Cytokines and Onchocerciasis-Associated Epilepsy, a Pilot Study and Review of the Literature
Abstract
:1. Introduction
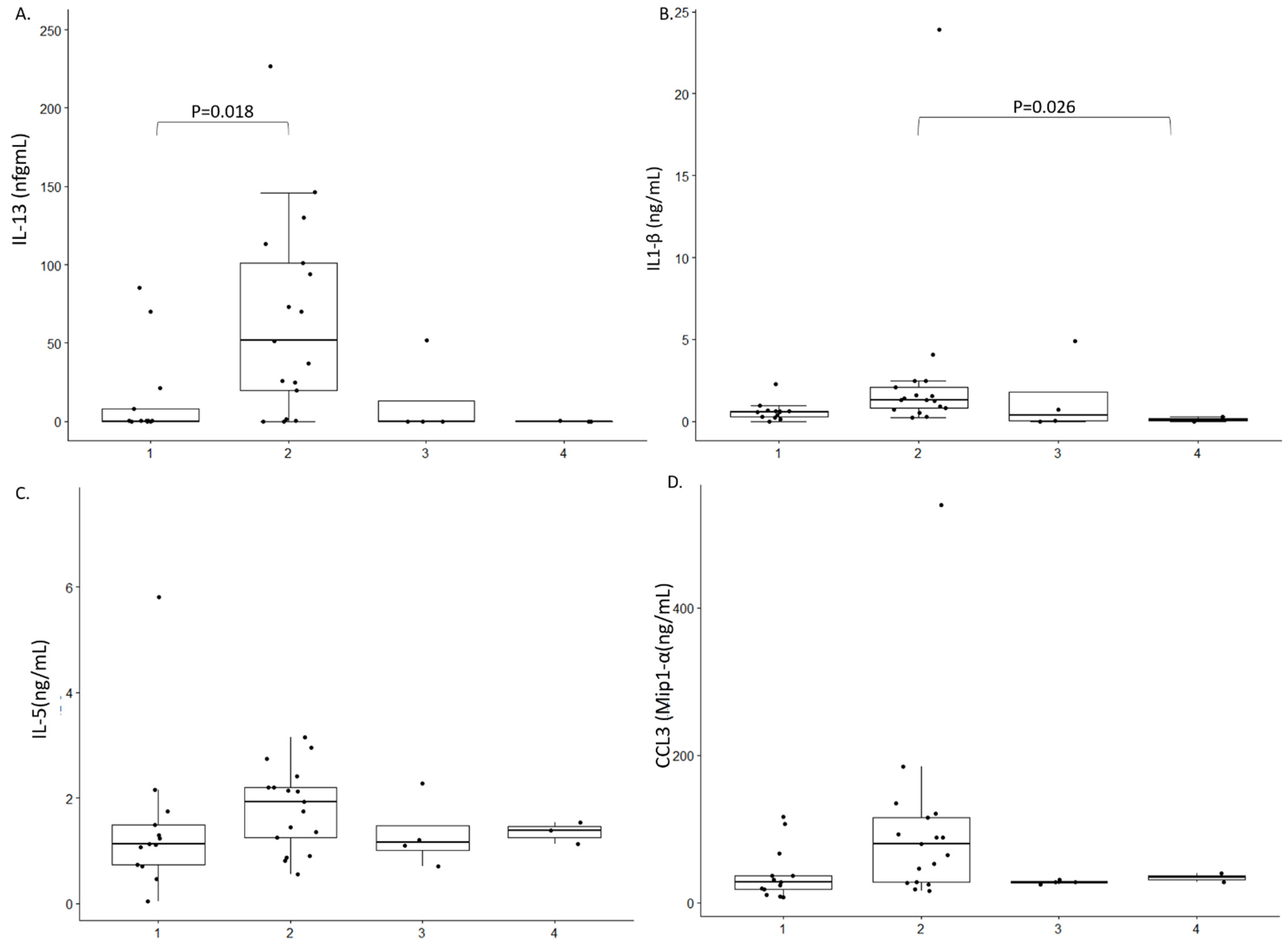
2. Results
3. Discussion
4. Materials and Methods
4.1. MSD Neuro-Inflammatory Panel
4.2. Statistical Analysis
4.3. Litterature Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colebunders, R.; Njamnshi, A.K.; Menon, S.; Newton, C.R.; Hotterbeekx, A.; Preux, P.-M.; Hopkins, A.; Vaillant, M.; Fodjo, J.N.S. Onchocerca volvulus and epilepsy: A comprehensive review using the Bradford Hill criteria for causation. PLoS Neglected Trop. Dis. 2021, 15, e0008965. [Google Scholar] [CrossRef]
- Gumisiriza, N.K.C.; Asaba, G.; Onen, H.; Mubiru, F.; Kisembo, D.; Fodjo, J.N.S.; Colebunders, R. Onchocerciasis elimination drastically reduces epilepsy prevalence and incidence in Kabarole, Western Uganda. In Proceedings of the 4th African Epilepsy Conference, Entebbe, Uganda, 22–24 August 2019. [Google Scholar]
- Chesnais, C.B.; Nana-Djeunga, H.C.; Njamnshi, A.K.; Lenou-Nanga, C.G.; Boullé, C.; Bissek, A.-C.Z.-K.; Kamgno, J.; Colebunders, R.; Boussinesq, M. The temporal relationship between onchocerciasis and epilepsy: A population-based cohort study. Lancet Infect. Dis. 2018, 18, 1278–1286. [Google Scholar] [CrossRef]
- Chesnais, C.B.; Bizet, C.; Campillo, J.T.; Njamnshi, W.Y.; Bopda, J.; Nwane, P.; Pion, S.D.; Njamnshi, A.K.; Boussinesq, M. A Second Population-based cohort study in cameroon confirms the temporal relationship between onchocerciasis and epilepsy. Open Forum Infect. Dis. 2020, 7, ofaa206. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Lammens, M.; Idro, R.; Akun, P.R.; Lukande, R.; Akena, G.; Nath, A.; Taylor, J.; Olwa, F.; Kumar-Singh, S.; et al. Neuroinflammation and not tauopathy is a predominant pathological signature of nodding syndrome. J. Neuropathol. Exp. Neurol. 2019, 78, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Duke, B.O.; Vincelette, J.; Moore, P.J. Microfilariae in the cerebrospinal fluid, and neurological complications, during treatment of onchocerciasis with diethylcarbamazine. Trop. Parasitol. 1976, 27, 123–132. [Google Scholar] [CrossRef]
- König, R.; Nassri, A.; Meindl, M.; Matuja, W.; Kidunda, A.R.; Siegmund, V.; Bretzel, G.; Löscher, T.; Jilek-Aall, L.; Schmutzhard, E.; et al. The role of Onchocerca volvulusin the development of epilepsy in a rural area of Tanzania. Parasitology 2010, 137, 1559–1568. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Raimon, S.; Abd-Elfarag, G.; Carter, J.Y.; Sebit, W.; Suliman, A.; Fodjo, J.N.S.; De Witte, P.; Logora, M.Y.; Colebunders, R.; et al. Onchocerca volvulus is not detected in the cerebrospinal fluid of persons with onchocerciasis-associated epilepsy. Int. J. Infect. Dis. 2020, 91, 119–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, T.P.; Tyagi, R.; Lee, P.R.; Lee, M.-H.; Johnson, K.R.; Kowalak, J.; Elkahloun, A.; Medynets, M.; Hategan, A.; Kubofcik, J.; et al. Nodding syndrome may be an autoimmune reaction to the parasitic worm Onchocerca volvulus. Sci. Transl. Med. 2017, 9, eaaf6953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollanen, M.S.; Onzivua, S.; Robertson, J.; McKeever, P.M.; Olawa, F.; Kitara, D.L.; Fong, A. Nodding syndrome in Uganda is a tauopathy. Acta Neuropathol. 2018, 136, 691–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, X.Y.; Koepp, M.; Duncan, J.S.; Fox, N.; Thompson, P.; Baxendale, S.; Liu, J.Y.W.; Reeves, C.; Michalak, Z.; Thom, M. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: A study of temporal lobe resections. Brain 2016, 139, 2441–2455. [Google Scholar] [CrossRef]
- Burfeind, K.G.; Kashama, J.-M.K.; Bora, B.K.; Murchison, C.F.; Ramos-Crawford, A.L.; Nseka, M.T.; Kunyu, S.B.; Okitundu, D.L.; Mashukano, N.L.; Banea, J.-P.M.; et al. Baseline characterization of epilepsy in an onchocerciasis endemic area of the Democratic Republic of Congo. Brain Res. Bull. 2019, 145, 45–52. [Google Scholar] [CrossRef]
- Levite, M.; Zelig, D.; Friedman, A.; Ilouz, N.; Eilam, R.; Bromberg, Z.; Lasu, A.A.R.; Arbel-Alon, S.; Edvardson, S.; Tarshish, M.; et al. Dual-Targeted Autoimmune Sword in Fatal Epilepsy: Patient’s glutamate receptor AMPA GluR3B peptide autoimmune antibodies bind, induce Reactive Oxygen Species (ROS) in, and kill both human neural cells and T cells. J. Autoimmun. 2020, 112, 102462. [Google Scholar] [CrossRef]
- Mărginean, C.O.; Man, L.; Pitea, A.M.; Man, A.; Mărginean, C.L.; Cotoi, O.S. The assessment between IL-6 and IL-8 and anthropometric status in malnourished children. Rom. J. Morphol. Embryol. 2013, 54, 935–938. [Google Scholar] [PubMed]
- Ogwang, R.; Muhanguzi, D.; Mwikali, K.; Anguzu, R.; Kubofcik, J.; Nutman, T.B.; Taylor, M.; Newton, C.R.; Vincent, A.; Conroy, A.L.; et al. Systemic and cerebrospinal fluid immune and complement activation in Ugandan children and adolescents with long-standing nodding syndrome: A case-control study. Epilepsia Open 2020. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Wynn, T.A.; Donaldson, D.D.; Urban, J.F. The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infections. Curr. Opin. Immunol. 1999, 11, 420–426. [Google Scholar] [CrossRef]
- Muhsin, M.; Ajendra, J.; Gentil, K.; Berbudi, A.; Neumann, A.-L.; Klaas, L.; Schmidt, K.E.; Hoerauf, A.; Hübner, M.P. IL-6 is required for protective immune responses against early filarial infection. Int. J. Parasitol. 2018, 48, 925–935. [Google Scholar] [CrossRef]
- Kamaşak, T.; Dilber, B.; Yaman, S.; Durgut, B.D.; Kurt, T.; Çoban, E.; Arslan, E.A.; Şahin, S.; Karahan, S.C.; Cansu, A. HMGB-1, TLR4, IL-1R1, TNF-α, and IL-1β: Novel epilepsy markers? Epileptic Disord 2020, 22, 183–193. [Google Scholar] [CrossRef]
- Hernandez, M.X.; Namiranian, P.; Nguyen, E.; Fonseca, M.I.; Tenner, A.J. C5a increases the injury to primary neurons elicited by fibrillar amyloid beta. ASN Neuro 2017, 9, 1759091416687871. [Google Scholar] [CrossRef]
- Morgan, B.P. The role of complement in neurological and neuropsychiatric diseases. Expert Rev. Clin. Immunol. 2015, 11, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Meri, T.; Bialonski, A.; Jokiranta, T.S.; Hellwage, J.; Zipfel, P.F.; Meri, S. Onchocerca volvulus microfilariae avoid complement attack by direct binding of factor H. J. Infect. Dis. 2002, 185, 1786–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brattig, N.W.; Lepping, B.; Timmann, C.; Büttner, D.W.; Marfo, Y.; Hamelmann, C.; Horstmann, R.D. Onchocerca volvulus–exposed persons fail to produce interferon-γ in response to O. volvulus antigen but mount proliferative responses with interleukin-5 and IL-13 production that decrease with increasing microfilarial density. J. Infect. Dis. 2002, 185, 1148–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liba, Z.; Nohejlova, H.; Capek, V.; Krsek, P.; Sediva, A.; Kayserova, J. Utility of chemokines CCL2, CXCL8, 10 and 13 and interleukin 6 in the pediatric cohort for the recognition of neuroinflammation and in the context of traditional cerebrospinal fluid neuroinflammatory biomarkers. PLoS ONE 2019, 14, e0219987. [Google Scholar] [CrossRef] [Green Version]
- Mai, C.S.; Hamm, D.M.; Banla, M.; Agossou, A.; Schulz-Key, H.; Heuschkel, C.; Soboslay, P.T. Onchocerca volvulus-specific antibody and cytokine responses in onchocerciasis patients after 16 years of repeated ivermectin therapy. Clin. Exp. Immunol. 2007, 147, 504–512. [Google Scholar] [CrossRef]
- De Vries, E.E.; van den Munckhof, B.; Braun, K.P.; van Royen-Kerkhof, A.; de Jager, W.; Jansen, F.E. Inflammatory mediators in human epilepsy: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 63, 177–190. [Google Scholar] [CrossRef]
- Youn, Y.; Sung, I.K.; Lee, I.G. The role of cytokines in seizures: Interleukin (IL)-1β, IL-1Ra, IL-8, and IL-10. Korean J. Pediatr. 2013, 56, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Hotterbeekx, A.; Dusabimana, A.; Mandro, M.; Abhafule, G.M.; Deogratias, W.; Fodjo, J.N.S.; Abrams, S.; Colebunders, R. Urinary N-acetyltyramine-O,β-glucuronide in persons with onchocerciasis-associated Epilepsy. Pathogens 2020, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Mukendi, D.; Tepage, F.; Akonda, I.; Siewe, J.N.F.; Rotsaert, A.; Ndibmun, C.N.; Laudisoit, A.; Couvreur, S.; Kabutako, B.; Menon, S.; et al. High prevalence of epilepsy in an onchocerciasis endemic health zone in the Democratic Republic of the Congo, despite 14 years of community-directed treatment with ivermectin: A mixed-method assessment. Int. J. Infect. Dis. 2019, 79, 187–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| No. | Age (Years) | Sex | ASO (Years) | Seizure Frequency (Monthly) | Generalized TCS | NS | Mf Density (Per SS) | Cognitive Impairment | Onchocerca Skin Lesion | Muscle Wasting |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 | F | 14 | 1 | YES | NO | 99.5 | NO | NO | NO |
| 2 | 11 | M | 5 | 150 | YES | YES | 122 | YES | YES | YES |
| 3 | 18 | M | 6 | 120 | YES | YES | 117 | YES | YES | YES |
| 4 | 9 | M | 7 | 120 | YES | YES | 84 | YES | YES | YES |
| 5 | 16 | M | 4 | 90 | YES | YES | 86 | YES | YES | YES |
| 6 | 28 | F | 1 | <1 | NO | NO | 118.5 | NO | NO | NO |
| 7 | 12 | M | 10 | 4 | NO | YES | 102 | NO | NO | NO |
| 8 | 15 | F | 7 | 24 | YES | YES | 85 | NO | YES | NO |
| 9 | 19 | F | 15 | 12 | YES | YES | 110 | NO | NO | NO |
| 10 | 11 | F | 5 | 8 | YES | NO | 105 | NO | NO | NO |
| 11 | 24 | M | 14 | 90 | NO | NO | 108 | NO | NO | NO |
| 12 * | 8 | M | NK | NK | NK | NK | NK | NK | NK | NK |
| 13 * | 28 | F | NK | NK | NK | NK | NK | NK | NK | NK |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieri, M.K.; Hotterbeekx, A.; Raimon, S.; Abd-Elfarag, G.; Mukendi, D.; Carter, J.Y.; Kumar-Singh, S.; Colebunders, R. Cytokines and Onchocerciasis-Associated Epilepsy, a Pilot Study and Review of the Literature. Pathogens 2021, 10, 310. https://doi.org/10.3390/pathogens10030310
Vieri MK, Hotterbeekx A, Raimon S, Abd-Elfarag G, Mukendi D, Carter JY, Kumar-Singh S, Colebunders R. Cytokines and Onchocerciasis-Associated Epilepsy, a Pilot Study and Review of the Literature. Pathogens. 2021; 10(3):310. https://doi.org/10.3390/pathogens10030310
Chicago/Turabian StyleVieri, Melissa Krizia, An Hotterbeekx, Stephen Raimon, Gasim Abd-Elfarag, Deby Mukendi, Jane Y. Carter, Samir Kumar-Singh, and Robert Colebunders. 2021. "Cytokines and Onchocerciasis-Associated Epilepsy, a Pilot Study and Review of the Literature" Pathogens 10, no. 3: 310. https://doi.org/10.3390/pathogens10030310
APA StyleVieri, M. K., Hotterbeekx, A., Raimon, S., Abd-Elfarag, G., Mukendi, D., Carter, J. Y., Kumar-Singh, S., & Colebunders, R. (2021). Cytokines and Onchocerciasis-Associated Epilepsy, a Pilot Study and Review of the Literature. Pathogens, 10(3), 310. https://doi.org/10.3390/pathogens10030310







