Photodynamic Eradication of Trichophyton rubrum and Candida albicans
Abstract
:1. Introduction
2. Results and Discussion
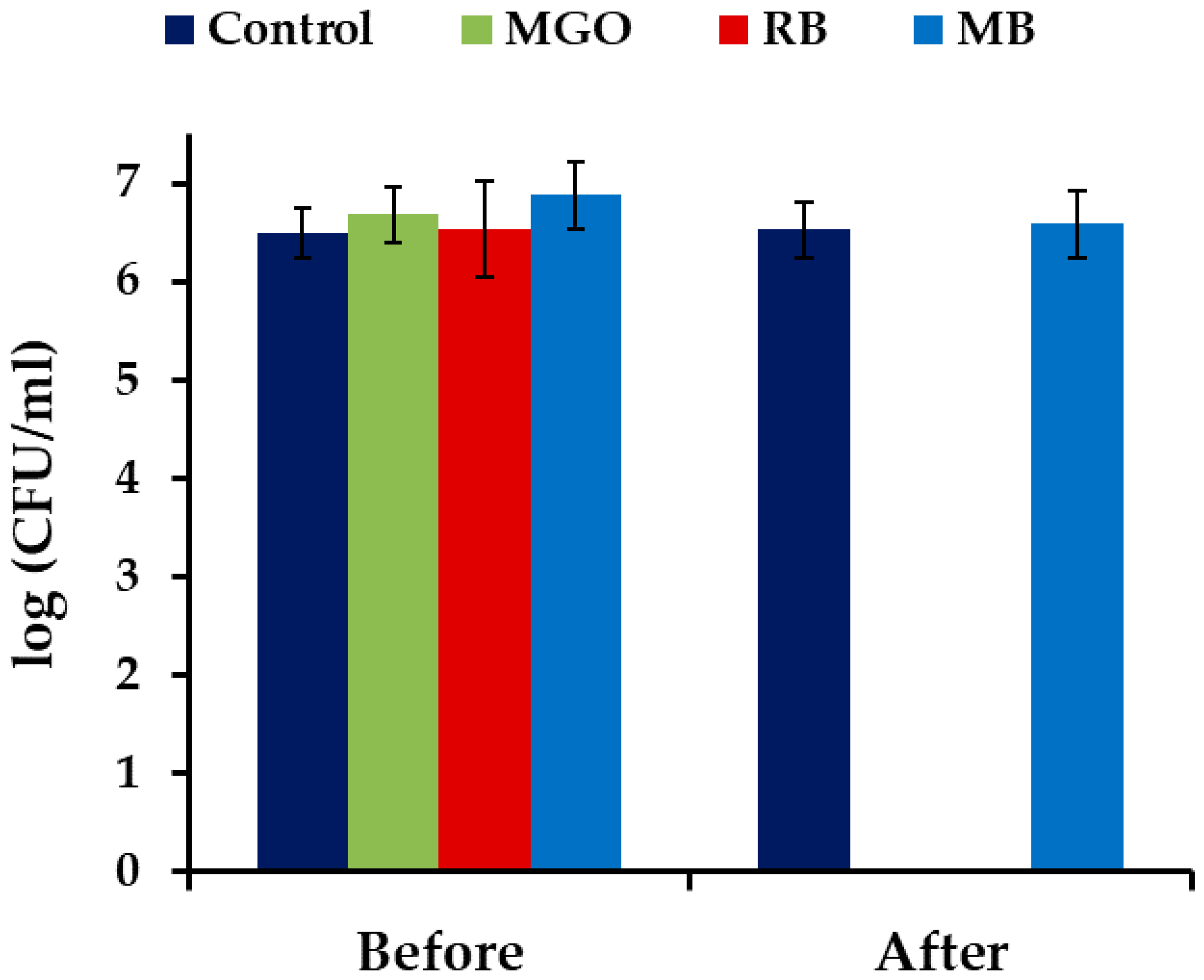
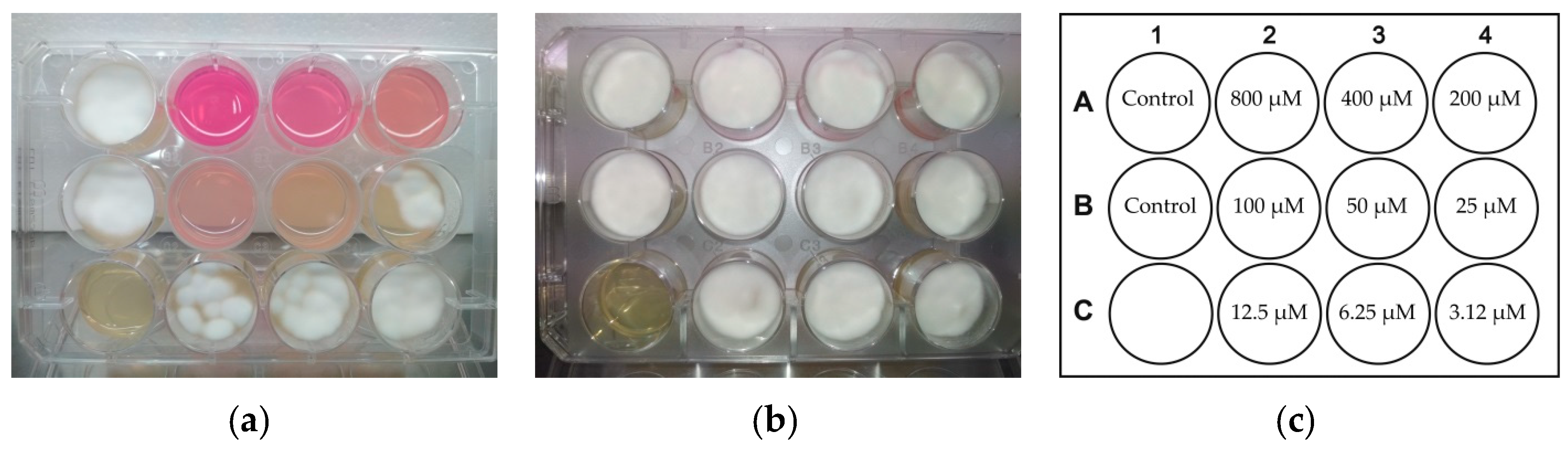
2.1. Photodynamic Treatment of Planktonic Culture of Candida albicans
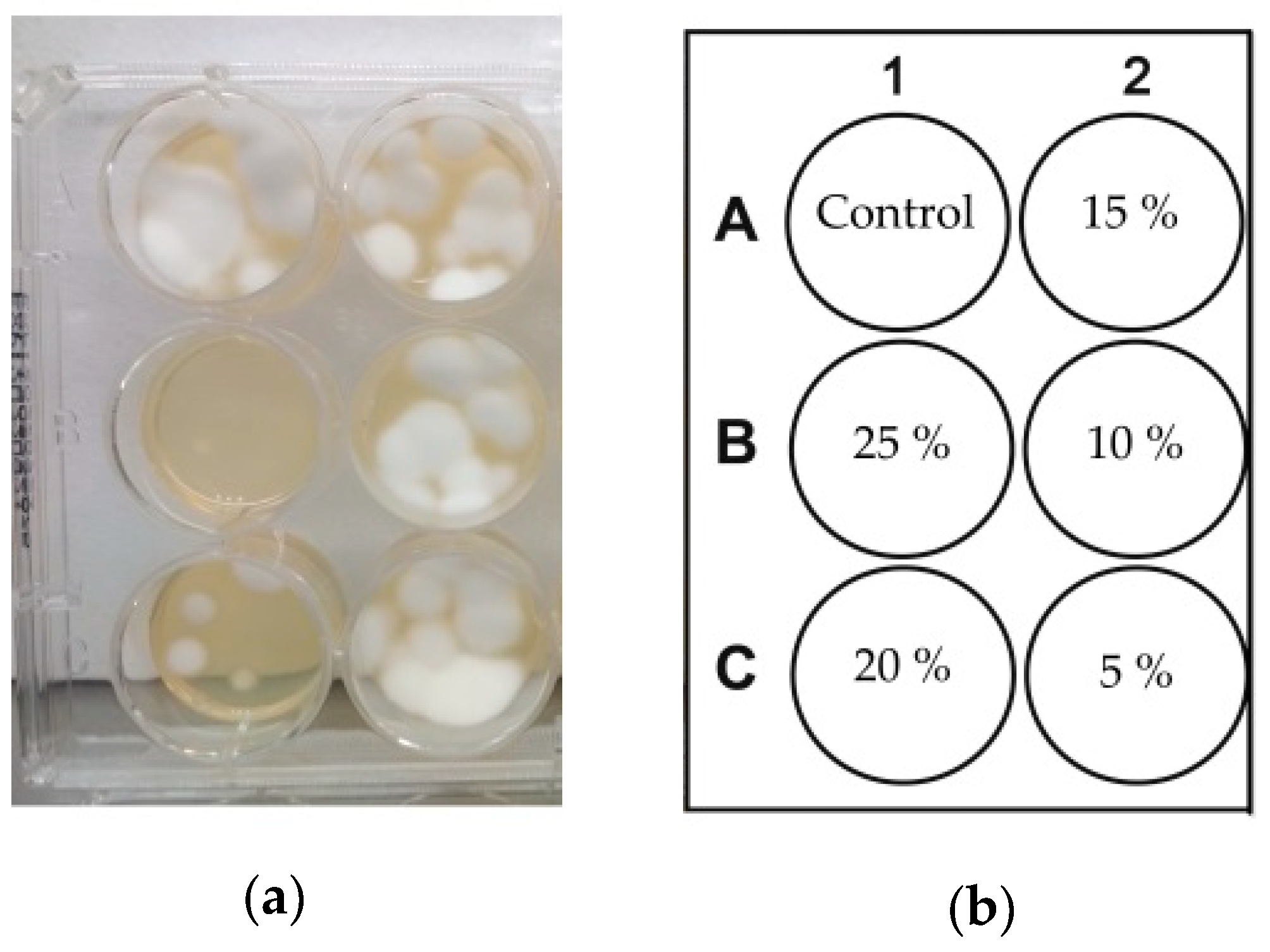
2.2. Photodynamic Treatment of Trichophyton rubrum
2.3. Stability of Formulations
3. Materials and Methods
3.1. Fungal Strains
3.2. Preparation and Growth of Fungi
3.3. Photodynamic Treatment of Planktonic Cultures of Candida albicans
3.4. Photodynamic Treatment of Trichophyton rubrum
3.5. Stability of Formulations
3.6. Statistical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elewski, B.E.; Hay, R.J. Update on the management of onychomycosis: Highlights on the Third Annual International Summit on Cutaneous Antifungal Therapy. Clin. Infect. Dis. 1996, 23, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burzykowski, T.; Molenberghs, G.; Abeck, D.; Haneke, E.; Hay, R.; Katsambas, A.; Roseeuw, D.; Kerkhof, P.; Aelst, R.; Marynissen, G. High prevalence of foot diseases in Europe: Results of the Achilles Project. Mycoses 2003, 46, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Levy, L.A. Epidemiology of onychomycosis in special-risk populations. J. Am. Podiatr. Med. Assoc. 1997, 87, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E. Once-weekly fluconazole in the treatment of onychomycosis: Introduction. J. Am. Acad. Dermatol. 1998, 38, S73–S76. [Google Scholar] [CrossRef]
- Rich, P. Special patient populations: Onychomycosis in the diabetic patient. J. Am. Acad. Dermatol. 1996, 35, 10–12. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Hodak, E.; Vardi, P.; Shraga, I.; Karp, M.; Sprecher, E.; David, M. The Prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care 1998, 21, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Cribier, B.J.; Bakshi, R. Terbinafine in the treatment of onychomycosis: A review of its efficacy in high-risk populations and in patients with nondermatophyte infections. Br. J. Dermatol. 2004, 150, 414–420. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ryder, J.E.; Johnson, A.M. Cumulative meta-analysis of systemic antifungal agents for the treatment of onychomycosis. Br. J. Dermatol. 2004, 150, 537–544. [Google Scholar] [CrossRef]
- Angelo, T.; Borgheti-Cardoso, L.N.; Gelfuso, G.M.; Taveira, S.F.; Gratieri, T. Chemical and physical strategies in onychomycosis topical treatment: A review. Med. Mycol. 2016, 55, 461–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Lu, L.M.; Chen, Y.; Lin, Y.K. Photodynamic therapy as an antifungal treatment. Exp. Ther. Me. 2016, 12, 23–27. [Google Scholar] [CrossRef]
- Cohen, P.R.; Scher, R.K. Topical and surgical treatment of onychomycosis. J. Am. Acad. Dermatol. 1994, 31, S74–S77. [Google Scholar] [CrossRef]
- Elewski, B.E. Onychomycosis: Pathogenesis, diagnosis, and management (Review). Clin. Microbiol. Rev. 1998, 11, 415–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odom, R.B. New therapies for onychomycosis. J. Am. Acad. Dermatol. 1996, 35, S26–S30. [Google Scholar] [CrossRef]
- Katz, H.I. Possible drug interactions in oral treatment of onychomycosis. J. Am. Podiatr. Med. Assoc. 1997, 87, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J. New developments in antifungals. Int. J. Dermatol. 1999, 38, 65–69. [Google Scholar] [PubMed]
- Tom, C.M.; Kane, M.P. Management of toenail onychomycosis. Am. J. Heal. Pharm. 1999, 56, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Nahabedian, M.Y. Multiple-Digit Onychomycosis: A Simple Surgical Cure. Ann. Plast. Surg. 2000, 45, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Braathen, L.R.; Morton, C.A.; Basset-Seguin, N.; Bissonnette, R.; Gerritsen, M.J.P.; Gilaberte, Y.; Calzavara-Pinton, P.; Sidoroff, A.; Wulf, H.C.; Szeimies, R.M. Photodynamic therapy for skin field cancerization: An international consensus. International Society for Photodynamic Therapy in Dermatology. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1063–1066. [Google Scholar] [CrossRef]
- Dai, T.; Fuchs, B.B.; Coleman, J.J.; Prates, R.A.; Astrakas, C.; Denis, T.G.S.; Ribeiro, M.S.; Mylonakis, E.; Hamblin, M.R.; Tegos, G.P. Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front. Microbiol. 2012, 3, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Allison, R.R.; Moghissi, K. Photodynamic therapy (PDT): PDT mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Valkov, A.; Nakonechny, F.; Nisnevitch, M. Antibacterial properties of Rose Bengal immobilized in polymer supports. Appl. Mech. Mater. 2015, 719–720, 21–24. [Google Scholar] [CrossRef]
- Valkov, A.; Raik, K.A.; Mualem-Sinai, Y.; Nakonechny, F.; Nisnevitch, M. Water disinfection by immobilized photosensitizers. Water 2018, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ilizirov, Y.; Formanovsky, A.; Mikhura, I.; Paitan, Y.; Nakonechny, F.; Nisnevitch, M. Effect of photodynamic antibacterial chemotherapy combined with antibiotics on Gram-positive and Gram-negative bacteria. Molecules 2018, 23, 3152. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Xu, H.; Deng, J.; Dan, H.; Ji, P.; Chen, Q.; Zeng, X. Photodynamic therapy for oral potentially malignant disorders. Photodiagnosis Photodyn. Ther. 2019, 28, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Nakonechny, F.; Nisnevitch, M. Aspects of photodynamic inactivation of bacteria. In Microorganisms; Blumenberg, M., Ed.; InTech Open Access Publisher: Rijeka, Croatia, 2019; Volume 7, pp. 131–151. [Google Scholar]
- Nguyen, K.; Khachemoune, A. An update on topical photodynamic therapy for clinical dermatologists. J. Dermatolog. Treat. 2019, 30, 732–744. [Google Scholar] [CrossRef]
- Pertiwi, Y.D.; Chikama, T.; Sueoka, K.; Ko, J.; Kiuchi, Y.; Onodera, M.; Sakaguchi, T. Photodynamic antimicrobial chemotherapy with the photosensitizer TONS504 eradicates Acanthamoeba. Photodiagnosis Photodyn. Ther. 2019, 28, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Bokan, M.; Nakonechny, F.; Talalai, E.; Kobzev, D.; Gellerman, G.; Patsenker, L. Photodynamic effect of novel hexa-iodinated quinono-cyanine dye on Staphylococcus aureus. Photodiagnosis Photodyn. Ther. 2020, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghannoum, M.; Isham, N.; Herbert, J.; Henry, W.; Yurdakul, S. Activity of TDT 067 (terbinafine in transfersome) against agents of onychomycosis, as determined by minimum inhibitory and fungicidal concentrations. J. Clin. Microbiol. 2011, 49, 1716–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K.; Simpson, F.C. New therapeutic options for onychomycosis. Expert Opin. Pharmacother. 2012, 13, 1131–1142. [Google Scholar] [CrossRef]
- Lusiana; Reichl, S.; Müller-Goymann, C.C. Keratin film made of human hair as a nail plate model for studying drug permeation. Eur. J. Pharm. Biopharm. 2011, 78, 432–440. [Google Scholar] [CrossRef]
- Lusiana; Reichl, S.; Müller-Goymann, C.C. Infected nail plate model made of human hair keratin for evaluating the efficacy of different topical antifungal formulations against Trichophyton rubrum in vitro. Eur. J. Pharm. Biopharm. 2013, 84, 599–605. [Google Scholar] [CrossRef]
- Valkov, A.; Zinigrad, M.; Sobolev, A.; Nisnevitch, M. Keratin biomembranes as a model for studying onychomycosis. Int. J. Mol. Sci. 2020, 21, 3512. [Google Scholar] [CrossRef]
- Nisnevitch, M.; Nakonechny, F.; Nitzan, Y. Photodynamic antimicrobial chemotherapy by liposome-encapsulated water-soluble photosensitizers. Russ. J. Bioorganic Chem. 2010, 36, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Prates, R.A.; Yamada, A.M., Jr.; Suzuki, L.C.; Hashimoto, M.C.E.; Cai, S.; Gouw-Soares, S.; Gomes, L.; Ribeiro, M.S. Bactericidal effect of malachite green and red laser on Actinobacillus actinomycetemcomitans. J. Photochem. Photobiol. B 2007, 86, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Nisnevitch, M.; Nakonechny, F.; Firer, M.; Nitzan, Y. Photodynamic antimicrobial chemotherapy with photosensitizers in liposomes under external and chemoluminescent excitation. FEBS J. 2009, 276 S1, 332. [Google Scholar]
- Nakonechny, F.; Nisnevitch, M.; Nitzan, Y. Antimicrobial properties of photosensitizers methylene blue and neutral red encapsulated in liposomes. FEBS J. 2009, 276, 330–331. [Google Scholar]
- Valkov, A.; Nakonechny, F.; Nisnevitch, M. Polymer-immobilized photosensitizers for continuous eradication of bacteria. Int. J. Mol. Sci. 2014, 15, 14984–14996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.C.B.P.; Rasteiro, V.M.C.; Pereira, C.A.; Rossoni, R.D.; Junqueira, J.C.; Jorge, A.O.C. The effects of rose bengal- and erythrosine-mediated photodynamic therapy on Candida albicans. Mycoses 2011, 55, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Freire, F.; Costa, A.; Pereira, C.; Junior, M.; Junqueira, J.; Jorge, A. Comparison of the effect of rose bengal- and eosin Y-mediated photodynamic inactivation on planktonic cells and biofilms of Candida albicans. Lasers Med. Sci. 2014, 29, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.D.; Giri, A.; Singh, L. Transungual drug delivery: A newer approach. World J. Pharm. Pharm. Sci. 2014, 3, 781–794. [Google Scholar]
- Houang, J.; Halliday, C.; Chen, S.; Ho, C.-H.; Bekmukhametova, A.; Lauto, A. Effective photodynamic treatment of Trichophyton species with Rose Bengal. J. Biophotonics. 2020, 14, 1–7. [Google Scholar] [CrossRef]
- Houang, J.; Perrone, G.G.; Pedrinazzi, C.; Longo, L.; Mawad, D.; Boughton, P.C.; Ruys, A.J.; Lauto, A. Genetic tolerance to Rose Bengal photodynamic therapy and antifungal clinical application for onychomycosis. Adv. Ther. 2019, 2, 1–13. [Google Scholar] [CrossRef]
- Quintanar-Guerrero, D.; Ganem-Quintanar, A.; Tapia-Olguín, P.; Kalia, Y.N.; Buri, P. The effect of keratolytic agents on the permeability of three imidazole antimycotic drugs through the human nail. Drug Dev. Ind. Pharm. 1998, 24, 685–690. [Google Scholar] [CrossRef]
- Bunyaratavej, S.; Leeyaphan, C.; Rujitharanawong, C.; Surawan, T.; Muanprasat, C.; Matthapan, L. Efficacy of 5% amorolfine nail lacquer in Neoscytalidium dimidiatum onychomycosis. J. Dermatolog. Treat. 2016, 27, 359–363. [Google Scholar] [CrossRef]
- Escalante, K.; Herrera, E.O.M.; Torres-Guerrero, E.; Arroyo, S.; Arenas, R. Onychomycosis with dermatophytoma. A comparison among the results of treatments with oral terbinafine, topical 40% urea in monotherapy and combination therapy. Dermatologia Klin. 2013, 15, 67–70. [Google Scholar]
- Clever, H.L.; Battino, R.; Miyamoto, H.; Yampolski, Y.; Young, C.L. IUPAC-NIST Solubility Data Series. 103. Oxygen and ozone in water, aqueous solutions, and organic liquids (Supplement to Solubility Data Series Volume 7). J. Phys. Chem. 2014, 43, 1–209. [Google Scholar]
- Foote, C.S. Type I and Type II Mechanisms of Photodynamic Action. Am. Chem. Soc. 1987, 339, 22–38. [Google Scholar]
- Macdonald, I.J.; Douherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Chaturvedi, V.; Espinel-Ingroff, A.; Ghannoum, M.A.; Gosey, L.L.; Odds, F.C.; Rex, J.H.; Rinaldi, M.G.; Sheehan, D.J.; Walsh, T.J.; et al. Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved Standard—Second Edition. NCCLS Doc. M27-A2 2002, 22, 1–29. [Google Scholar]









| Solvent | Sample Number | Urea (g) | Thiourea (g) | Rose Bengal (g) | λmax (nm) |
|---|---|---|---|---|---|
| Water 1 | 1 | 0.1 | 190 | ||
| 2 | 0.1 | 196, 236 | |||
| 3 | 0.1 | 212, 546 | |||
| 4 | 0.1 | 0.1 | 0.1 | 230, 546 | |
| Glycerol/Water 1 70/30%, w/w | 5 | 0.1 | 202 | ||
| 6 | 0.1 | 230 | |||
| 7 | 0.1 | 214, 556 | |||
| 8 | 0.1 | 0.1 | 0.1 | 230, 556 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valkov, A.; Zinigrad, M.; Nisnevitch, M. Photodynamic Eradication of Trichophyton rubrum and Candida albicans. Pathogens 2021, 10, 263. https://doi.org/10.3390/pathogens10030263
Valkov A, Zinigrad M, Nisnevitch M. Photodynamic Eradication of Trichophyton rubrum and Candida albicans. Pathogens. 2021; 10(3):263. https://doi.org/10.3390/pathogens10030263
Chicago/Turabian StyleValkov, Anton, Michael Zinigrad, and Marina Nisnevitch. 2021. "Photodynamic Eradication of Trichophyton rubrum and Candida albicans" Pathogens 10, no. 3: 263. https://doi.org/10.3390/pathogens10030263
APA StyleValkov, A., Zinigrad, M., & Nisnevitch, M. (2021). Photodynamic Eradication of Trichophyton rubrum and Candida albicans. Pathogens, 10(3), 263. https://doi.org/10.3390/pathogens10030263







