Detection of Babesia odocoilei in Ixodes scapularis Ticks Collected in Southern Ontario, Canada
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tick Collection
2.2. DNA Extraction, PCR, and Sequencing
2.3. Phylogenetic Analysis
3. Results
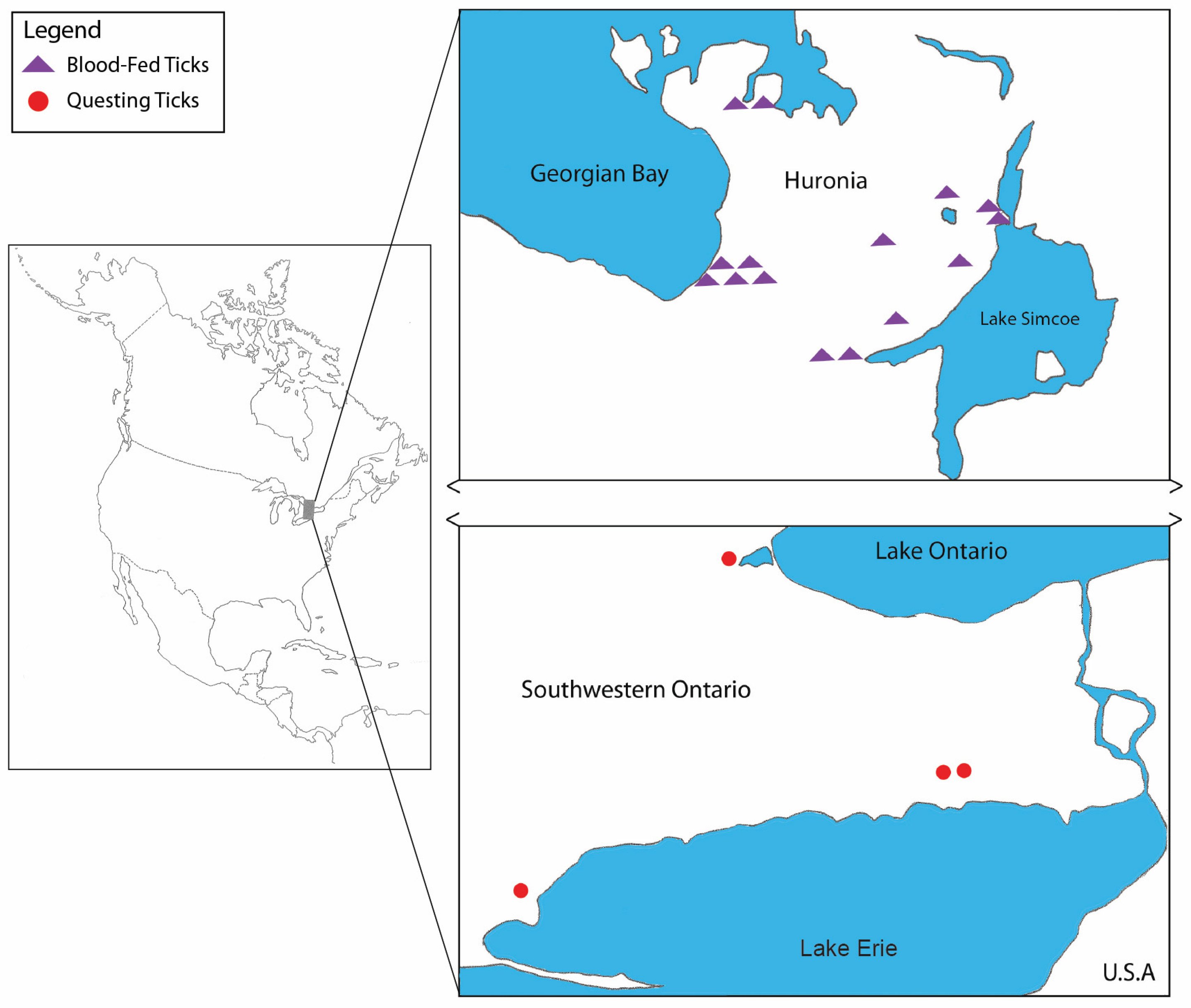
3.1. Tick Collection
3.2. Babesia Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schnittger, L.; Rodriguez, A.E.; Florin-Christensen, M.; Morrison, D.A. Babesia: A world emerging. Infect. Gen. Evol. 2012, 12, 1788–1809. [Google Scholar] [CrossRef] [PubMed]
- Reubush, T.K., II; Cassaday, P.S.; Marsh, H.J.; Lisker, S.A.; Voorhees, D.B.; Maloney, E.B.; Healy, G.R. Human babesiosis on Nantucket Island. Clinical features. Ann. Intern. Med. 1977, 86, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Vannier, E.; Krause, P.J. Human babesiosis. N. Eng. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herwaldt, B.L.; Cacciò, S.; Gherlinzoni, F.; Aspöck, H.; Siemenda, S.B.; Piccaluga, P.; Martinelli, G.; Edelhofer, R.; Hollenstein, U.; Poletti, G.; et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg. Infect. Dis. 2003, 9, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Škrabalo, Z.; Deanović, Z. Piroplasmosis in man: Report on a case. Doc. Med. Geogr. Trop. 1957, 9, 11–16. [Google Scholar] [PubMed]
- Anderson, J.F.; Mintz, E.D.; Gadbaw, J.J.; Magnarelli, L.A. Babesia microti, human babesiosis, and Borrelia burgdorferi in Connecticut. J. Clin. Microbiol. 1991, 29, 2779–2783. [Google Scholar] [CrossRef] [Green Version]
- Herwaldt, B.L.; Persing, D.H.; Precigout, E.A.; Goff, W.L.; Mathiesen, D.A.; Taylor, P.W.; Eberhard, M.L.; Gorenflot, A.F. A fatal case of babesiosis in Missouri: Identification of another piroplasm that infects humans. Ann. Intern. Med. 1996, 124, 643–650. [Google Scholar] [CrossRef]
- Homer, M.J.; Aquilar-Delfin, I.; Telford, S.R., III; Krause, P.J.; Persing, D.H. Babesiosis. Clin. Microbiol. Rev. 2000, 13, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, J.C.; Greenberg, P.D.; Antique, J.; Jimenez-Lucho, V.E. Severe babesiosis in Long Island: Review of 34 cases and their complications. Clin. Infect. Dis. 2001, 32, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Centeno-Lima, S.; Do Rosário, V.; Parreira, R.; Maia, A.J.; Freudenthal, A.M.; Nijhof, A.M.; Jongejan, F. A fatal case of human babesiosis in Portugal: Molecular and phylogenetic analysis. Trop. Med. Int. Health 2003, 8, 760–764. [Google Scholar] [CrossRef]
- Herwaldt, B.L.; De Bruyn, G.; Pieniazek, N.J.; Homer, M.; Lofy, K.H.; Siemenda, S.B.; Fritsche, T.R.; Persing, D.H.; Limaye, A.P. Babesia divergens-like infection, Washington State. Emerg. Infect. Dis. 2004, 10, 622–629. [Google Scholar] [CrossRef]
- Gelfand, J.A. Babesia. In Mandell, Douglas and Bennett’s Principles of Practice of Infectious Diseases, 4th ed.; Mandell, G.L., Bennett, J.E., Dolin, R., Eds.; Churchill Livingstone: New York, NY, USA, 1995; Volume 2, pp. 2497–2500. [Google Scholar]
- Gubernot, D.M.; Nakhasi, H.L.; Mied, P.A.; Asher, D.M.; Epstein, J.S.; Kumar, S. Transfusion-transmitted babesiosis in the United States: Summary of workshop. Transfusion 2009, 49, 2759–2771. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.M.; Herwaldt, B.L.; Leiby, D.A.; Shaieb, A.; Herron, R.M.; Chervenak, M.; Reed, V.V.; Hunter, R.; Ryals, R.; Hagar, W.; et al. The third described case of transfusion-transmitted Babesia duncani. Transfusion 2012, 52, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, T.; Karp, J.K. Transfusion-transmitted babesiosis. Arch. Pathol. Lab. Med. 2019, 143, 130–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, M.B.; Herwaldt, B.L.; Kazmierczak, J.J.; Weiss, J.W.; Klein, C.L.; Leith, C.P.; He, R.; Oberley, M.J.; Tonnetti, L.; Wilkins, P.P.; et al. Transmission of Babesia microti parasites by solid organ transplantation. Emerg. Infect. Dis. 2016, 22, 1869–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, L.M.; Winger, S.; Ahmed, A.; Arnold, A.; Chou, J.; Rhein, L.; Levy, O. Neonatal babesiosis: Case report and review of the literature. Pediatr. Infect. Dis. J. 2006, 25, 169–173. [Google Scholar] [CrossRef]
- Cornett, J.K.; Malhotra, A.; Hart, D. Vertical transmission of babesiosis from a pregnant, splenectomized mother to her neonate. Infect. Dis. Clin. Pract. 2012, 20, 408–410. [Google Scholar] [CrossRef]
- Kjemtrup, A.M.; Conrad, P.A. Human babesiosis: An emerging tick-borne disease. Int. J. Parasitol. 2000, 30, 1323–1337. [Google Scholar] [CrossRef]
- Scott, J.D. First record of locally acquired human babesiosis in Canada caused by Babesia duncani: A case report. SAGE Open Med. Case Rep. 2017, 5, 2050313–17725645. [Google Scholar]
- Shih, C.M.; Liu, L.P.; Chung, W.C.; Ong, S.J.; Wang, C.C. Human babesiosis in Taiwan: Asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J. Clin. Microbiol. 1997, 35, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Ord, R.L.; Lobo, C.A. Human babesiosis: Pathogens, prevalence, diagnosis, and treatment. Cur. Clin. Microbiol. Rep. 2015, 2, 173–181. [Google Scholar] [CrossRef]
- Emerson, H.R.; Wright, W.T. The isolation of a Babesia in white-tailed deer. Bull. Wildl. Dis. Assoc. 1968, 4, 142–143. [Google Scholar] [CrossRef] [Green Version]
- Emerson, H.R.; Wright, W.T. Correction. J. Wildl. Dis. 1970, 6, 519. [Google Scholar] [CrossRef]
- Holman, P.J.; Madeley, J.; Craig, T.M.; Allsopp, B.A.; Allsopp, M.T.; Petrini, K.R.; Waghela, S.D.; Wagner, G.G. Antigenic, phenotypic and molecular characterization confirms Babesia odocoilei isolated from three cervids. J. Wildl. Dis. 2000, 36, 518–530. [Google Scholar] [CrossRef]
- Pattullo, K.M.; Wobeser, G.; Lockerbie, B.P.; Burgess, H.J. Babesia odocoilei infection in a Saskatchewan elk (Cervus elaphus canadensis) herd. J. Vet. Diagn. Investig. 2013, 25, 535–540. [Google Scholar] [CrossRef]
- Waldrup, K.A.; Kocan, A.A.; Barker, R.W.; Wagner, G.G. Transmission of Babesia odocoilei in white-tailed deer (Odocoileus virginianus) by Ixodes scapularis (Acari: Ixodidae). J. Wildl. Dis. 1990, 26, 390–391. [Google Scholar] [CrossRef] [Green Version]
- Perry, B.D.; Nichols, D.K.; Cullom, E.S. Babesia odocoilei Emerson and Wright, 1970 in white-tailed deer, Odocoileus virginianus (Zimmermann), in Virginia. J. Wildl. Dis. 1985, 21, 149–152. [Google Scholar] [CrossRef] [Green Version]
- Holman, P.J.; Waldrup, K.A.; Wagner, G.G. In vitro cultivation of a Babesia isolated from a white-tailed deer (Odocoileus virginianus). J. Parasitol. 1988, 74, 111–115. [Google Scholar] [CrossRef]
- Holman, R.J.; Craig, T.M.; Doan-Crider, D.L.; Petrini, K.R. Culture isolation of partial characterization of a Babesia sp. from a North American elk (Cervus elaphus). J. Wildl. Dis. 1994, 30, 460. [Google Scholar] [CrossRef] [PubMed]
- Holman, P.J.; Bendele, K.G.; Schoelkopf, L.; Jones, R.L. Ribosomal RNA analysis of Babesia odocoilei isolates from farmed reindeer (Rangifer tarandus tarandus) and elk (Cervus elaphus canadensis) in Wisconsin. Parasitol. Res. 2003, 91, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Schoelkopf, L.; Hutchinson, C.E.; Bendele, K.G.; Goff, W.L.; Willette, M.; Rasmussen, J.M.; Holman, P.J. New ruminant hosts and wider geographic range identified for Babesia odocoilei (Emerson and Wright 1970). J. Wildl. Dis. 2005, 41, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, S.L.; Abou-Madi, N.; Messick, J.B.; Birkenheuer, A. Diagnosis and treatment of Babesia odocoilei in captive reindeer (Rangifer tarandus tarandus) and recognition of three novel host species. J. Zoo Wildl. Med. 2009, 40, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Keirans, J.E.; Hutcheson, H.J.; Durden, L.A.; Klompen, J.S.H. Ixodes (Ixodes) scapularis (Acari: Ixodidae): Redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J. Med. Entomol. 1996, 33, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Pascoe, E.L.; Sajid, M.S.; Foley, J.E. Detection of Babesia odocoilei in Ixodes scapularis ticks collected from songbirds in Ontario and Quebec, Canada. Pathogens 2020, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Morzaria, S.P. Biology of Babesia. Parasitol. Today 1986, 2, 211–219. [Google Scholar] [CrossRef]
- Mehlhorn, H.; Shein, E. The piroplasms: Life cycle and sexual stages. Adv. Parasitol. 1985, 23, 37–103. [Google Scholar]
- Nicholson, W.A.; Sonenshine, D.E.; Noden, B.H. Ticks (Ixodida). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press/Elsevier: London, UK, 2019; pp. 603–672. [Google Scholar]
- Scott, J.D.; Clark, K.L.; Coble, N.M.; Ballantyne, T.R. Detection and transstadial passage of Babesia species and Borrelia burgdorferi sensu lato in ticks collected from avian and mammalian hosts in Canada. Healthcare 2019, 7, 155. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.D.; Clark, K.L.; Coble, N.M.; Ballantyne, T.R. Presence of Babesia odocoilei and Borrelia burgdorferi sensu stricto in a tick and dual parasitism of Amblyomma inornatum and Ixodes scapularis on a bird in Canada. Healthcare 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milnes, E.L.; Thornton, G.; Léveillé, A.N.; Delinatte, P.; Barta, J.R.; Smith, D.A.; Nemeth, N. Babesia odocoilei and zoonotic pathogens identified from Ixodes scapularis ticks in southern Ontario, Canada. Ticks Tick Borne Dis. 2019, 10, 670–676. [Google Scholar] [CrossRef]
- Keirans, J.E.; Clifford, C.M. The genus Ixodes in the United States: A scanning electron microscope study and key to the adults. J. Med. Entomol. 1978, 15, 1–38. [Google Scholar] [CrossRef]
- Casati, S.; Sager, H.; Gern, L.; Piffaretti, J.-C. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann. Agric. Environ. Med. 2016, 13, 65–70. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.P., Jr.; Elias, S.P.; Borelli, T.J.; Missagthi, R.; York, B.J.; Kessler, R.A.; Lubelczyk, C.B.; Lacome, E.H.; Hayes, C.M.; Coulter, M.S.; et al. Human babesiosis. Emerg. Infect. Dis. 2014, 20, 1727–1730. [Google Scholar] [PubMed]
- Stock, B.C.; Moncayo, A.; Cohen, S.; Mitchell, E.A.; Williamson, P.C.; Lopez, G.; Garrison, L.E.; Yabsley, M.J. Diversity of piroplasms detected in blood-fed and questing ticks from several states in the United States. Ticks Tick Borne Dis. 2014, 5, 373–380. [Google Scholar]
- Steiner, F.E.; Pinger, R.R.; Vann, C.N.; Abley, M.J.; Sullivan, B.; Grindle, N.; Clay, K.; Fuqua, C. Detection of Anaplasma phagocytophilum and Babesia odocoilei DNA in Ixodes scapularis (Acari: Ixodidae) collected in Indiana. J. Med. Entomol. 2006, 43, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Penzhorn, B.L.; Oosthuizen, M.C. Babesia species of domestic cats: Molecular characterization has opened Pandor’s box. Front. Vet. Sci. 2020, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Caccio, S.M.; Antunovic, B.; Moretti, A.; Mangili, V.; Marinculic, A.; Baric, R.R.; Siemenda, S.B.; Pieniazek, N.J. Molecular characterisation of Babesia canis canis and Babesia canis vogeli from naturally infected European dogs. Vet. Parasitol. 2002, 106, 285–292. [Google Scholar] [CrossRef]
- Kjemtrup, A.M.; Wainwright, K.; Miller, M.; Penzhorn, B.L.; Carreno, R.A. Babesia conradae, sp. nov., a small canine Babesia identified in California. Vet. Parasitol. 2006, 138, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, H.; Yoshizaki, Y.; Shimada, Y.; Shimada, Y.; Sakata, Y.; Okuda, M.; Onishi, T. Epidemiological survey of Babesia species in Japan performed with specimens from ticks collected from dogs and detection of new Babesia DNA closely related to Babesia odocoilei and Babesia divergens DNA. J. Clin. Microbiol. 2003, 41, 3494–3498. [Google Scholar] [CrossRef] [Green Version]
- Lempereur, L.; De Cat, A.; Caron, Y.; Madder, M.; Claerebout, E.; Saegerman, C.; Losson, B. First molecular evidence of potentially zoonotic Babesia microti and Babesia sp. EU1 in Ixodes ricinus in Belgium. Vector Borne Zoonotic Dis. 2011, 11, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, I.N.; Parveen, T.; Abrar, A.; Mehmood, A.K.; Iqubal, F. Molecular detection of Babesia microti in dogs and cat blood samples collected from Punjab (Pakistan). Trop. Biomed. 2019, 36, 304–309. [Google Scholar] [PubMed]
- Zhang, X.-L.; Li, X.-W.; Li, W.-J.; Huang, H.-L.; Huang, S.-J.; Shao, J.-W. Molecular evidence of Babesia in pet cats in mainland China. BMC Vet. Res. 2019, 15, 476. [Google Scholar] [CrossRef]
- Steiner, F.E.; Pinger, R.R.; Vann, C.N.; Grindle, N.; Civetello, D.; Clay, K.; Fuqua, C. Infection and co-infection rates of Anaplasma phagocytophilum variant, Babesia spp., Borrelia burgdorferi, and rickettsial endosymbiont in Ixodes scapularis (Acari: Ixodidae) from sites in Indiana, Maine, Pennsylvania, and Wisconsin. J. Med. Entomol. 2008, 45, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldrup, K.A.; Kocan, A.A.; Qureshi, T.; Baggett, D.; Wagner, G.G. Serological prevalence and isolation of Babesia odocoilei among white-tailed deer (Odocoileus virginianus) in Texas and Oklahoma. J. Wild. Dis. 1989, 25, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.D.; Fernando, K.; Banerjee, S.N.; Durden, L.A.; Byrne, S.K.; Banerjee, M.; Mann, R.B.; Morshed, M.G. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J. Med. Entomol. 2001, 38, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.G.; Scott, J.D.; Fernando, K.; Beati, L.; Mazerolle, D.F.; Geddes, G.; Durden, L.A. Migratory songbirds disperse ticks across Canada, and first isolation of the Lyme disease spirochete, Borrelia burgdorferi, from the avian tick, Ixodes auritulus. J. Parasitol. 2005, 91, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Durden, L.A. First isolation of Lyme disease spirochete, Borrelia burgdorferi, from ticks collected from songbirds in Ontario, Canada. N. Am. Bird Bander 2009, 34, 97–101. [Google Scholar]
- Scott, J.D.; Lee, M.-K.; Fernando, K.; Durden, L.A.; Jorgensen, D.R.; Mak, S.; Morshed, M.G. Detection of Lyme disease spirochete, Borrelia burgdorferi sensu lato, including three novel genotypes in ticks (Acari: Ixodidae) collected from songbirds (Passeriformes) across Canada. J. Vector Ecol. 2010, 35, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Anderson, J.F.; Durden, L.A. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J. Parasitol. 2012, 98, 49–59. [Google Scholar] [CrossRef]
- Scott, J.D.; Durden, L.A. New records of the Lyme disease bacterium in ticks collected from songbirds in central and eastern Canada. Int. J. Acarol. 2015, 41, 241–249. [Google Scholar] [CrossRef]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Bierman, B.C.; Durden, L.A. Far-reaching dispersal of Borrelia burgdorferi sensu lato-infected blacklegged ticks by migratory songbirds in Canada. Healthcare 2018, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Anderson, J.F.; Bierman, B.C.; Durden, L.A. Extensive distribution of the Lyme disease bacterium, Borrelia burgdorferi sensu lato, in multiple tick species parasitizing avian and mammalian hosts across Canada. Healthcare 2018, 6, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.D.; Durden, L.A. Amblyomma dissimile (Acari: Ixodidae) parasitizes bird captured in Canada. Syst. Appl. Acarol. 2015, 20, 854–860. [Google Scholar]
- Anderson, J.F.; Magnarelli, L.A. Avian and mammalian hosts for spirochete-infected ticks and insects in a Lyme disease focus in Connecticut. Yale J. Biol. Med. 1984, 57, 627–641. [Google Scholar]
- Anderson, J.F.; Magnarelli, L.A.; Stafford, K.C., III. Bird-feeding ticks transstadially transmit Borrelia burgdorferi that infect Syrian hamsters. J. Wildl. Dis. 1990, 26, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.D.; Scott, C.M.; Anderson, J.F. The establishment of a blacklegged tick population by migratory songbirds in Ontario. J. Vet. Sci. Med. 2014, 2, 5. [Google Scholar] [CrossRef]
- Scott, J.D.; Pascoe, E.L.; Sajid, M.S.; Foley, J.E. Monitoring of nesting songbirds detects established population of blacklegged ticks and associated Lyme disease endemic area in Canada. Healthcare 2020, 8, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Number of B. odocoilei-Positive Ticks (%) | |||
|---|---|---|---|
| Source | Female(s) | Male(s) | Total Tick(s) |
| General sampling | |||
| Cat | 2/4 (50) | 0 (0) | 2/4 (50) |
| Dog | 13/17 (76.5) | 0 (0) | 13/17 (76.5) |
| Vegetation | 1/19 (5.3) | 3/13 (23.1) | 4/32 (12.5) |
| Cat and dog sampling | |||
| Barrie | 2/2 (100) | 0 (0) | 2/2 (100) |
| East Wasaga | 0/1 (0) | 0 (0) | 0/1 (0) |
| Orillia | 1/2 (50) | 0 (0) | 1/2 (50) |
| Oro Medonte | 3/3 (100) | 0 (0) | 3/3 (100) |
| Penetanguishene | 2/3 (66.7) | 0 (0) | 2/3 (66.7) |
| Severn Township | 1/1 (100) | 0 (0) | 1/1 (100) |
| Warmister | 1/1 (100) | 0 ((0) | 1/1 (100) |
| Wasaga Beach | 5/7 (71.4) | 0 (0) | 5/7 (71.4) |
| Woods Beach | 0/1 (0) | 0 (0) | 0/1 (0) |
| Flagging vegetation | |||
| Dundas | 03 (0) | 1/3 (33.3) | 1/6 (16.7) |
| Port Burwell | 0/6 (0) | 0 (0) | 0/6 (0) |
| Thorold | 0/3 (0) | 0/3 (0) | 0/6 (0) |
| Turkey Point | 0/4 (0) | 1/4 (25.0) | 1/8 (12.5) |
| Wainfleet Bog | 1/3 (33.3) | 1/3 (33.3) | 2/6 (33.3) |
| Date | Sequence | BLAST Results | GenBank | |||||
|---|---|---|---|---|---|---|---|---|
| Tick ID | Location | Source | Collected | Length | % of Type Strain | Score | E-Value | Accession no. |
| CN19-2-2 | Dundas | flagging | 24 Apr | 263 | 100 | 521 | 7e-144 | MW182495 |
| CN19-5-2 | Wainfleet Bog | flagging | 24 Apr | 100 | 100 | 198 | 4e-47 | MW182496 |
| CN19-6-1 | Wainfleet Bog | flagging | 24 Apr | 163 | 99.37 | 307 | 1e-79 | MW182497 |
| CN19-8-2 | Turkey Point | flagging | 25 Apr | 152 | 100 | 301 | 7e-78 | MW182498 |
| CN19-79 | Penetanguishene | cat | 02 May | 265 | 100 | 525 | 5e-145 | MW182499 |
| CN19-80 | Penetanguishene | dog | 14 May | 212 | 100 | 416 | 2e-112 | MW182500 |
| CN19-86 | Wasaga Beach | dog | 30 Apr | 357 | 100 | 706 | 0 | MW182501 |
| CN19-87 | Wasaga Beach | dog | 03 May | 361 | 99.72 | 708 | 0 | MW182502 |
| CN19-88 | Wasaga Beach | dog | 09 May | 358 | 99.72 | 200 | 0 | MW182503 |
| CN19-89 | Wasaga Beach | dog | 10 May | 440 | 100 | 872 | 0 | MW182504 |
| CN19-90 | Wasaga Beach | dog | 10 May | 434 | 100 | 860 | 0 | MW182505 |
| CN19-91 | Severn Township | dog | 07 May | 440 | 100 | 872 | 0 | MW182506 |
| CN19-92 | Oro Medonte | dog | 14 May | 387 | 100 | 767 | 0 | MW182507 |
| CN19-93 | Barrie | dog | 15 May | 429 | 100 | 848 | 0 | MW182508 |
| CN19-94 | Orillia | dog | 16 May | 429 | 100 | 850 | 0 | MW182509 |
| CN19-95 | Warmister | dog | 17 May | 429 | 100 | 850 | 0 | MW182510 |
| CN19-96 | Oro Medonte | dog | 19 May | 429 | 100 | 850 | 0 | MW182511 |
| CN19-98 | Oro Medonte | dog | 23 May | 429 | 100 | 850 | 0 | MW182512 |
| CN19-99 | Barrie | cat | 24 May | 429 | 100 | 850 | 0 | MW182513 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, J.D.; Pascoe, E.L.; Sajid, M.S.; Foley, J.E. Detection of Babesia odocoilei in Ixodes scapularis Ticks Collected in Southern Ontario, Canada. Pathogens 2021, 10, 327. https://doi.org/10.3390/pathogens10030327
Scott JD, Pascoe EL, Sajid MS, Foley JE. Detection of Babesia odocoilei in Ixodes scapularis Ticks Collected in Southern Ontario, Canada. Pathogens. 2021; 10(3):327. https://doi.org/10.3390/pathogens10030327
Chicago/Turabian StyleScott, John D., Emily L. Pascoe, Muhammad S. Sajid, and Janet E. Foley. 2021. "Detection of Babesia odocoilei in Ixodes scapularis Ticks Collected in Southern Ontario, Canada" Pathogens 10, no. 3: 327. https://doi.org/10.3390/pathogens10030327
APA StyleScott, J. D., Pascoe, E. L., Sajid, M. S., & Foley, J. E. (2021). Detection of Babesia odocoilei in Ixodes scapularis Ticks Collected in Southern Ontario, Canada. Pathogens, 10(3), 327. https://doi.org/10.3390/pathogens10030327





