Microneme Protein 6 Is Involved in Invasion and Egress by Neospora caninum
Abstract
:1. Introduction
2. Results
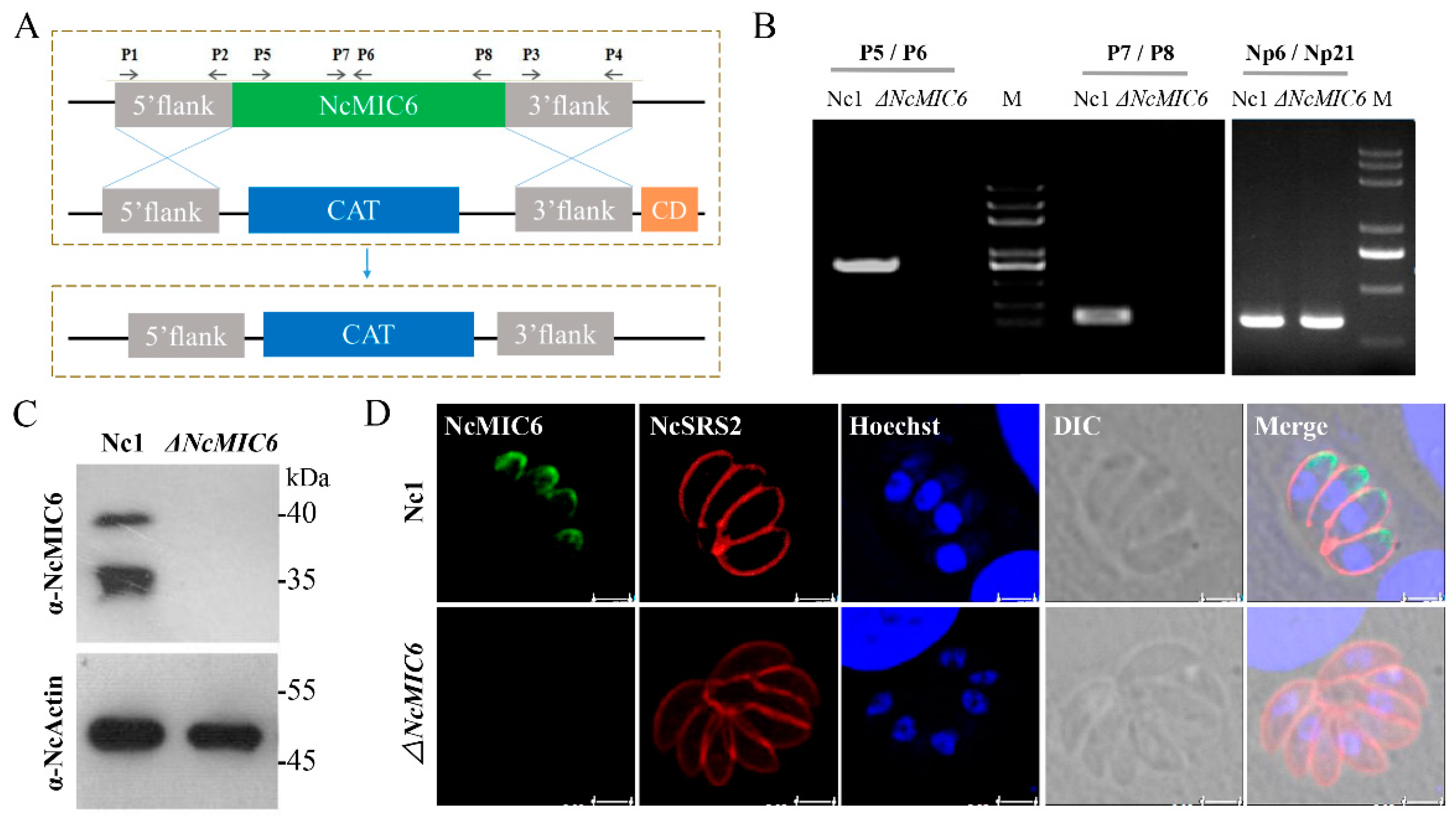
2.1. Successful Construction of an NcMIC6-Knockout Strain
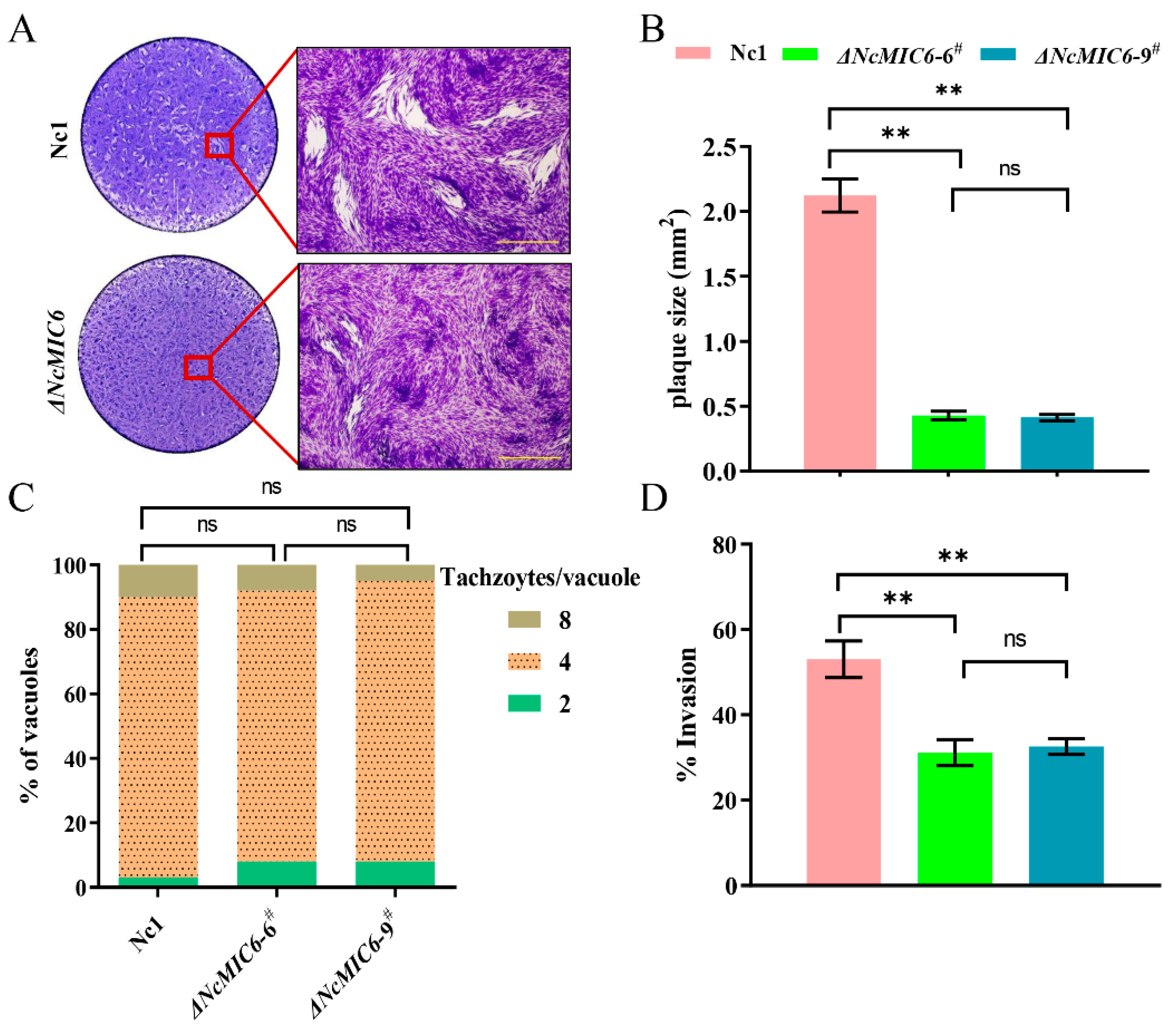
2.2. NcMIC6 Was Involved in the Invasion by N. caninum
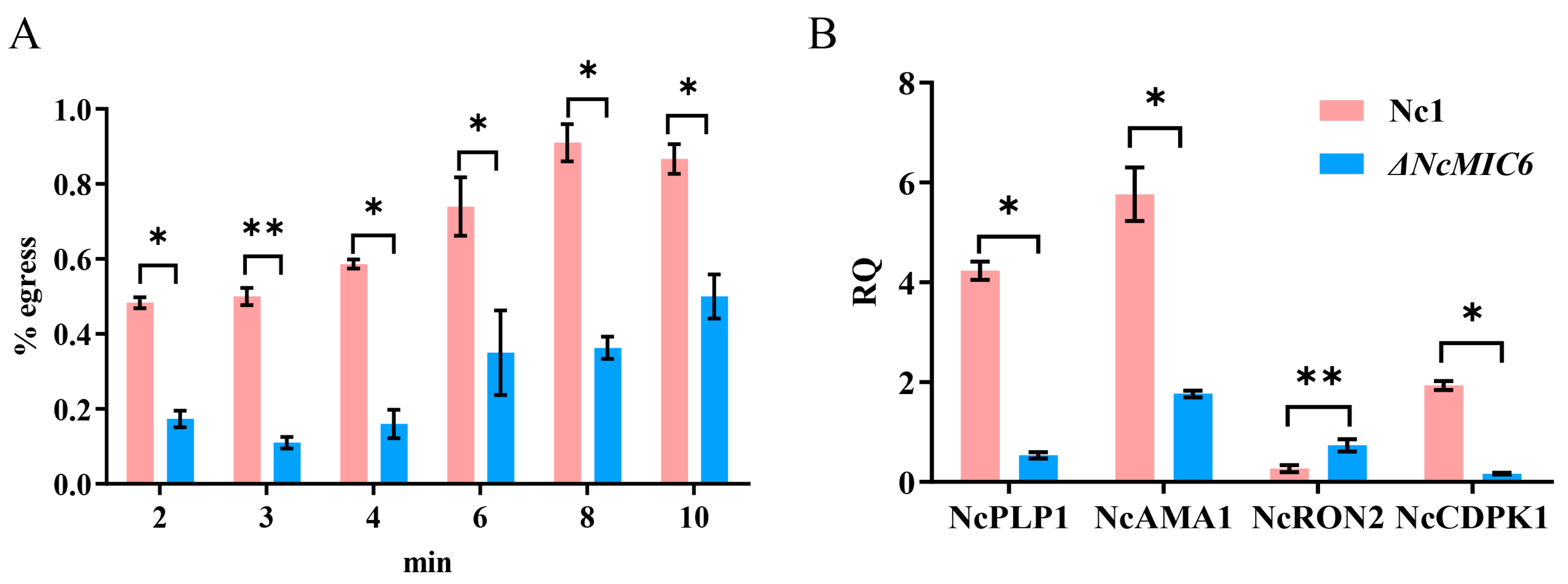
2.3. Absence of NcMIC6 Reduced the Expression and Secretion of NcMIC1 and NcMIC4
2.4. NcMIC6 Was Engaged in Egress by N. caninum
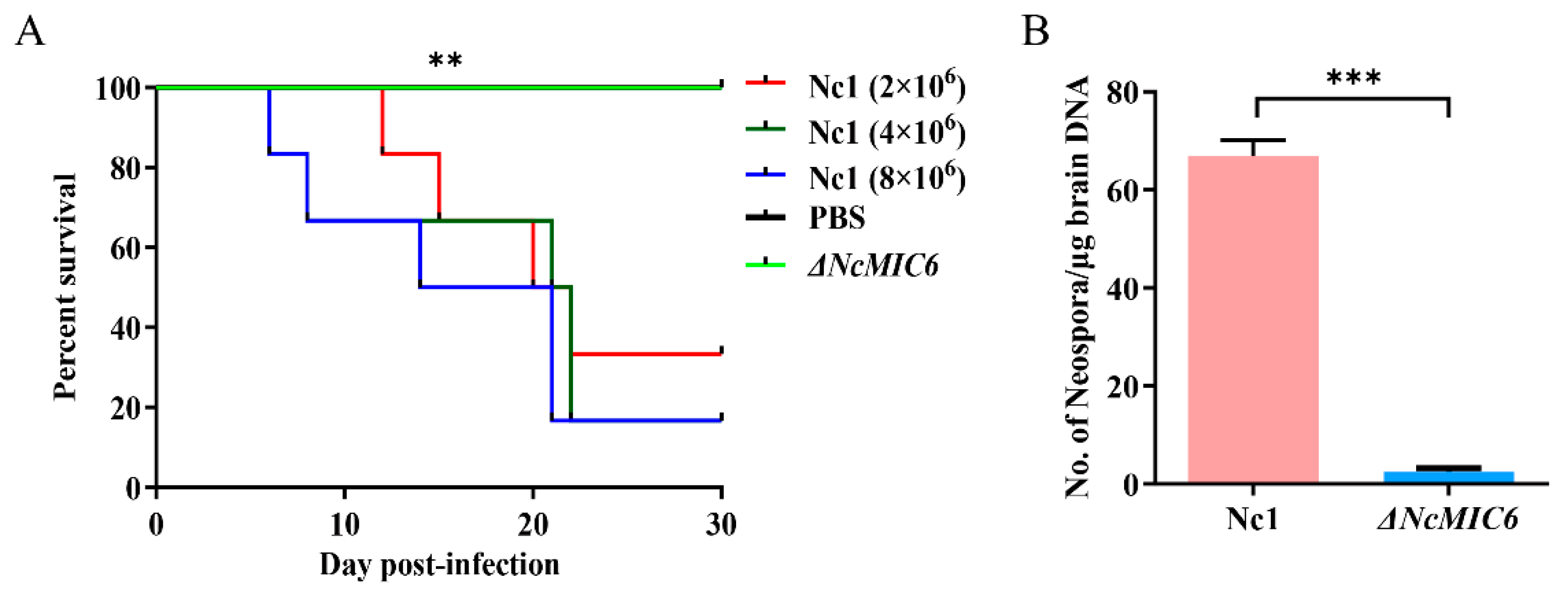
2.5. The Absence of NcMIC6 Weakened the Virulence in Mice
3. Discussion
4. Materials and Methods
4.1. Host Cells and N. caninum Culture
4.2. Generation of the NcMIC6-Knockout Strain
4.3. PCR
4.4. Western Blotting
4.5. Immunofluorescence Assay
4.6. Plaque Assay
4.7. Proliferation Assay
4.8. Invasion Assay
4.9. Secretion Assay
4.10. Egress Assay
4.11. QRT-PCR
4.12. Virulence Assay in Mice
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 2003, 41, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Dubey, J.P.; Schares, G. Neosporosis in animals—The last five years. Vet. Parasitol. 2011, 180, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Bisio, H.; Soldati-Favre, D. Signaling Cascades Governing Entry into and Exit from Host Cells by Toxoplasma gondii. Annu. Rev. Microbiol. 2019, 73, 579–599. [Google Scholar] [CrossRef] [Green Version]
- Boucher, L.E.; Bosch, J. The apicomplexan glideosome and adhesins—Structures and function. J. Struct. Biol. 2015, 190, 93–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naguleswaran, A.; Cannas, A.; Keller, N.; Vonlaufen, N.; Schares, G.; Conraths, F.J.; Björkman, C.; Hemphill, A. Neospora caninum Microneme Protein NcMIC3: Secretion, Subcellular Localization, and Functional Involvement in Host Cell Interaction. Infect. Immun. 2001, 69, 6483–6494. [Google Scholar] [CrossRef] [Green Version]
- Lovett, J.L.; Howe, D.K.; Sibley, L.D. Molecular characterization of a thrombospondin-related anonymous protein homologue in Neospora caninum. Mol. Biochem. Parasitol. 2000, 107, 33–43. [Google Scholar] [CrossRef]
- Wang, J.; Tang, D.; Li, W.; Xu, J.; Liu, Q.; Liu, J. A new microneme protein of Neospora caninum, NcMIC8 is involved in host cell invasion. Exp. Parasitol. 2017, 175, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Compaore, M.K.A.; Lee, E.-g.; Liao, M.; Zhang, G.; Sugimoto, C.; Fujisaki, K.; Nishikawa, Y.; Xuan, X. Apical membrane antigen 1 is a cross-reactive anti-gen between Neospora caninum and Toxoplasma gondii, and the anti-NcAMA1 antibody inhibits host cell invasion by both parasites. Mol. Biochem. Parasitol. 2007, 151, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Naguleswaran, A.; Cannas, A.; Keller, N.; Vonlaufen, N.; Björkman, C.; Hemphill, A. Vero cell surface proteoglycan interaction with the microneme protein NcMIC(3) mediates adhesion of Neospora caninum tachyzoites to host cells unlike that in Toxo-plasma gondii. Int. J. Parasitol. 2002, 32, 695–704. [Google Scholar] [CrossRef]
- Yang, D.; Liu, J.; Hao, P.; Wang, J.; Lei, T.; Shan, D.; Liu, Q. MIC3, a novel cross-protective antigen expressed in Toxoplasma gondii and Neospora caninum. Parasitol. Res. 2015, 114, 3791–3799. [Google Scholar] [CrossRef]
- Keller, N.; Naguleswaran, A.; Cannas, A.; Vonlaufen, N.; Bienz, M.; Björkman, C.; Bohne, W.; Hemphill, A. Identification of a Neospora caninum micro-neme protein (NcMIC1) which interacts with sulfated host cell surface glycosaminoglycans. Infect. Immun. 2002, 70, 3187–3198. [Google Scholar] [CrossRef] [Green Version]
- Keller, N.; Riesen, M.; Naguleswaran, A.; Vonlaufen, N.; Stettler, R.; Leepin, A.; Wastling, J.M.; Hemphill, A. Identification and characterization of a Ne-ospora caninum microneme-associated protein (NcMIC4) that exhibits unique lactose-binding properties. Infect. Immun. 2004, 72, 4791–4800. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liu, J.; Wang, J.; Fu, Y.; Nan, H.; Liu, Q. Identification and characterization of a microneme protein (NcMIC6) in Neospora caninum. Parasitol. Res. 2015, 114, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, J.H.; Taubert, A.; Zahner, H.; Hermosilla, C. Studies on synchronous egress of coccidian parasites (Neospora caninum, Toxoplasma gondii, Eimeria bovis) from bovine endothelial host cells mediated by calcium ionophore A23187. Veter. Res. Commun. 2008, 32, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Dubois, D.J.; Soldati-Favre, D. Biogenesis and secretion of micronemes inToxoplasma gondii. Cell. Microbiol. 2019, 21, e13018. [Google Scholar] [CrossRef] [Green Version]
- Frénal, K.; Dubremetz, J.-F.; Lebrun, M.; Soldati-Favre, D. Gliding motility powers invasion and egress in Apicomplexa. Nat. Rev. Genet. 2017, 15, 645–660. [Google Scholar] [CrossRef] [Green Version]
- Saouros, S.; Edwards-Jones, B.; Reiss, M.; Sawmynaden, K.; Cota, E.; Simpson, P.; Dowse, T.J.; Jäkle, U.; Ramboarina, S.; Shivarattan, T.; et al. A novel galectin-like domain from Toxoplas-ma gondii micronemal protein 1 assists the folding, assembly, and transport of a cell adhesion complex. J. Biol. Chem. 2005, 280, 38583–38591. [Google Scholar] [CrossRef] [Green Version]
- Reiss, M.; Viebig, N.; Brecht, S.; Fourmaux, M.-N.; Soete, M.; Di Cristina, M.; Dubremetz, J.F.; Soldati, D. Identification and Characterization of an Escorter for Two Secretory Adhesins in Toxoplasma gondii. J. Cell Biol. 2001, 152, 563–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Abdelnabi, G.H.; Lee, S.H.; Li, G.; Jin, H.; Lillehoj, H.S.; Suo, X. Enhanced Egress of Intracellular Eimeria tenella Sporozoites by Splenic Lymphocytes from Coccidian-Infected Chickens. Infect. Immun. 2011, 79, 3465–3470. [Google Scholar] [CrossRef] [Green Version]
- Mossaad, E.; Asada, M.; Nakatani, D.; Inoue, N.; Yokoyama, N.; Kaneko, O.; Kawazu, S.-I. Calcium ions are involved in egress of Babesia bovis merozoites from bovine erythrocytes. J. Vet. Med. Sci. 2015, 77, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, D.A.; Clark, D.P. Host Cell Fate on Cryptosporidium parvum Egress from MDCK Cells. Infect. Immun. 2003, 71, 5422–5426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullen, H.E.; Bisio, H.; Soldati-Favre, D. The triumvirate of signaling molecules controlling Toxoplasma microneme exocytosis: Cyclic GMP, calcium, and phosphatidic acid. PLoS Pathog. 2019, 15, e1007670. [Google Scholar] [CrossRef] [PubMed]
- Blader, I.J.; Coleman, B.I.; Chen, C.-T.; Gubbels, M.-J. Lytic Cycle of Toxoplasma gondii: 15 Years Later. Annu. Rev. Microbiol. 2015, 69, 463–485. [Google Scholar] [CrossRef] [Green Version]
- Hoff, E.F.; Carruthers, V.B. Is Toxoplasma egress the first step in invasion? Trends Parasitol. 2002, 18, 251–255. [Google Scholar] [CrossRef]
- Lovett, J.L. Intracellular calcium stores in Toxoplasma gondii govern invasion of host cells. J. Cell Sci. 2003, 116, 3009–3016. [Google Scholar] [CrossRef] [Green Version]
- Del Carmen, M.G.; Mondragon, M.; González, S.; Mondragón, R. Induction and regulation of conoid extrusion inToxoplasma gondii. Cell. Microbiol. 2009, 11, 967–982. [Google Scholar] [CrossRef] [PubMed]
- Radke, J.R.; Striepen, B.; Guerini, M.N.; Jerome, M.E.; Roos, D.S.; White, M.W. Defining the cell cycle for the tachyzoite stage of Toxoplasma gondii. Mol. Biochem. Parasitol. 2001, 115, 165–175. [Google Scholar] [CrossRef]
- Andersonwhite, B.R.; Beck, J.R.; Chen, C.-T.; Meissner, M.; Bradley, P.J.; Gubbels, M.-J. Cytoskeleton Assembly in Toxoplasma gondii Cell Division. Int. Rev. Cell Mol. Biol. 2012, 298, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Kafsack, B.F.C.; Pena, J.D.O.; Coppens, I.; Ravindran, S.; Boothroyd, J.C.; Carruthers, V.B. Rapid Membrane Disruption by a Perforin-Like Protein Facilitates Parasite Exit from Host Cells. Science 2009, 323, 530–533. [Google Scholar] [CrossRef] [Green Version]
- Bargieri, D.Y.; Andenmatten, N.; Lagal, V.; Thiberge, S.; Whitelaw, J.A.; Tardieux, I.; Meissner, M.; Ménard, R. Apical membrane antigen 1 mediates apicomplexan parasite attachment but is dispensable for host cell invasion. Nat. Commun. 2013, 4, 2552. [Google Scholar] [CrossRef] [Green Version]
- Lourido, S.; Shuman, J.; Zhang, C.; Shokat, K.M.; Hui, R.; Sibley, L.D. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nat. Cell Biol. 2010, 465, 359–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosetti, N.; Pacheco, N.D.S.; Soldati-Favre, D.; Jacot, D. Three F-actin assembly centers regulate organelle inheritance, cell-cell communication and motility in Toxoplasma gondii. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Ojo, K.K.; Reid, M.C.; Siddaramaiah, L.K.; Müller, J.; Winzer, P.; Zhang, Z.; Keyloun, K.R.; Vidadala, R.S.R.; Merritt, E.A.; Hol, W.G.J.; et al. Neospora caninum Calcium-Dependent Protein Kinase 1 Is an Effective Drug Target for Neosporosis Therapy. PLoS ONE 2014, 9, e92929. [Google Scholar] [CrossRef] [Green Version]
- Lamarque, M.H.; Roques, M.; Kong-Hap, M.; Tonkin, M.L.; Rugarabamu, G.; Marq, J.-B.; Penarete-Vargas, D.M.; Boulanger, M.J.; Soldati-Favre, D.; Lebrun, M. Plasticity and redundancy among AMA–RON pairs ensure host cell entry of Toxoplasma parasites. Nat. Commun. 2014, 5, 4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Liu, J.; Li, M.; Fu, Y.; Zhang, X.; Liu, Q. Rhoptry protein 5 (ROP5) Is a Key Virulence Factor in Neospora caninum. Front. Microbiol. 2017, 8, 370. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Lei, T.; Liu, J.; Li, M.; Nan, H.; Liu, Q. A Nuclear Factor of High Mobility Group Box Protein in Toxoplasma gondii. PLoS ONE 2014, 9, e111993. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Pace, D.; Dou, Z.; King, T.P.; Guidot, D.; Li, Z.-H.; Carruthers, V.B.; Moreno, S.N.J. A vacuolar-H(+) -pyrophosphatase (TgVP1) is required for microneme secretion, host cell invasion, and extracellular survival of Toxoplasma gondii. Mol. Microbiol. 2014, 93, 698–712. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, H.; Fu, Y.; Liu, J.; Liu, Q. Effects of Estradiol and Progesterone-Induced Intracellular Calcium Fluxes on Toxoplasma gondii Gliding, Microneme Secretion, and Egress. Front. Microbiol. 2018, 9, 1266. [Google Scholar] [CrossRef] [Green Version]
- Gagnaire, A.; Gorvel, L.; Papadopoulos, A.; Von Bargen, K.; Mège, J.-L.; Gorvel, J.-P. COX-2 Inhibition Reduces Brucella Bacterial Burden in Draining Lymph Nodes. Front. Microbiol. 2016, 7, 1987. [Google Scholar] [CrossRef] [Green Version]
- Collantes-Fernández, E.; Zaballos, A.; Alvarez-García, G.; Ortega-Mora, L.M. Quantitative detection of Neospora caninum in bo-vine aborted fetuses and experimentally infected mice by real-time PCR. J. Clin. Microbiol. 2002, 40, 1194–1198. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tang, D.; Wang, F.; Jin, G.; Wang, L.; Liu, Q.; Liu, J. Microneme Protein 6 Is Involved in Invasion and Egress by Neospora caninum. Pathogens 2021, 10, 201. https://doi.org/10.3390/pathogens10020201
Wang X, Tang D, Wang F, Jin G, Wang L, Liu Q, Liu J. Microneme Protein 6 Is Involved in Invasion and Egress by Neospora caninum. Pathogens. 2021; 10(2):201. https://doi.org/10.3390/pathogens10020201
Chicago/Turabian StyleWang, Xianmei, Di Tang, Fei Wang, Gaowei Jin, Lifang Wang, Qun Liu, and Jing Liu. 2021. "Microneme Protein 6 Is Involved in Invasion and Egress by Neospora caninum" Pathogens 10, no. 2: 201. https://doi.org/10.3390/pathogens10020201
APA StyleWang, X., Tang, D., Wang, F., Jin, G., Wang, L., Liu, Q., & Liu, J. (2021). Microneme Protein 6 Is Involved in Invasion and Egress by Neospora caninum. Pathogens, 10(2), 201. https://doi.org/10.3390/pathogens10020201




