Geographic Distribution, Genetic Variability and Biological Properties of Rice Orange Leaf Phytoplasma in Southeast Asia
Abstract
:1. Introduction
2. Results
2.1. Field Survey of ROLP in the Philippines, Vietnam and Cambodia
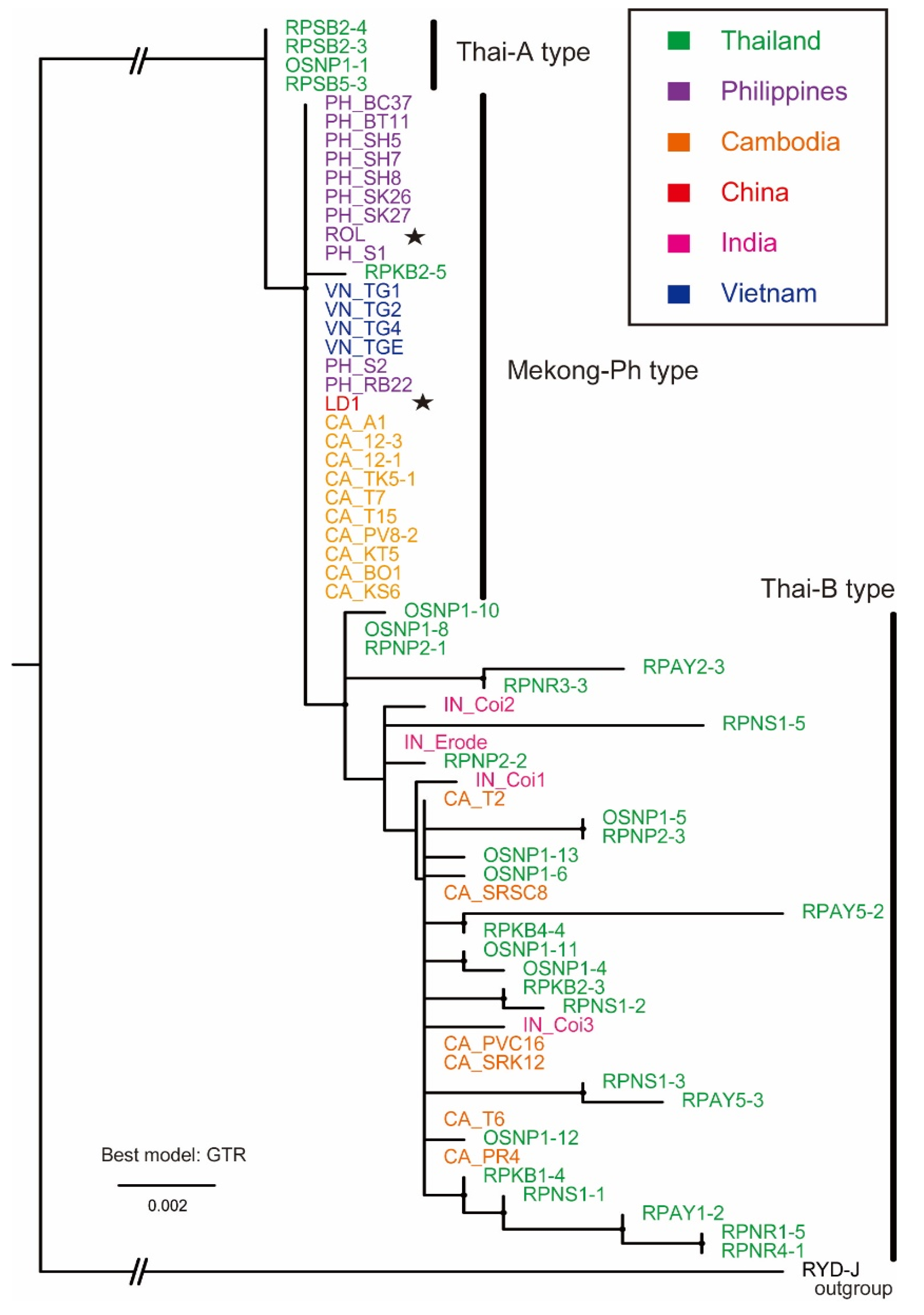
2.2. Phylogenetic Analysis of ROLP Based on 16S rDNA Sequences
2.3. Insect Population Dynamics and Detection of ROLP from Field-Collected Insects
2.4. Phloematic Accumulation of ROLP
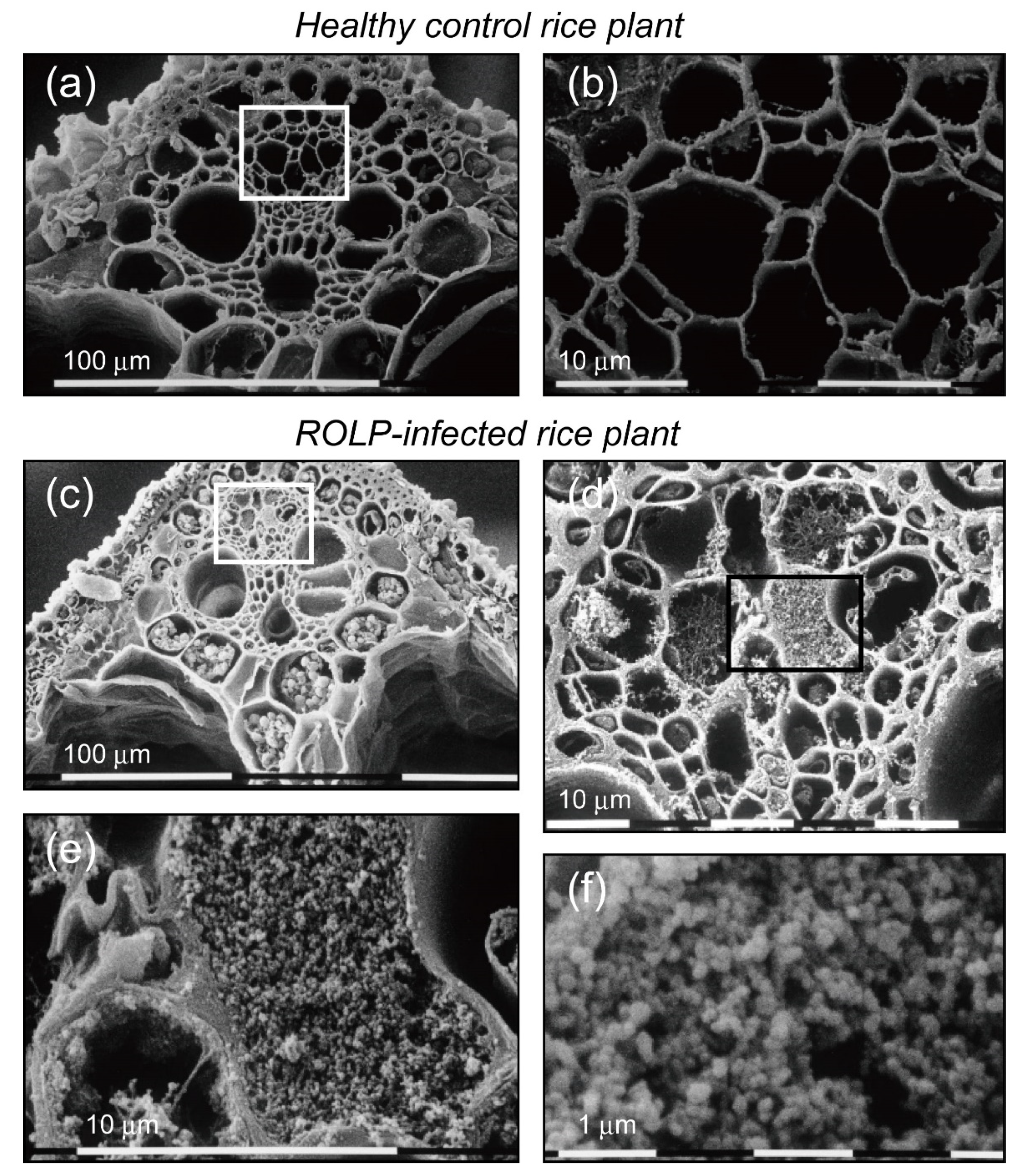
2.5. Histological Observation of ROLP-Infected Rice
3. Discussion
3.1. Global Population Structure of ROLP Based on Currently Available 16S rDNA Sequences
3.2. Paddy Leafhopper and Planthopper Populations and the Transmission of ROLP in Southeast Asia
3.3. Biological Properties of ROLP in the Host Rice Plants and Future Research Prospects
4. Materials and Methods
4.1. Field Survey of ROLP-Infested Rice Paddies
4.2. DNA Extraction, Nested PCR and LAMP
4.3. Sequencing and Phylogenetic Analysis
4.4. Insect Population Analysis
4.5. Microscopic Observation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Namba, S. Molecular and biological properties of phytoplasmas. Proc. Jpn. Acad. Ser. B 2019, 95, 401–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The IRPCM Phytoplasma/Spiroplasma Working Team—Phytoplasma Taxonomy Group. ‘Candidatus Phytoplasma’, a taxon for the wall-less, non-helical prokaryotes that colonize plant phloem and insects. Int. J. Syst. Evol. Microbiol. 2004, 54, 1243–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.-Y.; Sawayanagi, T.; Wongkaew, P.; Kakizawa, S.; Nishigawa, H.; Wei, W.; Oshima, K.; Miyata, S.-I.; Ugaki, M.; Hibi, T. ‘Candidatus Phytoplasma oryzae’, a novel phytoplasma taxon associated with rice yellow dwarf disease. Int. J. Syst. Evol. Microbiol. 2003, 53, 1925–1929. [Google Scholar] [CrossRef]
- Muniyappa, V.; Raychaudhuri, S. Rice yellow dwarf disease. In Mycoplasma Diseases of Crops; Springer: New York, NY, USA, 1988; pp. 233–284. [Google Scholar]
- Duan, Y.; Hibino, H. Orange leaf symptoms on rice. Int. Rice Res. Newsl. 1988, 13, 34. [Google Scholar]
- Li, S.; Hao, W.; Lu, G.; Huang, J.; Liu, C.; Zhou, G. Occurrence and identification of a new vector of rice orange leaf phytoplasma in South China. Plant Dis. 2015, 99, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Maejima, K.; Oshima, K.; Namba, S. Exploring the phytoplasmas, plant pathogenic bacteria. J. Gen. Plant Pathol. 2014, 80, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Gundersen, D.; Lee, I. Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopathol. Mediterr. 1996, 35, 144–151. [Google Scholar]
- Lee, I.-M.; Hammond, R.; Davis, R.; Gundersen, D. Universal amplification and analysis of pathogen 16S rDNA for classification and identification of mycoplasmalike organisms. Phytopathology 1993, 83, 834–842. [Google Scholar] [CrossRef]
- Bertaccini, A.; Fiore, N.; Zamorano, A.; Tiwari, A.K.; Rao, G.P. Molecular and Serological Approaches in Detection of Phytoplasmas in Plants and Insects. In Phytoplasmas: Plant Pathogenic Bacteria—III: Genomics, Host Pathogen Interactions and Diagnosis; Bertaccini, A., Oshima, K., Kube, M., Rao, G.P., Eds.; Springer: Singapore, 2019; pp. 105–136. [Google Scholar] [CrossRef]
- Nakashima, K.; Murata, N. Destructive plant diseases caused by mycoplasma-like organisms in Asia. Outlook Agric. 1993, 22, 53–58. [Google Scholar] [CrossRef]
- Nakashima, K.; Hayashi, T. Multiplication and Distribution of Rice Yellow Dwarf Phytoplasma in Infected Tissues of Rice and Green Rice Leafhopper Nephotettix cincticeps. Ann. Phytopathol. Soc. Jpn. 1995, 61, 451–455. [Google Scholar] [CrossRef]
- Cabauatan, P.; Koganezawa, H.; Cabunagan, R.; Sta Cruz, F. Rice dwarf (RDV), a new virus disease in the Philippines. Int. Rice Res. Notes 1993, 18, 50. [Google Scholar]
- Hibino, H.; Saleh, N.; Roechan, M. Transmission of two kinds of RTD viruses. Phytopathology 1979, 69, 792–794. [Google Scholar] [CrossRef]
- Azzam, O.; Chancellor, T.-C. The biology, epidemiology, and management of rice tungro disease in Asia. Plant Dis. 2002, 86, 88–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, K. Orange Leaf Disease. In Rice Virus Diseases; Ling, K., Ed.; International Rice Research Institute: Los Banõs, Philippines, 1972; pp. 79–81. [Google Scholar]
- Rivera, C.; Ou, S.; Pathak, M. Transmission studies of the orange leaf disease of rice. Plant Dis. Rep. 1963, 47, 1045–1048. [Google Scholar]
- Jonson, G.B.; Matres, J.M.; Ong, S.; Tanaka, T.; Choi, I.-R.; Chiba, S. Reemerging Rice Orange Leaf Phytoplasma with Varying Symptoms Expressions and Its Transmission by a New Leafhopper Vector—Nephotettix virescens Distant. Pathogens 2020, 9, 990. [Google Scholar] [CrossRef]
- Hibino, H.; Jonson, G.; Sta Cruz, F. Association of mycoplasmalike organisms with rice orange leaf in the Philippines. Plant Dis. 1987, 71, 792–794. [Google Scholar] [CrossRef]
- Saito, Y. Mycoplasma like bodies associated with rice orange leaf disease. Plant Dis. Rep. 1976, 60, 649–651. [Google Scholar]
- Valarmathi, P.; Rabindran, R.; Velazhahan, R.; Suresh, S.; Robin, S. First report of rice orange leaf disease phytoplasma (16 SrI) in rice (Oryza sativa) in India. Australas. Plant Dis. Notes 2013, 8, 141–143. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.T.; Hoat, T.X.; Quan, M.V. Molecular detection and characterization of 16SrI phytoplasma associated with rice orange leaf symptom in Vietnam. Phytopathog. Mol. 2016, 6, 29–32. [Google Scholar] [CrossRef]
- Klinkong, S.; Reanwarakorn, K.; Khwantongyim, C. Molecular classification of Phytoplasma associated with rice orange leaf disease in Thailand. Agric. Sci. J. (Thai.) 2016, 47, 57–68. [Google Scholar]
- Valarmathi, P.; Rabindran, R. Nested PCR assay for detection of Rice Orange Leaf phytoplasma in the Zigzag leafhopper vector, Recilia dorsalis (Motschulsky): A first report. Ecoscan 2015, 9, 837–839. [Google Scholar]
- Wang, Z.; Zhu, Y.; Li, Z.; Yang, X.; Zhang, T.; Zhou, G. Development of a Specific Polymerase Chain Reaction System for the Detection of Rice Orange Leaf Phytoplasma. Plant Dis. 2020, 104, 521–526. [Google Scholar] [CrossRef]
- He, Y.; Li, S.; Hao, W.; Zheng, J.; Zhong, B.; Zhou, G. Molecular detection of rice orange leaf disease and research on its occurrence and distribution in southern China. China Plant Prot. 2016, 2, 9–12. [Google Scholar]
- Khwantongyim, C.; Klinkong, S.; Reanwarakorn, K. Ultrastructure and molecular diversity of phytoplasma associated with rice orange leaf disease in the central part of Thailand. Agric. Sci. J. (Thai.) 2013, 44, 249–258. [Google Scholar]
- Zhu, Y.; He, Y.; Zheng, Z.; Chen, J.; Wang, Z.; Zhou, G. Draft genome sequence of rice orange leaf phytoplasma from Guangdong, China. Genome Announc. 2017, 5, e00430-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilet, F.; Quaicoe, R.N.; Osagie, I.J.; Freire, M.; Foissac, X. Multilocus sequence analysis reveals three distinct populations of ‘Candidatus Phytoplasma palmicola’ with a specific geographical distribution on the African continent. Appl. Environ. Microbiol. 2019, 85, e02716-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.-T.; Kung, H.-J.; Huang, W.; Hogenhout, S.A.; Kuo, C.-H. Species Boundaries and Molecular Markers for the Classification of 16SrI Phytoplasmas Inferred by Genome Analysis. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Malembic-Maher, S.; Desqué, D.; Khalil, D.; Salar, P.; Bergey, B.; Danet, J.-L.; Duret, S.; Dubrana-Ourabah, M.-P.; Beven, L.; Ember, I. When a Palearctic bacterium meets a Nearctic insect vector: Genetic and ecological insights into the emergence of the grapevine Flavescence dorée epidemics in Europe. PLoS Pathog. 2020, 16, e1007967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Oshima, K.; Kakizawa, S.; Arashida, R.; Jung, H.-Y.; Yamaji, Y.; Nishigawa, H.; Ugaki, M.; Namba, S. Interaction between the membrane protein of a pathogen and insect microfilament complex determines insect-vector specificity. Proc. Natl. Acad. Sci. USA 2006, 103, 4252–4257. [Google Scholar] [CrossRef] [Green Version]
- Matsukawa, M.; Ito, K.; Kawakita, K.; Tanaka, T. Current status of the occurrence and farmer perceptions of rice planthopper in Cambodia. Jpn. Agric. Res. Q. 2014, 48, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Richard, P.L.; Solieng, M. Rainfed Lowland Rice in Cambodia: A Baseline Survey; IRRI Research Paper Series; International Rice Research Institute: Manila, Philippines, 1994; p. 152. [Google Scholar]
- Kisimoto, R. Brown planthopper migration. In Proceedings of the Brown Planthopper: Threat to Rice Production in Asia; International Rice Research Institute: Los Banos, Philippines, 1979; pp. 113–124. [Google Scholar]
- Lee, S.; Kim, C.-E.; Cha, B. Migration and distribution of graft-inoculated jujube Witches’-broom Phytoplasma within a Cantharanthus roseus plant. Plant Pathol. J. 2012, 28, 191–196. [Google Scholar] [CrossRef]
- Gai, Y.P.; Han, X.J.; Li, Y.Q.; Yuan, C.Z.; Mo, Y.Y.; Guo, F.Y.; Liu, Q.X.; Ji, X.L. Metabolomic analysis reveals the potential metabolites and pathogenesis involved in mulberry yellow dwarf disease. Plant Cell Environ. 2014, 37, 1474–1490. [Google Scholar] [CrossRef]
- Ebadi, N.; Najafipour, G.; Faghihi, M.M.; Ayazpour, K.; Salehi, M. Interaction between ‘Candidatus Phytoplasma australasiae’ and Tomato yellow leaf curl virus in tomato plants. Eur. J. Plant Pathol. 2020, 158, 733–744. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musetti, R.; Buxa, S.V. DAPI and confocal laser-scanning microscopy for in vivo imaging of phytoplasmas. In Phytoplasmas; Humana Press: New York, NY, USA, 2019; pp. 301–306. [Google Scholar]
- Andrade, N.M.; Arismendi, N.L. DAPI staining and fluorescence microscopy techniques for phytoplasmas. In Phytoplasma; Humana Press: Totowa, NJ, USA, 2013; pp. 115–121. [Google Scholar]





| Country | Year | Province | District a | Detection b | Sequenced Isolates c | Note |
|---|---|---|---|---|---|---|
| Cambodia | 2016 | Svay Rieng | Svay Chrum | 13/20 | CA_A1 d, CA_B1 d, | This study |
| CA_12-1, CA_12-2 d, | ||||||
| CA_12-3 | ||||||
| 2017 | Takeo | Treang | 21/21 | CA_T7, CA_T15 | This study | |
| Takeo | Bati | 15/20 | CA_T6 | This study | ||
| Takeo | Angkorchey | 3/11 | CA_T2 | This study | ||
| Prey Veng | Peamrok | 18/20 | CA_PR4 | This study | ||
| Prey Veng | Prey Veng City | 20/20 | CA_PVC16 | This study | ||
| Svay Rieng | Svay Chrum | 18/20 | CA_SRSC8, CA_SRK12 | This study | ||
| 2018 | Kampong Thom | Steung Sen | 3/3 | CA_KS6 | This study | |
| Kampong Thom | Santuk | 2/4 | CA_KT5 | This study | ||
| 2019 | Phnom Penh | Dangkor | 5/6 | CA_BO1 | This study | |
| Prey Veng | Peamrok | 4/4 | CA_PV8-2 | This study | ||
| Takeo | Trumkok | 13/14 | CA_TK5-1 | This study | ||
| 2016–2019 | (Cambodia total) | 135/163 | Avg. detection rate: 82.8% e | |||
| Philippines | 2015 | Laguna | Los Banos | 2/2 | PH_602 d | This study |
| 2016 | Laguna | Los Banos | 2/2 | PH_A1 | This study | |
| 2017 | Laguna | Los Baños | 49/60 | PH_S1, PH_S2, PH_S9 d | Jonson et al. (2020) | |
| 2019 | Davao del Sur | Hagonoy | 8/8 | PH_SH5, PH_SH7, | Jonson et al. (2020) f | |
| PH_SH8 | ||||||
| Matanao | 2/2 | Jonson et al. (2020) | ||||
| Davao del Norte | Sto Tomas | 9/9 | PH_SK26, PH_SK27 | Jonson et al. (2020) f | ||
| Davao Oriental | Cabangcalan | 10/10 | PH_BC37, PH_BT11, | Jonson et al. (2020) f | ||
| PH_BT12 d, PH_RB22 | ||||||
| 2015–2019 | (Philippines total) | 82/93 | Avg. detection rate: 88.2% e | |||
| Vietnam | 2017 | Tien Giang | Tan Phuoc | 14/24 | VN_TG1, VN_TG2, | VN_TG4 (Thi et al., 2016) g |
| VN_TG4, VN_TGE-1 | ||||||
| Dong Thap | Tan Hong | 5/15 | VN_DT-13 d, VN_DT-3 d, | This study | ||
| VN_DT-5 d, VN_DT6 d | ||||||
| 2017 | (Vietnam total) | 19/39 | Avg. detection rate: 48.7% e | |||
| Cambodia, Philippines and Vietnam in total | 236/295 | Avg. detection rate: 80% e | ||||
| Racilia dorsalis (ZLH) | Nephotettix virescens (GLH) | ||||
|---|---|---|---|---|---|
| Province | District | Detection a | Rate | Detection a | Rate |
| Takeo | Bati | 11/11 | 100% | 3/7 | 42.9% |
| Prey Veng | Peamrok | 1/1 | 100% | 1/6 | 16.7% |
| Prey Veng | Prey Veng City | 5/8 | 62.5% | 1/6 | 16.7% |
| Svay Rieng | Svay Chrum | 4/5 | 80% | ||
| (total detection number and averaged detection rate) | 21/25 b | 84% c | 5/19 b | 26.3% c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, S.; Jonson, G.B.; Calassanzio, M.; Rin, S.; Chou, C.; Oi, T.; Sato, I.; Takemoto, D.; Tanaka, T.; Choi, I.-R.; et al. Geographic Distribution, Genetic Variability and Biological Properties of Rice Orange Leaf Phytoplasma in Southeast Asia. Pathogens 2021, 10, 169. https://doi.org/10.3390/pathogens10020169
Ong S, Jonson GB, Calassanzio M, Rin S, Chou C, Oi T, Sato I, Takemoto D, Tanaka T, Choi I-R, et al. Geographic Distribution, Genetic Variability and Biological Properties of Rice Orange Leaf Phytoplasma in Southeast Asia. Pathogens. 2021; 10(2):169. https://doi.org/10.3390/pathogens10020169
Chicago/Turabian StyleOng, Socheath, Gilda B. Jonson, Matteo Calassanzio, Soriya Rin, Cheythyrith Chou, Takao Oi, Ikuo Sato, Daigo Takemoto, Toshiharu Tanaka, Il-Ryong Choi, and et al. 2021. "Geographic Distribution, Genetic Variability and Biological Properties of Rice Orange Leaf Phytoplasma in Southeast Asia" Pathogens 10, no. 2: 169. https://doi.org/10.3390/pathogens10020169






