Q Fever: Seroprevalence, Risk Factors in Slaughter Livestock and Genotypes of Coxiella burnetii in South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Size and Study Population
2.3. Serological Testing
3. Molecular Characterization
3.1. DNA Extraction and PCR for Detection of C. burnetii
3.2. Sequence Confirmation of C. burnetii
3.3. MLVA Typing
| Name | Primer Sequence | Reference(s) |
|---|---|---|
| IS1111F | 5′ CGCAGCACGTCAAACCG3′ | [17] |
| IS1111R | 5′TATCTTTAACAGCGCTTGAACGTC3′ | [17] |
| MS23F | 5′CGCMTAGCGACACAACCAC3′ | [18,20] |
| MS23R | 5′GACGGGCTAAATTACACCTGCT3′ | [18,20] |
| MS24F | 5′TGGAGGGACTCCGATTAAAA3′ | [18,20] |
| MS24R | 5′GCCACACAACTCTGTTTTCAG3′ | [18,20] |
| MS27F | 5′TCTTTATTTCAGGCCGGAGT3′ | [18,20] |
| MS27R | 5′GAACGACTCATTGAACACACG3′ | [18,20] |
| MS28F | 5′AGCAAAGAAATGTGAGGATCG3′ | [18,20] |
| MS28R | 5′GCCAAAGGGATATTTTTGTCCTTC3′ | [18,20] |
| MS33F | 5′TCGCGTAGCGACACAACC3′ | [18,20] |
| MS33R | 5′GTAGCCCGTATGACGCGAAC3′ | [18,20] |
| MS34F | 5′TTCTTCGGTGAGTTGCTGTG3′ | [18,20] |
| MS34R | 5′GCAATGACTATCAGCGACTCGAA3′ | [18,20] |
4. Data Analysis
5. Results
5.1. Serology
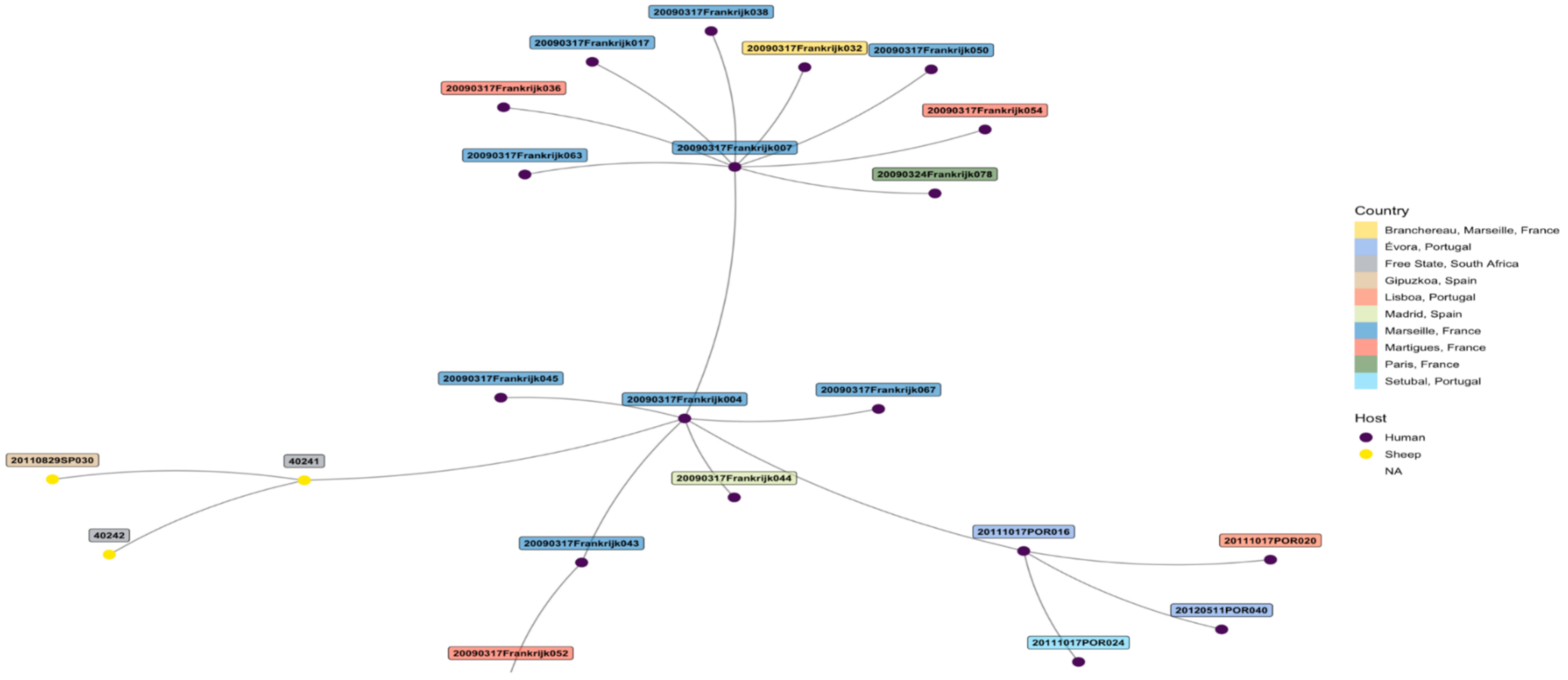
5.2. Molecular Detection and MLVA Typing of C. burnetii
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angelakis, E.; Raoult, D. Q Fever. Vet. Microbiol. 2010, 140, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, R.; Paulsen, I.T.; Eisen, J.A.; Read, T.D.; Nelson, K.E.; Nelson, W.C. Complete genome sequence of the Q fever pathogen Coxiella burnetii. Proc. Natl. Acad. Sci. USA 2003, 100, 5455–5460. [Google Scholar] [CrossRef]
- Davis, G.E.; Cox, H.R. A filter-passing infectious agent isolated from ticks. IN. Isolation from Dermacentor andersonii, reactions in animals and filtration. Public Health Rep. 1938, 53, 2259. [Google Scholar] [CrossRef]
- Heinzen, R.A.; Hackstadt, T.; Samuel, J.E. Developmental biology of Coxiella burnetii. TIMS 1999, 7, 149–154. [Google Scholar] [CrossRef]
- McCaul, T.F.; Williams, J.C. Developmental cycle of Coxiella burnetii: Structure and morphogenesis of vegetative and sporogenic differentiations. J. Bacteriol. 1981, 147, 1063–1076. [Google Scholar] [CrossRef]
- Amano, K.J.; Williams, C.; McCaul, T.E.; Peacock, M.G. Biochemical and immunological properties of Coxiella burnetii cell wall andpeptidoglycan-protein complex fractions. J. Bacteriol. 1984, 160, 982–988. [Google Scholar] [CrossRef]
- Marrie, T.J.; Schlech, W.F., 3rd; Williams, J.C.; Yates, L. Q fever pneumonia associated with exposure to wild rabbits. Lancet 1986, 1, 427–429. [Google Scholar] [CrossRef]
- Honarmand, H. Q fever: An old but still a poorly understood disease. Interdiscip. Perspect Infect Dis. 2012, 131932. [Google Scholar] [CrossRef]
- Whelan, J.; Schimmer, B.; Schneeberger, P.; Meekelenkamp, J.; Ijff, A.; Van der Hoek, W.; Van Beest, M.R. Q fever among culling workers, the Netherlands, 2009–2010. Emerg. Infect Dis. 2011, 17, 1719–1723. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoek, W.; Dijkstra, F.; Schimmer, B.; Schneeberger, P.M.; Vellema, P.; Wijkmans, C.; Ter Schegget, R.; Hackert, V.; Van Duynhoven, Y. Q fever in the Netherlands: An update on the epidemiology and control measures. Euro Surveill 2010, 15. Available online: http://www.njmonline.nl/getpdf.php?t=a&id=10000657 (accessed on 14 January 2021).
- Adesiyun, A.A.; Knobel, D.L.; Thompson, P.N.; Wentzel, J.; Kolo, F.B.; Kolo, A.O.; Conan, A.; Simpson, G.J.G. Sero-Epidemiological Study of Selected Zoonotic and Abortifacient Pathogens in Cattle at a Wildlife-Livestock Interface in South Africa. Vector Borne Zoonotic Dis. 2020, 4, 258–267. [Google Scholar] [CrossRef]
- Gummow, B.; Poerstamper, N.; Herr, S. The incidence of Coxiella burnetii antibodies in cattle in the Transvaal. Onderstepoort J. Vet. Res. 1987, 54, 569–571. [Google Scholar] [PubMed]
- Mtshali, K.; Khumalo, Z.; Nakao, R.; Grab, D.J.; Sugimoto, C.; Thekisoe, O. Molecular detection of zoonotic tick-borne pathogens from ticks collected from ruminants in four South African provinces. J. Vet. Med. Sci. 2016, 77, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Google Maps. Available online: https://maps.google.com/maps (accessed on 1 February 2021).
- Thrusfield, M. Veterinary Epidemiology; John Wiley & Sons: Hoboken, NJ, USA, 2013; p. 1289. [Google Scholar]
- Ozkaraca, M.; Ceribasi, S.; Ceribasi, A.O.; Kilic, A.; Altun, S.; Comakli, S.; Ongor, H. Determination of Coxiella burnetii in bovine foetuses using PCR and immunohistochemistry. Vet. Med. Czech 2017, 61, 421–427. [Google Scholar] [CrossRef]
- De Bruin, A.; Van Alphen, P.T.; Van der Plaats, R.Q.; De Heer, L.N.; Reusken, C.B.; Van Rotterdam, B.J.; Janse, I. Molecular typing of Coxiella burnetii from animal and environmental matrices during Q fever epidemics in the Netherlands. BMC Vet. Res. 2012, 8, 165. [Google Scholar] [CrossRef] [PubMed]
- Arricau-Bouvery, N.; Hauck, Y.; Bejaoui, A.; Frangoulidis, D.; Bodier, C.; Souriau, A.; Meyer, H.; Neubauer, H.; Rodolakis, A.; Vergnaud, G. Molecular characterization of Coxiella burnetii isolates by infrequent restriction site-PCR and MLVA typing. BMC Microbiol. 2006, 6, 38. [Google Scholar] [CrossRef]
- Santos, A.S.; Tilburg, J.J.; Botelho, A.; Barahona, M.J.; Nuncio, M.S.; Nabuurs-Franssen, M.H.; Klaassen, C.H. Genotypic diversity of clinical Coxiella burnetii isolates from Portugal based on MST and MLVA typing. Int. J. Med. Microbiol. 2012, 302, 253–256. [Google Scholar] [CrossRef]
- Svraka, S.; Toman, R.; Skultety, L.; Slaba, K.; Homan, W.L. Establishment of a genotyping scheme for Coxiella burnetii. FEMS Microbiol. Lett. 2006, 254, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ceglie, L.; Guerrini, E.; Rampazzo, E.; Barberio, A.; Tilburg, J.J.; Hagen, F.; Lucchese, L.; Zuliani, F.; Marangon, S.; Natale, A. Molecular characterization by MLVA of Coxiella burnetii strains infecting dairy cows and goats of North-eastern Italy. Microbes. Infect. 2015, 17, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Glazunova, O.; Roux, V.; Freylikman, O.; Sekeyova, Z.; Fournous, G.; Tyczka, J.; Tokarevich, N.; Kovacova, E.; Marrie, T.J.; Raoult, D. Coxiella burnetii genotyping. Emerg. Infect Dis. 2005, 11, 1211–1217. [Google Scholar] [PubMed]
- Boratyn, G.M.; Camacho, C.; Cooper, P.S.; Coulouris, G.; Fong, A.; Ma, N.; Madden, T.L.; Matten, W.T.; McGinnis, S.D.; Merezhuk, Y.; et al. BLAST: A more efficient report with usability improvements. Nucleic Acids Res. 2013, 41, W29–W33. [Google Scholar] [CrossRef]
- Tilburg, J.J.; Rossen, J.W.; Van Hannen, E.J.; Melchers, W.J.; Hermans, M.H.; Van de Bovenkamp, J.; Roest, H.J.; De Bruin, A.; Nabuurs-Franssen, M.H.; Horrevorts, A.M.; et al. H Genotypic diversity of Coxiella burnetii in the 2007–2010 Q fever outbreak episodes in The Netherlands. J. Clin. Microbiol. 2012, 50, 1076–1078. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maechler, M. Finding Groups in Data: Cluster Analysis Extended Rousseeuw et. R package Version 2; Wiley-Interscience: Hoboken, NJ, USA, 2019. [Google Scholar]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T.Y. gtree: An R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 52628. [Google Scholar] [CrossRef] [PubMed]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. InterJ. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Briatte, F. Ggnetwork: Geometries to Plot Networks with ’ggplot2’. 2016. R package version 0.5.1. pp. 27–29. Available online: https://github.com/briatte/ggnetwork (accessed on 14 January 2021).
- Team R Core. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria. 2013. Available online: http://www.R-project.org (accessed on 29 October 2020).
- McCaughey, C.; Murray, L.J.; McKenna, J.P.; Menzies, F.D.; McCullough, S.J.; O′Neill, H.J.; Wyatt, D.E.; Cardwell, C.R.; Coyle, P.V. Coxiella burnetii (Q fever) seroprevalence in cattle. Epidemiol. Infect. 2010, 138, 21–27. [Google Scholar] [CrossRef]
- Kelly, P.J.; Matthewman, L.A.; Mason, P.R.; Raoult, D. Q fever in Zimbabwe. A review of the disease and the results of a serosurvey of humans, cattle, goats and dogs. S. Afr. Med. J. 1993, 83, 21–25. [Google Scholar] [PubMed]
- Johnson, S.A.; Kaneene, J.B.; Asare-Dompreh, K.; Tasiame, W.; Mensah, I.G.; Afakye, K.; Simpson, S.V.; Addo, K. Seroprevalence of Q fever in cattle, sheep and goats in the Volta region of Ghana. Vet. Med. Sci. 2019, 5, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Adesiyun, A.A.; Cazabon, E.P. Seroprevalences of Brucellosis, Q fever and toxoplasmosis in slaughter livestock in Trinidad. Rev. Elev. Med. Vet. Pays Trop 1996, 49, 28–30. [Google Scholar]
- Paul, S.; Agger, J.F.; Agerholm, J.S.; Markussen, B. Prevalence and risk factors of Coxiella burnetii seropositivity in Danish beef and dairy cattle at slaughter adjusted for test uncertainty. Prev. Vet. Med. 2014, 113, 504–511. [Google Scholar] [CrossRef]
- Kruszewska, D.; Tylewska-Wierzbanowska, S. Isolation of Coxiella burnetii from bull semen. Res. Vet Sci. 1997, 62, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Cumbassá, A.; Barahona, M.J.; Cunha, M.V.; Azórin, B.; Fonseca, C.; Rosalino, L.M.; Tilburg, J.; Hagen, F.; Santos, A.S.; Botelho, A. Coxiella burnetii DNA detected in domestic ruminants and wildlife from Portugal. Vet. Microbiol. 2015, 180, 136–141. [Google Scholar]





| Province | Sample Type | Sample Name | Number (n) |
|---|---|---|---|
| Gauteng | Serum | Serum | 507 |
| Gauteng | Tissue | Penis | 355 |
| Gauteng | Tissue | Testes | 355 |
| Gauteng | Tissue | Uterus | 145 |
| Gauteng | Tissue | Mammary gland | 79 |
| Gauteng | Tissue | Ovary | 80 |
| Gauteng | Tissue | Oviduct | 4 |
| Free state | Pooled tissues | Spleen, liver, lung | 2 |
| Variable | Level | Prevalence (%) | 95%CI* | p-Value |
|---|---|---|---|---|
| Species | Bovine | 9.4 | 6.5–13.0 | 0.003 |
| Ovine | 4.3 | 0.9–12.0 | ||
| Porcine | 0.9 | 0.002–5.0 | ||
| Sex | Male | 4.8 | 2.8–7.6 | 0.007 |
| Female | 11.8 | 7.2–18.1 | ||
| Breed | Bonsmara | 6.6 | 3.8–10.5 | <0.001 |
| Jersey | 6.9 | 1.4–19.0 | ||
| Nguni | 26.7 | 14.7–42.0 | ||
| Dorper | 4.3 | 0.9–12.0 | ||
| Large white | 0.9 | 0.2–5.0 | ||
| District | Tshwane | 12.2 | 7.9–17.8 | 0.003 |
| Ekurhuleni | 3.4 | 0.7–9.7 | ||
| Metsweding | 10 | 1.2–32.0 | ||
| Sedibeng | 4.3 | 1.7–8.7 | ||
| West Rand | 0 | 0.0–7.1 | ||
| Abattoir throughput | High | 7.1 | 4.8–9.9 | 1 |
| Low | 5.8 | 1.6–143.9 | ||
| Origin of animals | Farm/feedlot | 4.8 | 2.9–7.3 | <0.001 |
| Auction | 16.7 | 9.6–26.0 | ||
| Total | 6.9 | 4.9–9.5 |
| Variable | Level | Odds Ratio | 95% CI* | p-Value |
|---|---|---|---|---|
| Species | Bovine | 1 * | 0.2–2.0 | 0.369 |
| Ovine | 0.6 | 0.006–0.4 | 0.003 | |
| Porcine | 0.04 | |||
| Abattoir throughput | High | 1 * | 1.2–14.0 | 0.023 |
| Low | 4.1 | |||
| Origin of animals | Farm/feedlot | 1 * | 2.6–12.4 | <0.001 |
| Auction | 5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangena, M.; Gcebe, N.; Pierneef, R.; Thompson, P.N.; Adesiyun, A.A. Q Fever: Seroprevalence, Risk Factors in Slaughter Livestock and Genotypes of Coxiella burnetii in South Africa. Pathogens 2021, 10, 258. https://doi.org/10.3390/pathogens10030258
Mangena M, Gcebe N, Pierneef R, Thompson PN, Adesiyun AA. Q Fever: Seroprevalence, Risk Factors in Slaughter Livestock and Genotypes of Coxiella burnetii in South Africa. Pathogens. 2021; 10(3):258. https://doi.org/10.3390/pathogens10030258
Chicago/Turabian StyleMangena, Maruping, Nomakorinte Gcebe, Rian Pierneef, Peter N. Thompson, and Abiodun A. Adesiyun. 2021. "Q Fever: Seroprevalence, Risk Factors in Slaughter Livestock and Genotypes of Coxiella burnetii in South Africa" Pathogens 10, no. 3: 258. https://doi.org/10.3390/pathogens10030258
APA StyleMangena, M., Gcebe, N., Pierneef, R., Thompson, P. N., & Adesiyun, A. A. (2021). Q Fever: Seroprevalence, Risk Factors in Slaughter Livestock and Genotypes of Coxiella burnetii in South Africa. Pathogens, 10(3), 258. https://doi.org/10.3390/pathogens10030258







