Genotype Diversity before and after the Introduction of a Rotavirus Vaccine into the National Immunisation Program in Fiji
Abstract
1. Introduction
2. Results
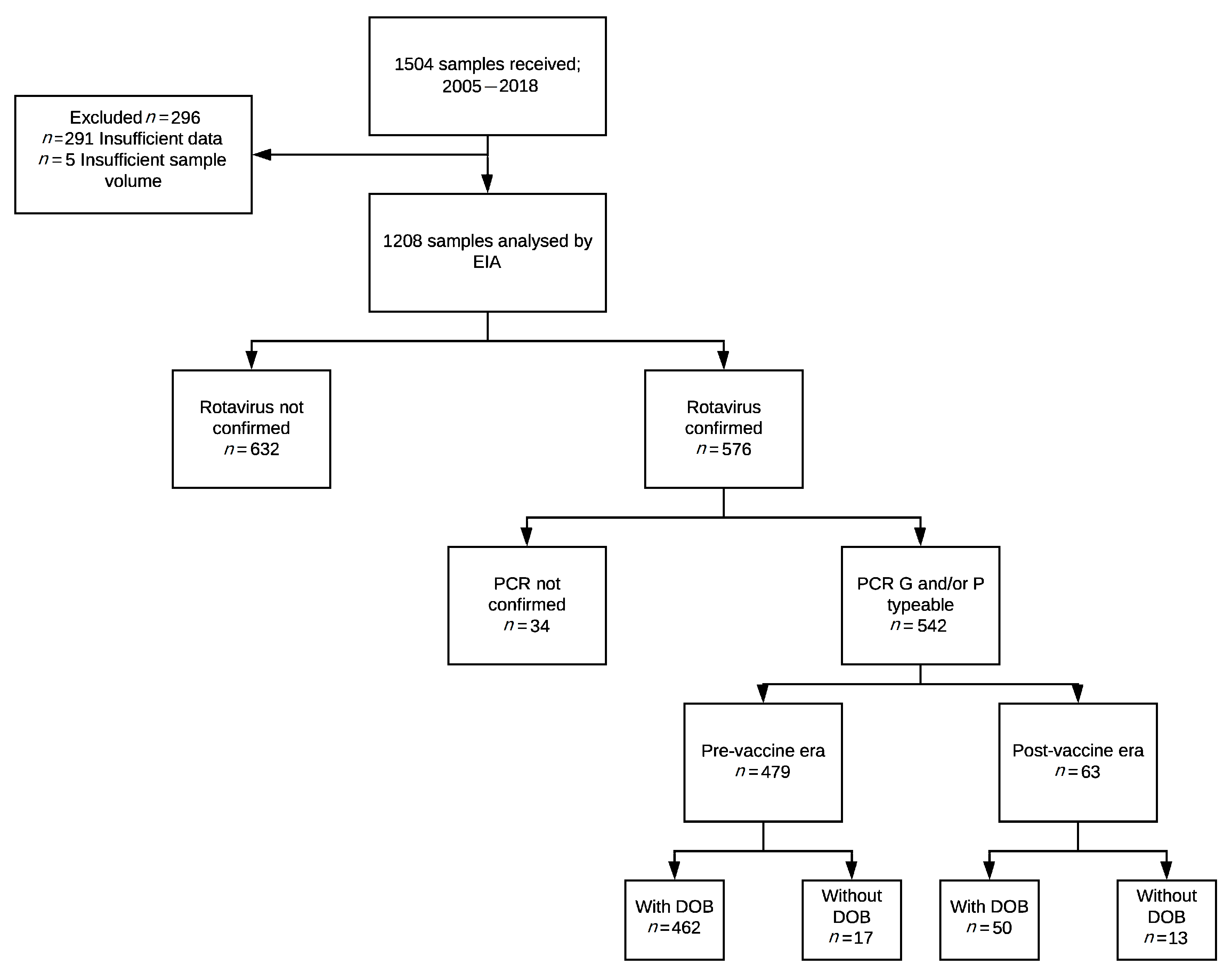
2.1. Study Samples
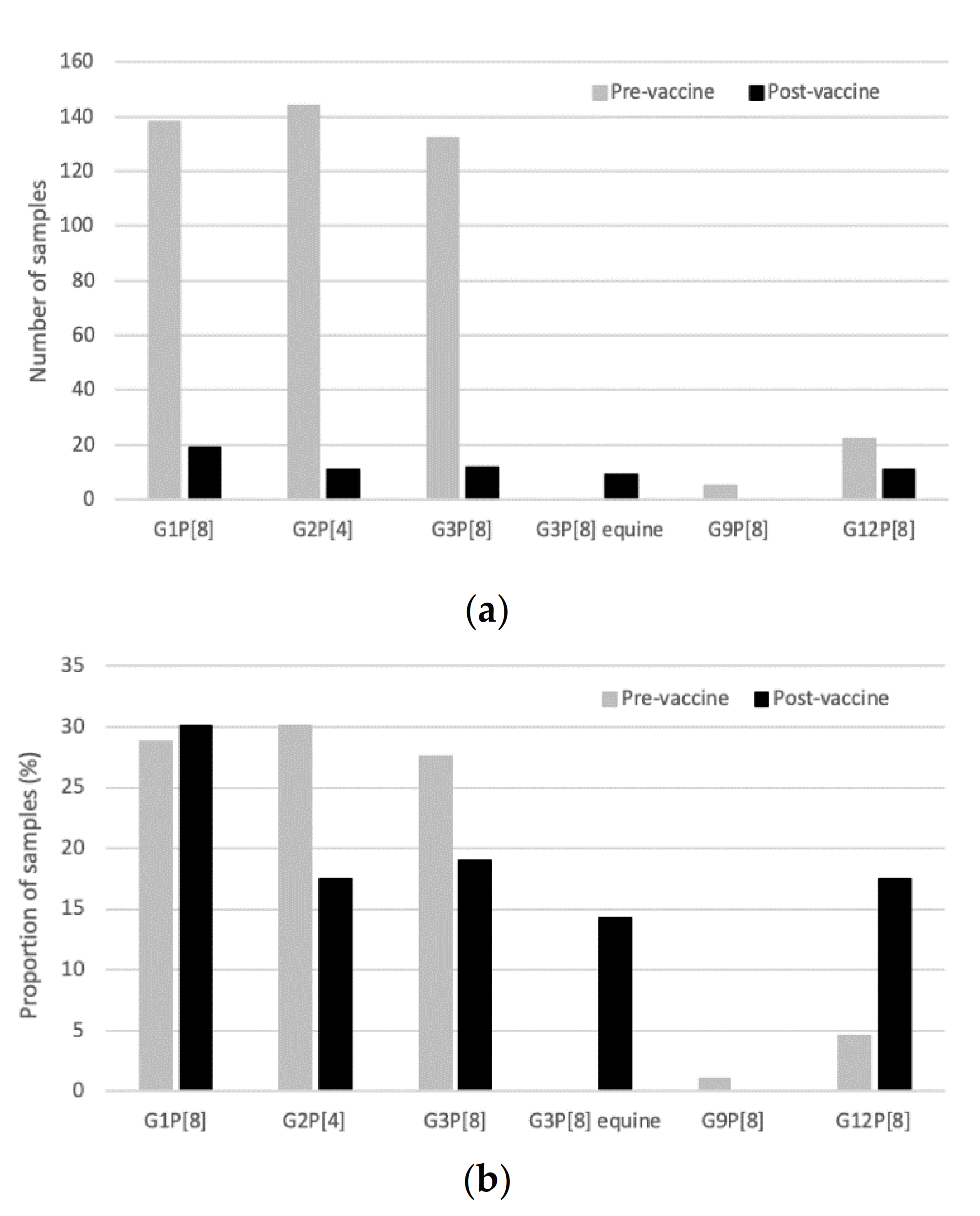
2.2. Genotype Distribution and the Impact of Vaccine Introduction
3. Discussion
4. Materials and Methods
4.1. Population and Study Sites
4.2. Participants
4.3. Genotyping
4.4. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Desselberger, U. Rotaviruses. Virus Res. 2014, 190, 75–96. [Google Scholar] [CrossRef]
- Rotavirus Classification Working Group. List of Accepted Genotypes. Available online: https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/rcwg (accessed on 1 October 2020).
- Clarke, E.; Desselberger, U. Correlates of protection against human rotavirus disease and the factors influencing protection in low-income settings. Mucosal Immunol. 2015, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Roczo-Farkas, S.; Kirkwood, C.D.; Cowley, D.; Barnes, G.L.; Bishop, R.F.; Bogdanovic-Sakran, N.; Boniface, K.; Donato, C.M.; Bines, J.E. The impact of rotavirus vaccines on genotype diversity: A comprehensive analysis of two decades of Australian surveillance data. J. Infect. Dis. 2018, 218, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, N.; Antoni, S.; Mwenda, J.M.; Weldegebriel, G.; Biey, J.N.M.; Cheikh, D.; Fahmy, K.; Teleb, N.; Ashmony, H.A.; Ahmed, H.; et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–2016: Findings from the Global Rotavirus Surveillance Network. Lancet Glob. Health 2019, 7, e893–e903. [Google Scholar] [CrossRef]
- United Nations Pacific. Socio-Economic Impact Assessment of COVID-19 in Fiji July 2020. Available online: https://www.pacific.undp.org/content/pacific/en/home/library/socio-economic-impact-assessment-of-covid-19-in-fiji.html (accessed on 8 December 2020).
- World Health Organization. Fiji Key Indicators. Available online: https://apps.who.int/gho/data/node.cco.ki-FJI?lang=en (accessed on 15 October 2020).
- Jenney, A.; Tikoduadua, L.; Buadromo, E.; Barnes, G.; Kirkwood, C.D.; Boniface, K.; Bines, J.; Mulholland, K.; Russell, F. The burden of hospitalised rotavirus infections in Fiji. Vaccine 2009, 27 (Suppl. 5), F108–F111. [Google Scholar] [CrossRef]
- World Health Organization. WHO and UNICEF Estimates of National Immunization Coverage. 2020. Available online: https://www.who.int/immunization/monitoring_surveillance/data/fji.pdf (accessed on 14 January 2020).
- Jenney, A.W.; Reyburn, R.; Ratu, F.T.; Tuivaga, E.; Nguyen, C.; Covea, S.; Thomas, S.; Rafai, E.; Devi, R.; Bright, K.; et al. The impact of the rotavirus vaccine on diarrhoea five years following national introduction in Fiji. Lancet Reg. Health-West. Pac. 2020. [Google Scholar] [CrossRef]
- Dóró, R.; Marton, S.; Bartókné, A.H.; Lengyel, G.; Agócs, Z.; Jakab, F.; Bányai, K. Equine-like G3 rotavirus in Hungary, 2015—Is it a novel intergenogroup reassortant pandemic strain? Acta Microbiol. Immunol. Hung. 2016, 63, 243–255. [Google Scholar] [CrossRef]
- Malasao, R.; Saito, M.; Suzuki, A.; Imagawa, T.; Nukiwa-Soma, N.; Tohma, K.; Liu, X.; Okamoto, M.; Chaimongkol, N.; Dapat, C.; et al. Human G3P[4] rotavirus obtained in Japan, 2013, possibly emerged through a human-equine rotavirus reassortment event. Virus Genes 2015, 50, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Luchs, A.; Da Costa, A.C.; Cilli, A.; Komninakis, S.C.V.; Carmona, R.D.C.C.; Boen, L.; Morillo, S.G.; Sabino, E.C.; Timenetsky, M.D.C.S.T. Spread of the emerging equine-like G3P[8] DS-1-like genetic backbone rotavirus strain in Brazil and identification of potential genetic variants. J. Gen. Virol. 2019, 100, 7–25. [Google Scholar] [CrossRef]
- Jain, S.; Vashistt, J.; Changotra, H. Rotaviruses: Is their surveillance needed? Vaccine 2014, 32, 3367–3378. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Bilcke, J.; Ciarlet, M.; Martella, V.; Bányai, K.; Rahman, M.; Zeller, M.; Beutels, P.; Van Damme, P.; Van Ranst, M. Rotavirus disease and vaccination: Impact on genotype diversity. Future Microbiol. 2009, 4, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Perkins, C.; Mijatovic-Rustempasic, S.; Ward, M.L.; Cortese, M.M.; Bowen, M.D. Genomic Characterization of the First Equine-Like G3P[8] Rotavirus Strain Detected in the United States. Genome Announc. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Markkula, J.; Hemming-Harlo, M.; Salminen, M.T.; Savolainen-Kopra, C.; Pirhonen, J.; Al-Hello, H.; Vesikari, T. Rotavirus epidemiology 5–6 years after universal rotavirus vaccination: Persistent rotavirus activity in older children and elderly. Infect. Dis. 2017, 49, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Luchs, A.; Cilli, A.; Morillo, S.G.; Gregório, D.D.S.; De Souza, K.A.F.; Vieira, H.R.; Fernandes, A.D.M.; Carmona, R.D.C.C.; Timenetsky, M.D.C.S.T. Detection of the emerging rotavirus G12P[8] genotype at high frequency in brazil in 2014: Successive replacement of predominant strains after vaccine introduction. Acta Trop. 2016, 156, 87–94. [Google Scholar] [CrossRef]
- Parry, C.M.; Crump, J.A.; Rosa, V.; Jenney, A.; Naidu, R.; Mulholland, K.; Strugnell, R.A. A retrospective study of patients with blood culture-confirmed typhoid fever in Fiji during 2014–2015: Epidemiology, clinical features, treatment and outcome. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 764–770. [Google Scholar]
- Kama, M.; Aubry, M.; Al, M.K.E.; Vanhomwegen, J.; Mariteragi-Helle, T.; Teissier, A.; Paoaafaite, T.; Hué, S.; Hibberd, M.L.; Manuguerra, J.-C.; et al. Sustained Low-Level Transmission of Zika and Chikungunya Viruses after Emergence in the Fiji Islands. Emerg. Infect. Dis. 2019, 25, 1535–1538. [Google Scholar] [CrossRef]
- Aubry, M.; Kama, M.; Henderson, A.D.; Teissier, A.; Vanhomwegen, J.; Mariteragi-Helle, T.; Paoaafaite, T.; Manuguerra, J.-C.; Christi, K.; Watson, C.H.; et al. Low chikungunya virus seroprevalence two years after emergence in Fiji. Int. J. Infect. Dis. 2020, 90, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Coulson, B.S.; Unicomb, L.E.; Pitson, G.A.; Bishop, R.F. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J. Clin. Microbiol. 1987, 25, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Gomara, M.I.; Cubitt, D.; Desselberger, U.; Gray, J. Amino acid substitution within the VP7 protein of G2 rotavirus strains associated with failure to serotype. J. Clin. Microbiol. 2001, 39, 3796–3798. [Google Scholar] [CrossRef]
- Simmonds, M.K.; Armah, G.; Asmah, R.; Banerjee, I.; Damanka, S.; Esona, M.; Gentsch, J.R.; Gray, J.J.; Kirkwood, C.; Page, N.; et al. New oligonucleotide primers for P-typing of rotavirus strains: Strategies for typing previously untypeable strains. J. Clin. Virol. 2008, 42, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, C.D.; Roczo-Farkas, S.; Australian Rotavirus Surveillance Group. Australian Rotavirus Surveillance Program annual report, 2013. Commun. Dis. Intell. Q. Rep. 2014, 38, E334–E342. [Google Scholar] [PubMed]
- Maes, P.; Matthijnssens, J.; Rahman, M.; Van Ranst, M. RotaC: A web-based tool for the complete genome classification of group a rotaviruses. BMC Microbiol. 2009, 9, 238. [Google Scholar] [CrossRef] [PubMed]
| G1P[8] | G2P[4] | G2P[8] | G3P[6] | G3P[8] | G3P[8] EQUINE | G8P[8] | G9P[8] | G12P[4] | G12P[8] | Mixed | Partially Typed | Total Genotyped | Negative | Total Samples | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n |
| 2005 | 1 | 20 | 1 | 20 | 1 | 20 | 2 | 40 | 5 | 71 | 2 | 29 | 7 | ||||||||||||||||
| 2006 | 1 | 1 | 74 | 94 | 4 | 5 | 79 | 99 | 1 | 1 | 80 | ||||||||||||||||||
| 2007 | 1 | 50 | 1 | 50 | 2 | 100 | 0 | 0.0 | 2 | ||||||||||||||||||||
| 2008 | 5 | 7 | 30 | 40 | 4 | 5 | 1 | 1 | 3 | 4 | 2 | 3 | 22 | 29 | 8 | 11 | 75 | 100 | 0 | 0.0 | 75 | ||||||||
| 2009 | 9 | 21 | 26 | 59 | 1 | 2 | 1 | 2 | 2 | 5 | 5 | 11 | 44 | 96 | 2 | 4 | 46 | ||||||||||||
| 2010 | 1 | 1 | 88 | 74 | 23 | 19 | 3 | 3 | 4 | 3 | 119 | 62 | 74 | 38 | 193 | ||||||||||||||
| 2011 | 127 | 85 | 15 | 10 | 6 | 4 | 1 | 1 | 149 | 37 | 255 | 63 | 404 | ||||||||||||||||
| 2012 | 4 | 67 | 2 | 33 | 6 | 30 | 14 | 70 | 20 | ||||||||||||||||||||
| Subtotal | 138 | 144 | 4 | 1 | 132 | 0 | 1 | 5 | 2 | 22 | 13 | 17 | 479 | 348 | 827 | ||||||||||||||
| Rotarix Vaccine Introduced | |||||||||||||||||||||||||||||
| 2013 | 1 | 8 | 5 | 42 | 6 | 50 | 12 | 67 | 6 | 33 | 18 | ||||||||||||||||||
| 2014 | 17 | 71 | 6 | 25 | 1 | 4 | 24 | 51 | 23 | 49 | 47 | ||||||||||||||||||
| 2015 | 1 | 17 | 5 | 83 | 6 | 4 | 158 | 96 | 164 | ||||||||||||||||||||
| 2016 | 4 | 100 | 4 | 15 | 22 | 85 | 26 | ||||||||||||||||||||||
| 2017 | 11 | 100 | 11 | 20 | 43 | 80 | 54 | ||||||||||||||||||||||
| 2018 | 6 | 100 | 6 | 16 | 32 | 84 | 38 | ||||||||||||||||||||||
| Subtotal | 19 | 11 | 0 | 0 | 12 | 9 | 0 | 0 | 0 | 11 | 1 | 0 | 63 | 284 | 347 | ||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.; Donato, C.M.; Covea, S.; Ratu, F.T.; Jenney, A.W.J.; Reyburn, R.; Sahu Khan, A.; Rafai, E.; Grabovac, V.; Serhan, F.; et al. Genotype Diversity before and after the Introduction of a Rotavirus Vaccine into the National Immunisation Program in Fiji. Pathogens 2021, 10, 358. https://doi.org/10.3390/pathogens10030358
Thomas S, Donato CM, Covea S, Ratu FT, Jenney AWJ, Reyburn R, Sahu Khan A, Rafai E, Grabovac V, Serhan F, et al. Genotype Diversity before and after the Introduction of a Rotavirus Vaccine into the National Immunisation Program in Fiji. Pathogens. 2021; 10(3):358. https://doi.org/10.3390/pathogens10030358
Chicago/Turabian StyleThomas, Sarah, Celeste M. Donato, Sokoveti Covea, Felisita T. Ratu, Adam W. J. Jenney, Rita Reyburn, Aalisha Sahu Khan, Eric Rafai, Varja Grabovac, Fatima Serhan, and et al. 2021. "Genotype Diversity before and after the Introduction of a Rotavirus Vaccine into the National Immunisation Program in Fiji" Pathogens 10, no. 3: 358. https://doi.org/10.3390/pathogens10030358
APA StyleThomas, S., Donato, C. M., Covea, S., Ratu, F. T., Jenney, A. W. J., Reyburn, R., Sahu Khan, A., Rafai, E., Grabovac, V., Serhan, F., Bines, J. E., & Russell, F. M. (2021). Genotype Diversity before and after the Introduction of a Rotavirus Vaccine into the National Immunisation Program in Fiji. Pathogens, 10(3), 358. https://doi.org/10.3390/pathogens10030358








