. 2016, 83, 165–191. [Google Scholar] [CrossRef] [Green Version]
- Corrêa-Moreira, D.; De Luca, P.M.; Romeo, O.; Menezes, R.C.; Paes, R.A.; Oliveira, R.Z.; De Moraes, A.M.; Neto, R.G.D.L.; Borba, C.D.M.; De Oliveira, M.M.E. Tregs in the immune response of BALB/c mice experimentally infected with species of the Sporothrix genus. Future Microbiol. 2020, 15, 1217–1225. [Google Scholar] [CrossRef]
- Oliveira, M.M.E.; Almeida-Paes, R.; Corrêa-Moreira, D.; Borba, C.D.M.; Menezes, R.C.; Freitas, D.F.S.; Do Valle, A.C.F.; Schubach, A.D.O.; Barros, M.B.D.L.; Nosanchuk, J.D.; et al. A case of sporotrichosis caused by different Sporothrix brasiliensis strains: Mycological, molecular, and virulence analyses. Memórias Inst. Oswaldo Cruz 2019, 114, e190260. [Google Scholar] [CrossRef] [PubMed]
- de Sequeira, D.C.; Peixoto, M.L.; De Luca, P.M.; Oliveira-Ferreira, J.; Antas, P.R.; Borba, C.M. Detection of antibody to Purpureocillium lilacinum by immunofluorescent assay and flow cytometry in serum of infected C57BL/6 mice. J. Immunol. Methods 2013, 396, 147–151. [Google Scholar] [CrossRef] [PubMed]
- De Sequeira, D.C.M.; Menezes, R.; Oliveira, M.M.E.; Antas, P.R.Z.; De Luca, P.M.; De Oliveira-Ferreira, J.; Borba, C.D.M. Experimental Hyalohyphomycosis by Purpureocillium lilacinum: Outcome of the Infection in C57BL/6 Murine Models. Front. Microbiol. 2017, 8, 1617. [Google Scholar] [CrossRef] [Green Version]
- Brito, M.M.S.; Conceição-Silva, F.; Morgado, F.N.; Raibolt, P.S.; Schubach, A.; Schubach, T.P.; Schäffer, G.M.V.; Borba, C.M. Comparison of virulence of different Sporothrix schenckii clinical isolates using experimental murine model. Med. Mycol. 2007, 45, 721–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos Brito, M.M.; da Silva Lima, M.; Morgado, F.N.; Raibolt, P.; Menezes, R.; Conceição-Silva, F.; de Moraes Borba, C. Characteristics of Paecilomyces lilacinus infection comparing immunocompetent with immunosuppressed murine model. Mycoses 2011, 54, e513–e521. [Google Scholar] [CrossRef] [PubMed]
- Romeo, O.; Scordino, F.; Criseo, G. New Insight into Molecular Phylogeny and Epidemiology of Sporothrix schenckii Species Complex Based on Calmodulin-Encoding Gene Analysis of Italian Isolates. Mycopathologia 2011, 172, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.; Dos Santos, P.O.; Rodrigues, A.M.; Sasaki, A.A.; Burger, E.; De Camargo, Z.P. Characterization of virulence profile, protein secretion and immunogenicity of different Sporothrix schenckii sensu stricto isolates compared with S. globosa and S. brasiliensis species. Virulence 2013, 4, 241–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makri, N.; Paterson, G.K.; Gregge, F.; Urquhart, C.; Nuttall, T. First case report of cutaneous sporotrichosis (Sporothrix species) in a cat in the UK. J. Feline Med. Surg. Open Rep. 2020, 6, 2055116920906001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida-Paes, R.; de Oliveira, L.C.; Oliveira, M.M.E.; Gutierrez-Galhardo, M.C.; Nosanchuk, J.D.; Zancopé-Oliveira, R.M. Phenotypic Characteristics Associated with Virulence of Clinical Isolates from the Sporothrix Complex. BioMed Res. Int. 2015, 2015, 212308. [Google Scholar] [CrossRef] [Green Version]
- Arrillaga-Moncrieff, I.; Capilla, J.; Mayayo, E.; Marimon, R.; Marine, M.; Genis, J.; Cano-Lira, J.F.; Guarro, J. Different virulence levels of the species of Sporothrix in a murine model. Clin. Microbiol. Infect. 2009, 15, 651–655. [Google Scholar] [CrossRef] [Green Version]
- Nobre, M.O.; Antunes, T.A.; Oliveira, I.A.; Berg, V.; Lucia, T., Jr.; Fernandes, C.G.; Meireles, M.C.A.; Ferreiro, L. Development of experimental sporothrichosis in a murine model with yeast and mycelial forms of Sporothrix schenckii. Acta Sci. Vet. 2018, 31, 161–166. [Google Scholar] [CrossRef]
- Batista-Duharte, A.; Téllez-Martínez, D.; de Andrade, C.R.; Portuondo, D.L.; Jellmayer, J.A.; Polesi, M.C.; Carlos, I.Z. Sporothrix brasiliensis induces a more severe disease associated with sustained Th17 and regulatory T cells responses than Sporothrix schenckii sensu stricto in mice. Fungal Biol. 2018, 122, 1163–1170. [Google Scholar] [CrossRef] [Green Version]
- Della Terra, P.P.; Rodrigues, A.M.; Fernandes, G.F.; Nishikaku, A.S.; Burger, E.; De Camargo, Z.P. Exploring virulence and immunogenicity in the emerging pathogen Sporothrix brasiliensis. PLoS Negl. Trop. Dis. 2017, 11, e0005903. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Khanna, N.; Stuehler, C.; Lünemann, A.; Wójtowicz, A.; Bochud, P.-Y.; LeibundGut-Landmann, S. Host response to fungal infections—How immunology and host genetics could help to identify and treat patients at risk. Swiss Med. Wkly. 2016, 146, w14350. [Google Scholar] [CrossRef] [PubMed]
- Shpilberg, Y.; Beaudry, J.L.; D’Souza, A.; Campbell, J.; Peckett, A.; Riddell, M.C. A rodent model of rapid-onset diabetes (ROD) induced by glucocorticoids and high-fat feeding. Dis. Models Mech. 2012, 5, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Peckett, A.J.; Wright, D.C.; Riddell, M.C. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism 2011, 60, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Matthews, L.C.; Hanley, N.A. The stress of starvation: Glucocorticoid restraint of beta cell development. Diabetologia 2010, 54, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Watts, L.M.; Manchem, V.P.; Leedom, T.A.; Rivard, A.L.; McKay, R.A.; Bao, D.; Neroladakis, T.; Monia, B.P.; Bodenmiller, D.M.; Cao, J.X.-C.; et al. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid. Diabetes 2005, 54, 1846–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, A.; Johnson, J.H.; Ohneda, M.; McAllister, C.T.; Inman, L.; Alam, T.; Unger, R.H. Roles of insulin resistance and beta-cell dysfunction in dexamethasone-induced diabetes Roles of insulin resistance and beta-cell dysfunction in dexamethasone-induced diabetes. J. Clin. Invest. 1992, 90, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Jahng, J.W.; Kim, N.Y.; Ryu, V.; Yoo, S.B.; Kim, B.-T.; Kang, D.-W.; Lee, J.-H. Dexamethasone reduces food intake, weight gain and the hypothalamic 5-HT concentration and increases plasma leptin in rats. Eur. J. Pharmacol. 2008, 581, 64–70. [Google Scholar] [CrossRef]
- Ogawa, M.M.; Mariano, M.; Silva, M.R.R.; Enokihara, M.M.S.E.S.; Michalany, N.S.; Nishikaku, A.S.; Silvestre, A.M.; Tomimori, J. Study of tissue inflammatory response in different mice strains infected by dematiaceous fungi Fonsecaea pedrosoi. An. Bras. Dermatol. 2019, 94, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Manente, F.A.; Quinello, C.; Ferreira, L.S.; de Andrade, C.R.; Jellmayer, J.A.; Portuondo, D.L.; Batista-Duharte, A.; Carlos, I.Z. Experimental sporotrichosis in a cyclophosphamide-induced immunosuppressed mice model. Med. Mycol. 2018, 56, 711–722. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.M.E.; de Almeida-Paes, R.; Muniz, M.D.M.; Barros, M.B.D.L.; Galhardo, M.C.G.; Zancope-Oliveira, R.M. Sporotrichosis Caused By Sporothrix globosa in Rio De Janeiro, Brazil: Case Report. Mycopathologia 2010, 169, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Goihman-Yahr, M.; Pine, L.; Albornoz, M.C.; Yarzábal, L.; De Gomez, M.H.; Martin, B.S.; Ocanto, A.; Molina, T.; Convit, J. Studies on plating efficiency and estimation of viability of suspensions of Paracoccidioides brasiliensis yeast cells. Mycopathologia 1980, 71, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.; Mathews, H.L.; Bezerra, L.M.L. Differences in virulence of Sporothrix schenckii conidia related to culture conditions and cell-wall components. J. Med. Microbiol. 1999, 48, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
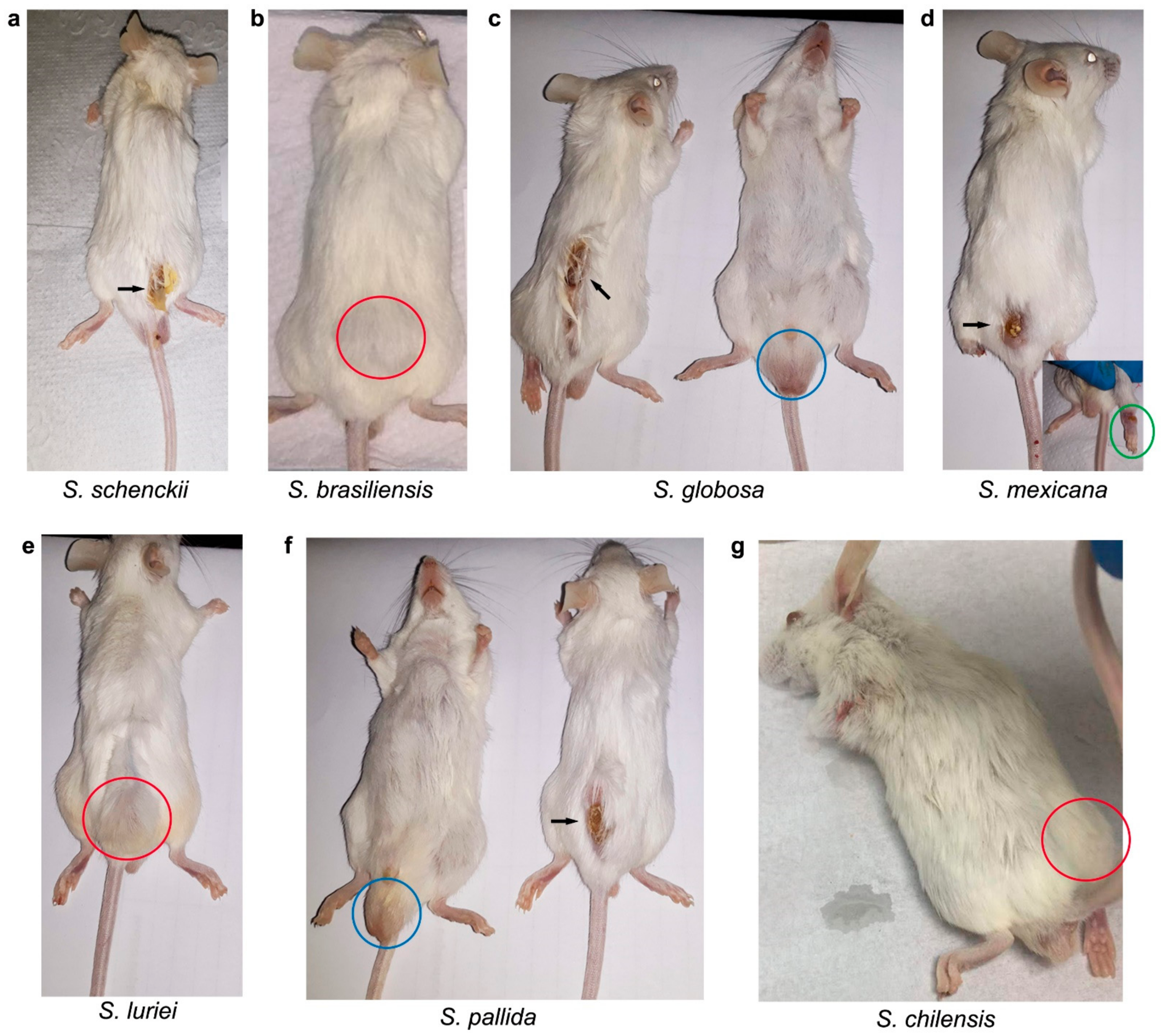




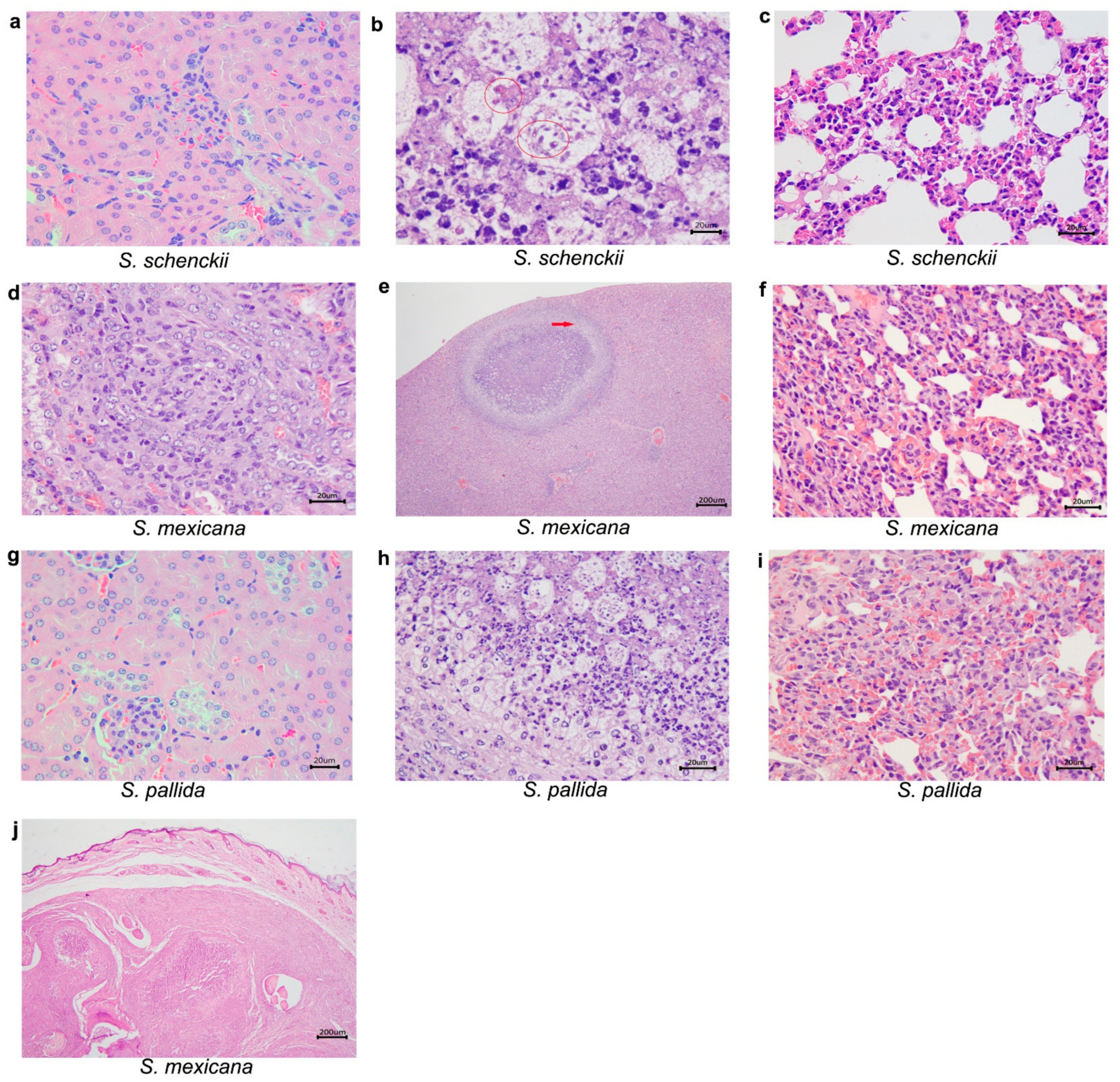
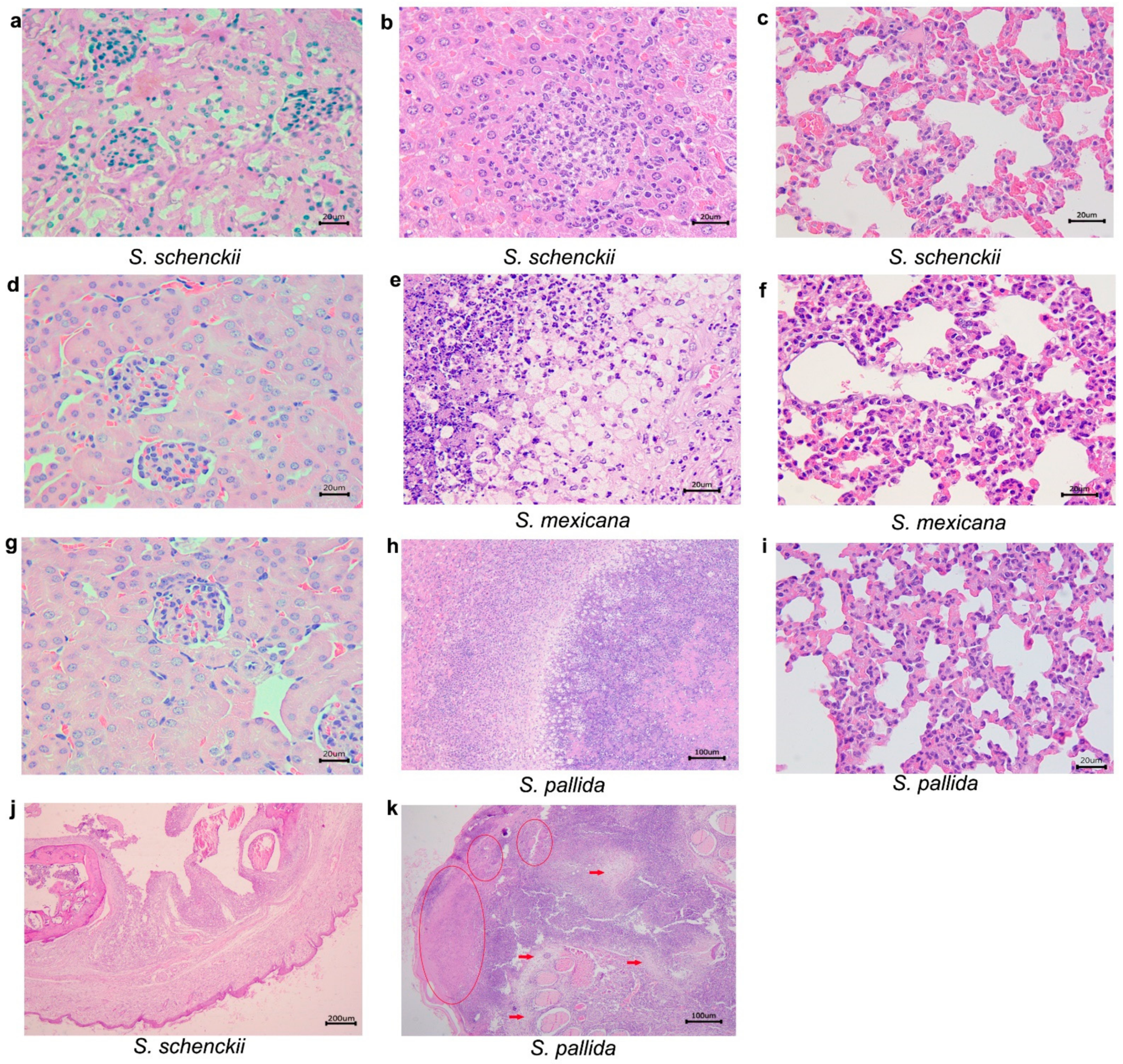
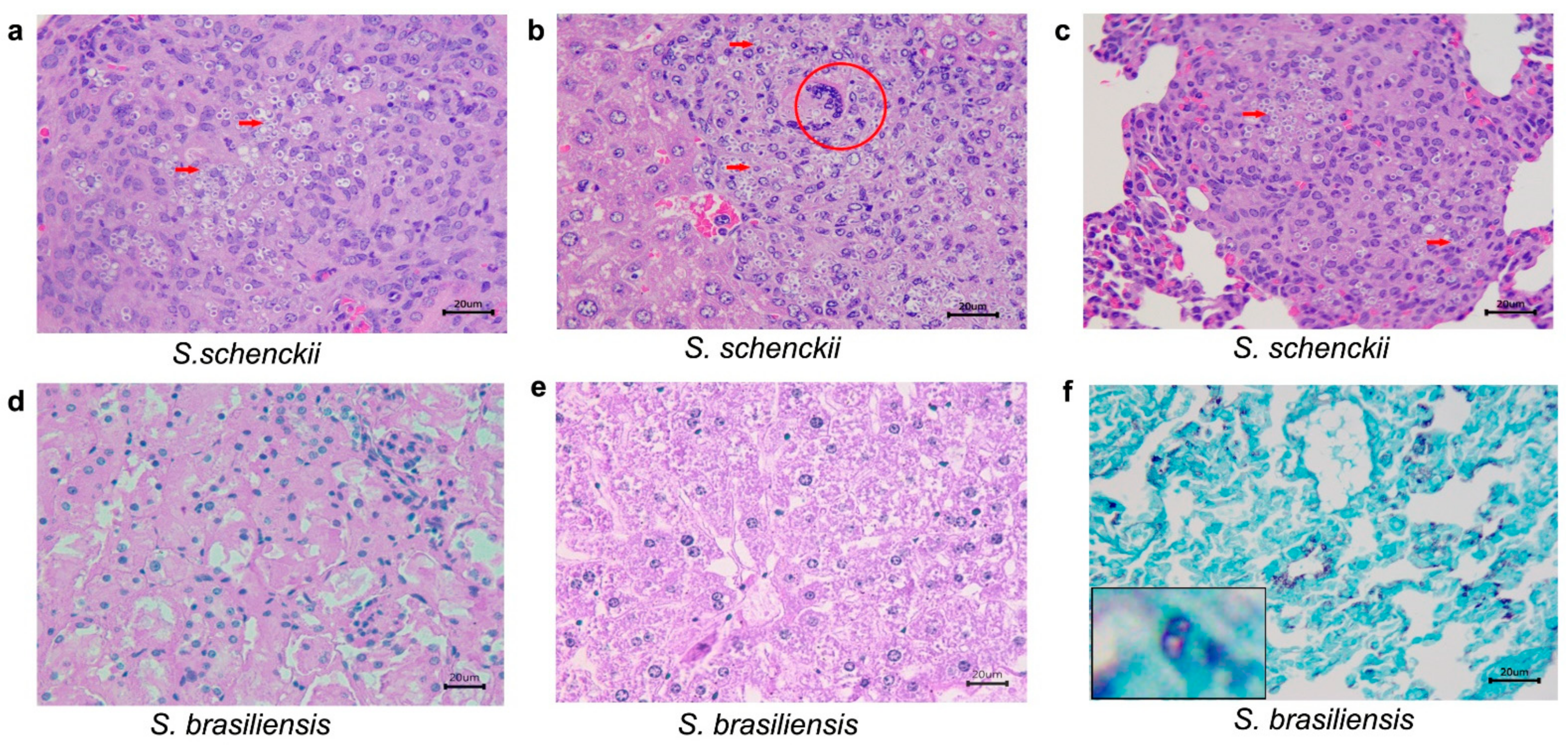


| Species | Days after Inoculation | Mean Weigh of Mice (g) ± SD | |
|---|---|---|---|
| Immunocompetent | Immunosuppressed | ||
| S. schenckii sensu stricto | 21 | 26.66 ± 1.35 | 22.86 ** ± 1.49 |
| 35 | 28.44 ± 1.78 | 19.96 ** ± 2.76 | |
| 49 | 27.37 ± 2.96 | Ø | |
| S. brasiliensis | 21 | 30.82 ± 3.28 | 23.83 ± 0.73 |
| 35 | 31.22 ± 1.56 | 25.92 ± 1.65 | |
| 49 | 31.26 ± 0.65 | 23.81 ± 1.44 | |
| S. globosa | 21 | 26.65 ± 1.86 | |
| 35 | 30.67 ± 1.35 | N.I. | |
| 49 | 29.66 ± 1.01 | ||
| S. mexicana | 21 | 26.57 ± 1.85 | |
| 35 | 26.51 ± 3.12 | N.I. | |
| 49 | 29.97 ± 1.85 | ||
| S. luriei | 21 | 27.61 ± 1.21 | |
| 35 | 30.31 ± 0.71 | N.I. | |
| 49 | 30.94 ± 0.64 | ||
| S. pallida | 21 | 27.23 ± 0.52 | |
| 35 | 29.52 ± 2.20 | N.I. | |
| 49 | 26.81 ± 2.37 | ||
| S. chilensis | 21 | 30.15 ± 1.65 | |
| 35 | 30.45 ± 2.01 | N.I. | |
| 49 | 28.53 ± 0.58 | ||
| CONTROL | 21 | 28.96 ± 0.42 | 24.30 ± 1.42 |
| 35 | 30.44 ± 20.1 | 23.15 ± 1.06 | |
| 49 | 29.30 ± 2.60 | 23.58 ± 0.97 | |
| Days after Inoculation | Sporothrix Species | Organs | ||
|---|---|---|---|---|
| Kidney | Liver | Lungs | ||
| 21 | S. schenckii sensu stricto | ABSENT | ABSENT | Pneumonia, pyogranulomatous, multifocal and mild. Poorly organized granuloma. GMS: rounded yeasts amid the inflammatory infiltrate. |
| S.mexicana | Interstitial nephritis, pyogranulomatous, focal and mild. Poorly organized granuloma. GMS: remaining fungal cell wall amid the inflammatory infiltrate. | Hepatitis, pyogranulomatous, necrotizing, multifocal and moderate, with areas of fibrosis. Poorly organized granuloma. GMS: Rounded yeasts within the granulomas. | ABSENT | |
| S. pallida | Interstitial nephritis, pyogranulomatous, multifocal and moderate in the cortex. Poorly organized granuloma. GMS: rare rounded yeasts amid the inflammatory infiltrate. | Hepatitis, granulomatous, focal and moderate. Well organized granuloma. GMS: Rounded and cigar shaped yeasts, amid the inflammatory infiltrate. | Pneumonia, granulomatous multifocal and moderate to severe. Well organized granuloma. GMS: rounded yeasts, amid the inflammatory infiltrate. | |
| 35 | S. schenckii sensu stricto | ABSENT | Hepatitis, pyogranulomatous, multifocal and moderate. Poorly organized granuloma. GMS: fungal structures were not detected. | Pneumonia, granulomatous, diffuse and moderate to severe. Poorly organized granuloma. GMS: fungal structures were not detected. |
| S. mexicana | Interstitial nephritis, pyogranulomatous, focal and discreet in the cortex. Poorly organized granuloma. GMS: fungal structures were not detected. | Hepatitis, pyogranulomatous, necrotizing, multifocal and moderate. Multiple fibrosis areas. Well organized granuloma. GMS: rounded with a narrow-based budding or cigar-shaped yeasts within granulomas. | Pneumonia, pyogranulomatous, diffuse and moderate to severe. Poorly organized granuloma. GMS: rare rounded yeast forms amid the inflammatory infiltrate. | |
| S. pallida | ABSENT | Hepatitis, suppurative or lymphoplasmacytic infiltrate, multifocal and mild. Poorly organized granuloma. GMS: Rounded yeasts within the granulomas. | Pneumonia, granulomatous, diffuse and moderate to severe. Poorly organized granuloma. GMS: fungal structures were not detected. | |
| 49 | S. schenckii sensu stricto | ABSENT | Hepatitis, pyogranulomatous, necrotizing, multifocal and moderate to severe. Well organized granulomas. GMS: rare round yeast forms within the granulomas. | Pneumonia, suppurative, diffuse and mild. GMS: fungal structures were not detected. |
| S. mexicana | ABSENT | Hepatitis, pyogranulomatous, multifocal and moderate. Multifocal areas of fibrosis. Poorly organized granuloma. GMS: rounded and cigar shaped yeasts, amid the inflammatory infiltrate. | Pneumonia, suppurative, diffuse and moderate. Poorly organized granuloma. GMS: fungal structures were not detected. | |
| S. pallida | ABSENT | Hepatitis, pyogranulomatous, multifocal and moderate to severe. Poorly organized granuloma. GMS: rounded or cigar shaped yeasts within the granuloma amid necrosis areas. | Pneumonia, suppurative, diffuse and moderate to severe. Poorly organized granuloma. GMS: fungal structures were not detected. | |
| CONTROL | ABSENT | ABSENT | ABSENT | |
| Days after Inoculation | Sporothrix Species | Organs | |||
|---|---|---|---|---|---|
| Kidney | Liver | Lungs | |||
| 21 | S. schenckii sensu stricto | ABSENT | ABSENT | ABSENT | |
| S. brasiliensis | ABSENT | ABSENT | ABSENT | ||
| 35 | S. schenckii sensu stricto | Interstitial nephritis, granulomatous, multifocal and severe in the capsule, cortex, medulla and pelvis. Multifocal areas of fibrosis. Poorly organized granuloma. GMS: abundant rounded yeasts with a narrow-based budding or cigar-shaped in in the capsule, cortical, medullary region and renal pelvis. | Hepatitis, pyogranulomatous, necrotizing, multifocal and moderate to severe. Well-organized granulomas. GMS: abundant rounded yeasts with a narrow-based budding or cigar-shaped, amid the inflammatory infiltrate | Pneumonia, pyogranulomatous, multifocal and moderate to severe. Abundant yeast-like structures amid the inflammatory infiltrate. GMS: abundant rounded yeasts with a narrow-based budding or cigar-shaped, amid the inflammatory infiltrate | |
| S. brasiliensis | ABSENT | ABSENT | ABSENT | ||
| 49 | S. schenckii sensu stricto | All animals had died by this point in the study, and histopathological analysis was not possible. | |||
| S. brasiliensis | ABSENT | ABSENT | Tissue alterations were not detected by HE staining. GMS: rounded yeasts with a narrow-based budding or cigar-shaped, amid the inflammatory infiltrate | ||
| CONTROL (of all species at different times) | ABSENT | ABSENT | ABSENT | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa-Moreira, D.; Menezes, R.C.; Romeo, O.; Borba, C.M.; Oliveira, M.M.E. Clinical and Anatomopathological Evaluation of BALB/c Murine Models Infected with Isolates of Seven Pathogenic Sporothrix Species. Pathogens 2021, 10, 1647. https://doi.org/10.3390/pathogens10121647
Corrêa-Moreira D, Menezes RC, Romeo O, Borba CM, Oliveira MME. Clinical and Anatomopathological Evaluation of BALB/c Murine Models Infected with Isolates of Seven Pathogenic Sporothrix Species. Pathogens. 2021; 10(12):1647. https://doi.org/10.3390/pathogens10121647
Chicago/Turabian StyleCorrêa-Moreira, Danielly, Rodrigo C. Menezes, Orazio Romeo, Cintia M. Borba, and Manoel M. E. Oliveira. 2021. "Clinical and Anatomopathological Evaluation of BALB/c Murine Models Infected with Isolates of Seven Pathogenic Sporothrix Species" Pathogens 10, no. 12: 1647. https://doi.org/10.3390/pathogens10121647
APA StyleCorrêa-Moreira, D., Menezes, R. C., Romeo, O., Borba, C. M., & Oliveira, M. M. E. (2021). Clinical and Anatomopathological Evaluation of BALB/c Murine Models Infected with Isolates of Seven Pathogenic Sporothrix Species. Pathogens, 10(12), 1647. https://doi.org/10.3390/pathogens10121647








