Ecoepidemiology and Potential Transmission of Vibrio cholerae among Different Environmental Niches: An Upcoming Threat in Egypt
Abstract
:1. Introduction
2. Results
2.1. Phenotypic and Genotypic Identification of V. cholerae in Different Environmental Niches
2.2. Ecoepidemiology of V. cholerae in Aquatic Nile Environment (Water and Sediments)
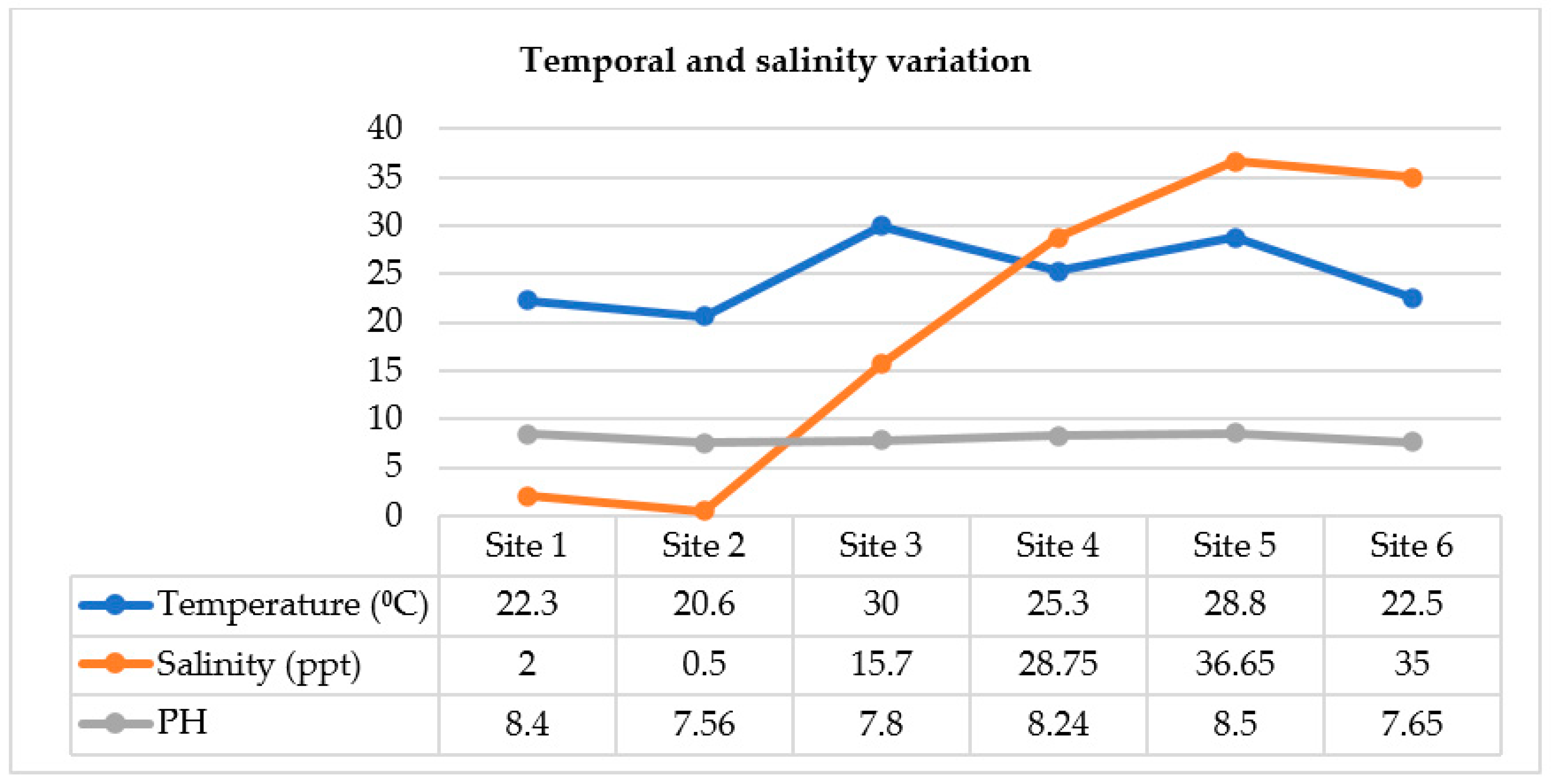
2.2.1. Occurrence of Cholerae and Spatial Variation (Salinity)
2.2.2. Occurrence of Cholerae Regarding Temporal Variation
2.3. Ecoepidemiology of V. cholerae and Aquatic Host Interactions
2.4. Occurrence of V. cholerae in Poultry Species and Waterfowls
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Study Location and Duration
4.3. Collection of Different Samples
4.3.1. Aquatic Sources (Water, Sediment, Finfish, and Crustacean Seafood)
- Water samples and sediments
- Finfish samples
- Crustacean fish (seafood)
4.3.2. Poultry Sources
4.4. Phenotypic Identification of V. cholerae (Isolation, Culturing, and Biochemical Characterization)
4.5. Molecular Identification of V. Cholerae
4.6. Sequence Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Bakhshi, B.; Barzelighi, H.M.; Adabi, M.; Lari, A.R.; Pourshafie, M.R. A molecular survey on virulence associated genotypes of non-O1 non-O139 Vibrio cholerae in aquatic environment of Tehran, Iran. Water Res. 2009, 43, 1441–1447. [Google Scholar] [CrossRef]
- Mendes-Marques, C.L.; Silveira Filho, V.D.M.; da Costa, A.P.R.; Nunes, M.D.L.; Silva Filho, S.V.D.; Figueirôa, Â.C.T.D.A.; Hofer, E.; de Almeida, A.M.P.; Leal, N.C. The aquatic environment as a reservoir of Vibrio cholerae O1 in hydrographic basins of the state of Pernambuco, Brazil. Sci. World J. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racault, M.F.; Abdulaziz, A.; George, G.; Menon, N.; Punathil, M.; McConville, K.; Loveday, B.; Platt, T.; Sathyendranath, S.; Vijayan, V. Environmental reservoirs of Vibrio cholerae: Challenges and opportunities for ocean-color remote sensing. Remote Sens. 2019, 11, 2763. [Google Scholar] [CrossRef] [Green Version]
- Almagro-Moreno, S.; Taylor, R.K. Cholera: Environmental reservoirs and impact on disease transmission. Microbiol. Spectrum 2013, 1. [Google Scholar] [CrossRef]
- Angelo, R.U.; Ramesh, S. Antibiogram and molecular characterization of Vibrio cholerae isolated from marine fish. Asian J. Pharm Clin. Res. 2017, 10, 146–149. [Google Scholar] [CrossRef] [Green Version]
- Gangarosa, E.J.; Saghari, H.; Emile, J.; Siadat, H. Detection of Vibrio cholerae biotype El Tor by purging. Bull. World Health Organ. 1966, 34, 363. [Google Scholar]
- Miller, C.J.; Drasar, B.S.; Feachem, R.G. Cholera and estuarine salinity in Calcutta and London. Lancet 1982, 1, 12–16. [Google Scholar] [CrossRef]
- Miller, C.I.; Feachem, R.G.; Drasar, B.S. Cholera epidemiology in developed and developing countries: New thoughts on transmission, seasonality, and control. Lancet 1985, 261, 3–17. [Google Scholar] [CrossRef]
- Islam, M.S.; Drasar, B.S.; Sack, R.B. The aquatic environment as a reservoir of Vibrio cholerae: A review. J. Diarrhoeal Dis. Res. 1993, 11, 197–206. [Google Scholar] [PubMed]
- Colwell, R.R. Global climate and infectious disease: The cholera paradigm. Science 1996, 274, 2025–2031. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Zaman, M.H.; Islam, M.S.; Ahmed, N.; Clemens, J.D. Environmental reservoirs of Vibrio cholerae. Vaccine 2020, 38, A52–A62. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.M.; Osman, M.A.; Potter, T.L.; Levin, R.E. Lead and cadmium in Nile River water and finished drinking water in Greater Cairo, Egypt. Environ. Int. 1998, 24, 767–772. [Google Scholar] [CrossRef]
- Abdel-Satar, A.M. Water quality assessment of river Nile from Idfo to Cairo. Egypt. J. Aquat. Res. 2005, 31, 200–223. [Google Scholar]
- Mahmoud, N.E.; Badawy, M.F.M.; Fahmy, M.M. Investigations on Mass Mortalities among Oreochromis niloticus at Mariotteya Stream, Egypt: Parasitic Infestation and Environmental Pollution Impacts. J. Aquac Res. Dev. 2014, 5, 219. [Google Scholar] [CrossRef] [Green Version]
- Broza, M.; Halpern, M. Chironomid egg masses and Vibrio cholerae. Nature 2001, 412, 40. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.; Gancz, H.; Broza, M.; Kashi, Y. Vibrio cholerae hemagglutinin/protease degrades chironomid egg masses. Appl. Environ. Microbiol. 2003, 69, 4200–4204. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, G.; Greenough, W. Food as a vehicle of transmission of Cholera. J. Diarrhoeal Dis. Res. 1999, 1, 1–9. [Google Scholar]
- Acosta, C.J.; Galindo, C.M.; Kimario, J.; Senkoro, K.; Urassa, H.; Casals, C.; Lwilla, F. Cholera outbreak in southern Tanzania: Risk factors and patterns of transmission. Emerg. Infect. Dis. 2001, 7, 583. [Google Scholar] [CrossRef]
- Forssman, B.; Mannes, T.; Musto, J.; Gottlieb, T.; Robertson, G.; Natoli, J.D.; Gupta, L. Vibrio cholerae O1 El Tor cluster in Sydney linked to imported whitebait. Med. J. Aust. 2007, 187, 345–347. [Google Scholar] [CrossRef] [Green Version]
- Akond, M.A.; Alam, S.; Hasan, S.M.R.; Uddin, S.N.; Shirin, M. Antibiotic resistance of Vibrio cholerae from poultry sources of Dhaka, Bangladesh. Adv. Biol. Res. 2008, 2, 60–67. [Google Scholar]
- Shanker, S.; Rosenfield, J.A.; Davey, G.R.; Sorrell, T.C. Campylobacter jejuni: Incidence in processed broilers and biotype distribution in human and broiler isolates. Appl. Environ. Microbiol. 1982, 43, 1219–1220. [Google Scholar] [CrossRef] [Green Version]
- Schürmann, D.; Ebert, N.; Kampf, D.; Baumann, B.; Frei, U.; Suttorp, N. Domestic cholera in Germany associated with fresh fish imported from Nigeria. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 827–828. [Google Scholar] [CrossRef]
- Coyle, N.M.; Bartie, K.L.; Bayliss, S.C.; Bekaert, M.; Adams, A.; McMillan, S.; Verner-Jeffreys, D.W.; Desbois, A.P.; Feil, E.J. A hopeful sea-monster: A very large homologous recombination event impacting the core genome of the marine pathogen Vibrio anguillarum. Front. Microbiol. 2020, 11, 1430. [Google Scholar] [CrossRef]
- Ahmed, Z.S.; Elshafiee, E.A.; Khalefa, H.S.; Kadry, M.; Hamza, D.A. Evidence of colistin resistance genes (mcr-1 and mcr-2) in wild birds and its public health implication in Egypt. Antimicrob. Resistance Infect. Control 2019, 8, 197. [Google Scholar] [CrossRef]
- Islam, M.S.; Drasar, B.S.; Sack, R.B. Probable role of blue-green algae in maintaining endemicity and seasonality of cholera in Bangladesh: A hypothesis. J. Diarrhoeal Dis Res. 1994, 12, 245–256. [Google Scholar]
- Colwell, R.R.; Huq, A. Environmental reservoir of Vibrio cholerae. The causative agent of cholera. Ann. NY Acad. Sci. 1994, 740, 44–54. [Google Scholar] [CrossRef]
- De Magny, G.C.; Colwell, R.R. Cholera and climate: A demonstrated relationship. Trans. Am. Clin. Climatol Assoc. 2009, 120, 119–128. [Google Scholar]
- Jutla, A.S.; Akanda, A.S.; Griffiths, J.K.; Colwell, R.R.; Islam, S. Warming oceans, phytoplankton, and river discharge: Implications for cholera outbreaks. Am. J. Trop Med. Hyg. 2011, 85, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Saad, S.M.; Samir, M.M.; El Abd, H.S.; Maksod, E. Incidence of Vibrio species in fish with special emphasis on the effect of heat treatments. Benha Vet. Med. J. 2015, 29, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Al-Agha, D.E.; Closas, A.; Molle, E. Survey of groundwater use in the central part of the Nile Delta. Water and salt management in the Nile Delta. Report, 2015; report number 6. [Google Scholar]
- Adeogun, A.O.; Chukwuka, A.V.; Ibor, O.R. Impact of abattoir and saw-mill effluent on water quality of upper ogun river (Abeokuta). Am. J. Environ. Sci. 2011, 7, 525–530. [Google Scholar]
- El-Amier, Y.A.; Al- Mamory, S.H. Macrophytic vegetation-environment relationship along rosetta branch of the River Nile in Egypt. J. Environ. Sci. 2016, 45, 299–314. [Google Scholar]
- Elshenawy, N.M.; Nesreen, K.I.; Gibreel, M.S.; Saad, Z.M. Ecological Studies of the Macrobenthic Fauna of Ashtoum El-Gamil Protectorate, Port Said Egypt. J. Aquat. Biol. Fish. 2015, 19, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.H.H. Assessment of some water quality characteristics and determination of some heavy metals in Lake Manzala, Egypt. Egypt. J. Aquat. Biol. Fish. 2008, 12, 133–154. [Google Scholar] [CrossRef] [Green Version]
- Fishar, M.R. Nile Delta (Egypt). In The Wetland Book; Finlayson, C., Milton, G., Prentice, R., Davidson, N., Eds.; Springer: Dordrecht, The Netherlands, 2018. [Google Scholar] [CrossRef]
- El-Mezayen, M.M.; Digna, T.R.; Essa, M.A.; Frank, E.M.; Elghobashy, A.E. Water quality observations in the marine aquaculture complex of the Deeba Triangle, Lake Manzala, Egyptian Mediterranean coast. Environ. Monit Assess. 2018, 190, 12. [Google Scholar] [CrossRef]
- Senderovich, Y.; Izhaki, I.; Halpern, M. Fish as reservoirs and vectors of Vibrio cholerae. PLoS ONE 2010, 5, e8607. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.; Hettiarachchi, H. Environmental damage caused by wastewater discharge into the Lake Manzala in Egypt. Am. J. Biosci. Bioeng. 2017, 5, 141–150. [Google Scholar] [CrossRef]
- Farouk, A.E.; AA Abdel-Hamid, E.; MT, M. Environmental studies on water quality, plankton and bacterial community in Mariout Lake, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 139–158. [Google Scholar] [CrossRef]
- Bergstad, O.A. Fish: Demersal Fish (Life Histories, Behavior, Adaptations). In Encyclopedia of Ocean Sciences, 2nd ed.; Steele, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 458–466. [Google Scholar]
- Pruzzo, C.; Vezzulli, L.; Colwell, R.R. Global impact of Vibrio cholerae interactions with chitin. Environ. Microbiol. 2008, 10, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Nandi, B.; Nandy, R.K.; Mukhopadhyay, S.; Nair, G.B.; Shimada, T.; Ghose, A.C. Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein OmpW. J. Clin. Microbiol. 2000, 38, 4145–4154. [Google Scholar] [CrossRef] [Green Version]
- Benskin, C.M.H.; Wilson, K.; Jones, K.; Hartley, I.R. Bacterial pathogens in wild birds: A review of the frequency and effects of infection. Biol. Rev. 2009, 84, 349–373. [Google Scholar] [CrossRef] [PubMed]
- Pretzer, C.; Druzhinina, I.S.; Amaro, C.; Benediktsdottir, E.; Hedenstrom, I.; Hervio-Heath, D.; Huhulescu, S.; Schets, F.M.; Farnleitner, A.H.; Kirschner, A.K. High genetic diversity of Vibrio cholerae in the European lake Neusiedler See is associated with intensive recombination in the reed habitat and the long-distance transfer of strains. Environ. Microbiol. 2017, 19, 328–344. [Google Scholar] [CrossRef] [Green Version]
- Halpern, M.; Senderovich, Y.; Izhaki, I. Waterfowl: The missing link in epidemic and pandemic cholera dissemination? PLoS Pathog. 2008, 4, e1000173. [Google Scholar] [CrossRef] [Green Version]
- Newsome, A.L.; Scott, T.M.; Benson, R.F.; Fields, B.S. Isolation of an amoeba naturally harboring a distinctive Legionella species. Appl. Environ. Microbiol. 1998, 64, 1688–1693. [Google Scholar] [CrossRef] [Green Version]
- Sakr, S.A.; Attia, F.A.; Millette, J.A. Vulnerability of the Nile Delta aquifer of Egypt to seawater intrusion. Prodceedings of the International conference on water resources of arid and semi-arid regions of Africa, Issues and challenges, Gaborone, Botswana, 3–4 August 2004. [Google Scholar]
- El Bedawy, R. Water resources management: Alarming crisis for Egypt. J. Manag. Sustain. 2014, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Beattie, A. Cairo: A Cultural History; Oxford University Press: Oxford, UK, 2005; ISBN 0-19-517892-0. [Google Scholar]
- Bird, K. Crossing Mandelbaum Gate: Coming of Age Between the Arabs and Israelis; Scribner: New York, NY, USA, 2010; pp. 1956–1978. ISBN 978-1-4165-4440-1. [Google Scholar]
- Moghazy, H.M.; Saleh, O.K.; Abd El Azim, N.F. Hydraulic analysis of El Mahmoudia canal. Trans. Ecol. Environ. 2013, 178, 41–52. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Amlacher, E. Textbook of Fish Diseases; TFH Publications Inc.: Neptune City, NJ, USA, 1970. [Google Scholar]
- APHA. Recommended Procedures for the Examination of Seawater and Shellfish, 4th ed.; American Public Health Association: Washington, DC, USA, 1970. [Google Scholar]
- Kaysner, C.A.; De Paola, A.J. Bacteriological Analytical Manual; U.S. Food and Drug Administration (FDA): Washington, DC, USA, 2004; Chapter 9.
- Wang, R.F.; Cao, W.W.; Cerniglia, C.E. PCR detection and quantitation of predominant anaerobic bacteria in human and animal fecal samples. Appl. Environ. Microbiol. 1996, 62, 1242–1247. [Google Scholar] [CrossRef] [Green Version]
- Montieri, S.; Suffredini, E.; Ciccozzi, M.; Croci, L. Phylogenetic and evolutionary analysis of Vibrio parahaemolyticus and Vibrio alginolyticus isolates based on toxR gene sequence. New Microbiol. 2010, 33, 359–372. [Google Scholar]




| Environmental Niche | No. of Examined Samples | V. cholerae Isolates (%) |
|---|---|---|
| Finfish and crustacean seafood | 185 | 83 (45%) |
| Water and sediment | 12 | 5 (42%) (4 sediments and 1 water) |
| Waterfowl, broiler, and turkey | 115 | 41 (36%) |
| Total | 312 | 129/312 (41%) |
| Nile Salinity | Location | V. cholerae Isolates (%) | |
|---|---|---|---|
| Water and Sediments | Fish Sources | ||
| Fresh water | Sites 1 and 2 | 25% | 33.3% b |
| Brackish water | Site 3 | 50% | 71.9% a |
| Marine water | Sites 4, 5, and 6 | 50% | 29.4% b |
| 0.773 (FET) | <0.0001 | ||
| Environmental Location | No. of Examined Samples | Specimens | No. of V. cholerae– Positive Samples/Pools | Total No. of V. cholerae Isolates |
|---|---|---|---|---|
| South Delta Site 1 Site 2 | 9 Nile tilapia (Oreochromis niloticus) | Gills | 3 | 12/36 b (33.3%) |
| Kidney | 3 | |||
| Liver | 3 | |||
| Intestine | 3 | |||
| Middle delta Site 3 | 16 Shield head fish, catfish (Synodontis schall) | Gills | 16 | 46/64 a (72%) |
| Kidney | 12 | |||
| Liver | 12 | |||
| Intestine | 6 | |||
| North Delta Site 4 Site 5 Site 6 | 75 Shrimp Penaeus japonicas | 14 pools | 4 pools (each 5 shrimps) = 20 shrimps | 25/85 b (29.4%) |
| 5 Cephalothorax | 3 | |||
| 5 Abdomen | 0 | |||
| 5 Tail | 2 | |||
| Total aquatic samples | 100 sample | 185 | 83 | 83/185 (45%) |
| p value | 0.0001 |
| Poultry Species | No. of Examined Samples | V. cholerae Isolates (%) |
|---|---|---|
| Duck | 35 | 14 (40%) a |
| Broiler chicken | 60 | 27 (45%) a |
| Turkey | 20 | 0 b |
| Total | 115 | 41 (36%) |
| P value | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, E.M.; Kadry, M.; Elshafiee, E.A.; Ragab, E.; Morsy, E.A.; Rizk, O.; Zaki, M.M. Ecoepidemiology and Potential Transmission of Vibrio cholerae among Different Environmental Niches: An Upcoming Threat in Egypt. Pathogens 2021, 10, 190. https://doi.org/10.3390/pathogens10020190
Ismail EM, Kadry M, Elshafiee EA, Ragab E, Morsy EA, Rizk O, Zaki MM. Ecoepidemiology and Potential Transmission of Vibrio cholerae among Different Environmental Niches: An Upcoming Threat in Egypt. Pathogens. 2021; 10(2):190. https://doi.org/10.3390/pathogens10020190
Chicago/Turabian StyleIsmail, Eman M., Mona Kadry, Esraa A. Elshafiee, Eman Ragab, Eman A. Morsy, Omar Rizk, and Manal M. Zaki. 2021. "Ecoepidemiology and Potential Transmission of Vibrio cholerae among Different Environmental Niches: An Upcoming Threat in Egypt" Pathogens 10, no. 2: 190. https://doi.org/10.3390/pathogens10020190
APA StyleIsmail, E. M., Kadry, M., Elshafiee, E. A., Ragab, E., Morsy, E. A., Rizk, O., & Zaki, M. M. (2021). Ecoepidemiology and Potential Transmission of Vibrio cholerae among Different Environmental Niches: An Upcoming Threat in Egypt. Pathogens, 10(2), 190. https://doi.org/10.3390/pathogens10020190






