New Cladosporium Species from Normal and Galled Flowers of Lamiaceae
Abstract
1. Introduction
2. Results
2.1. Cladosporium Isolates
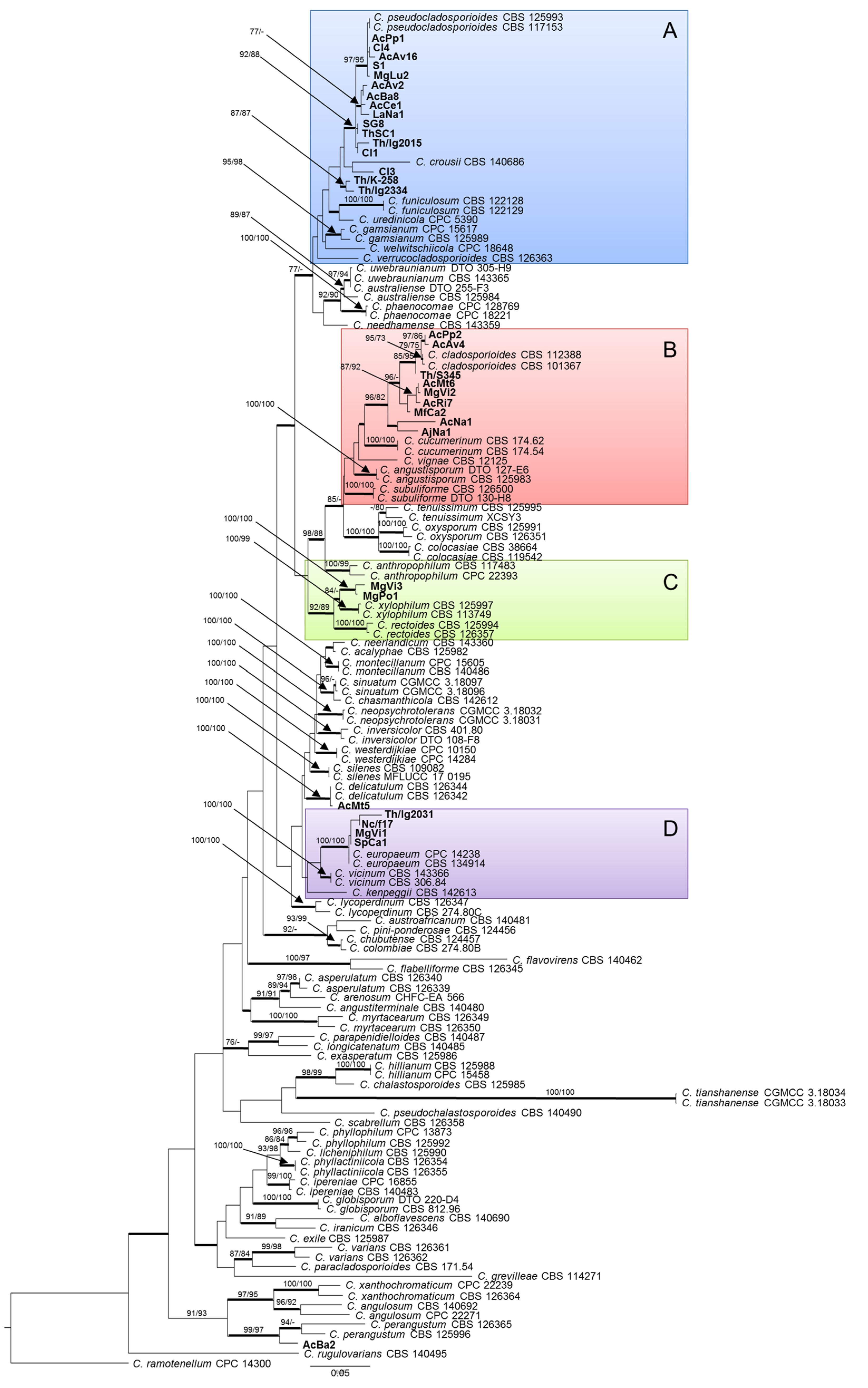
2.2. Phylogenetic Analysis
2.3. Species Delimitation Assay
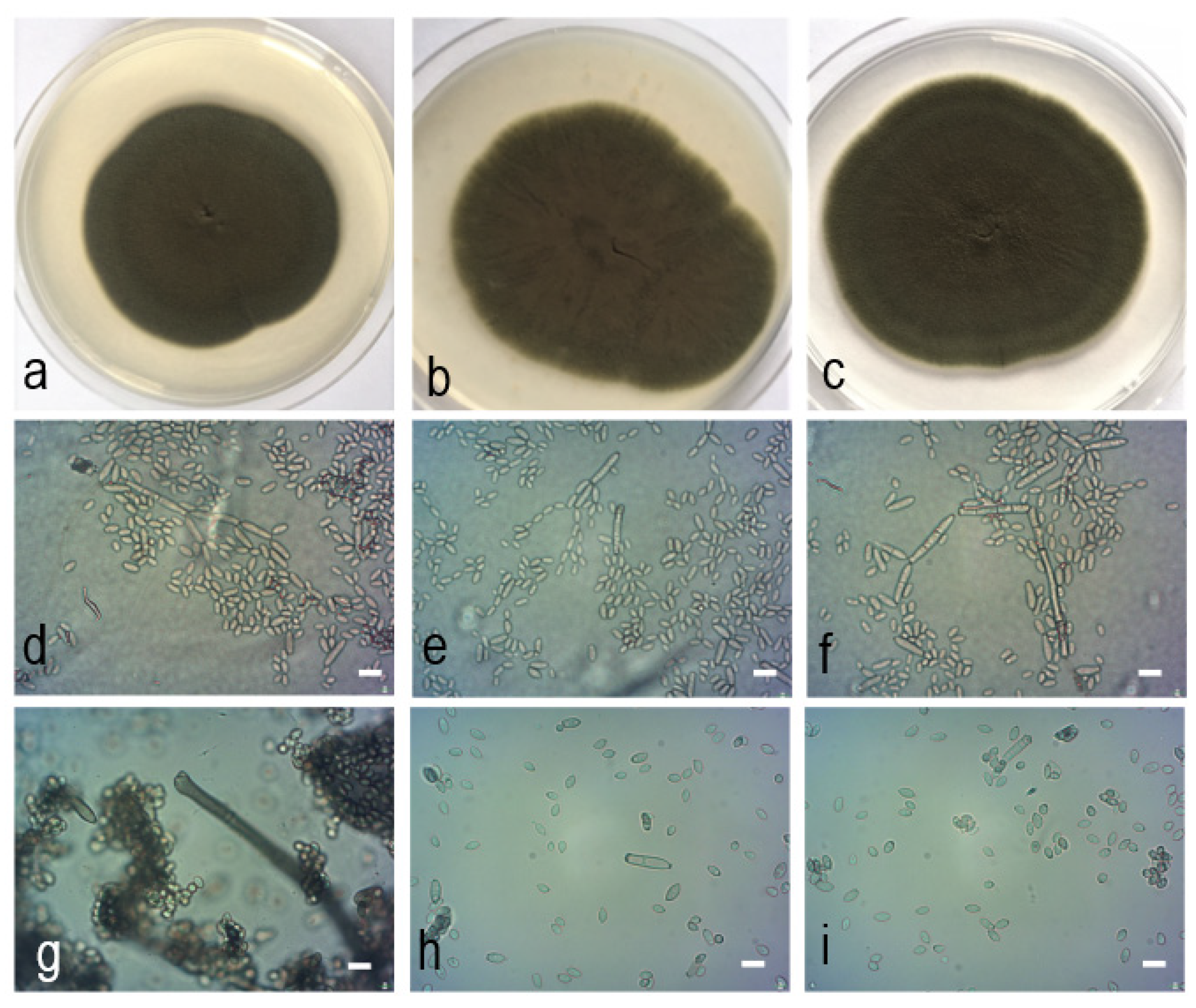
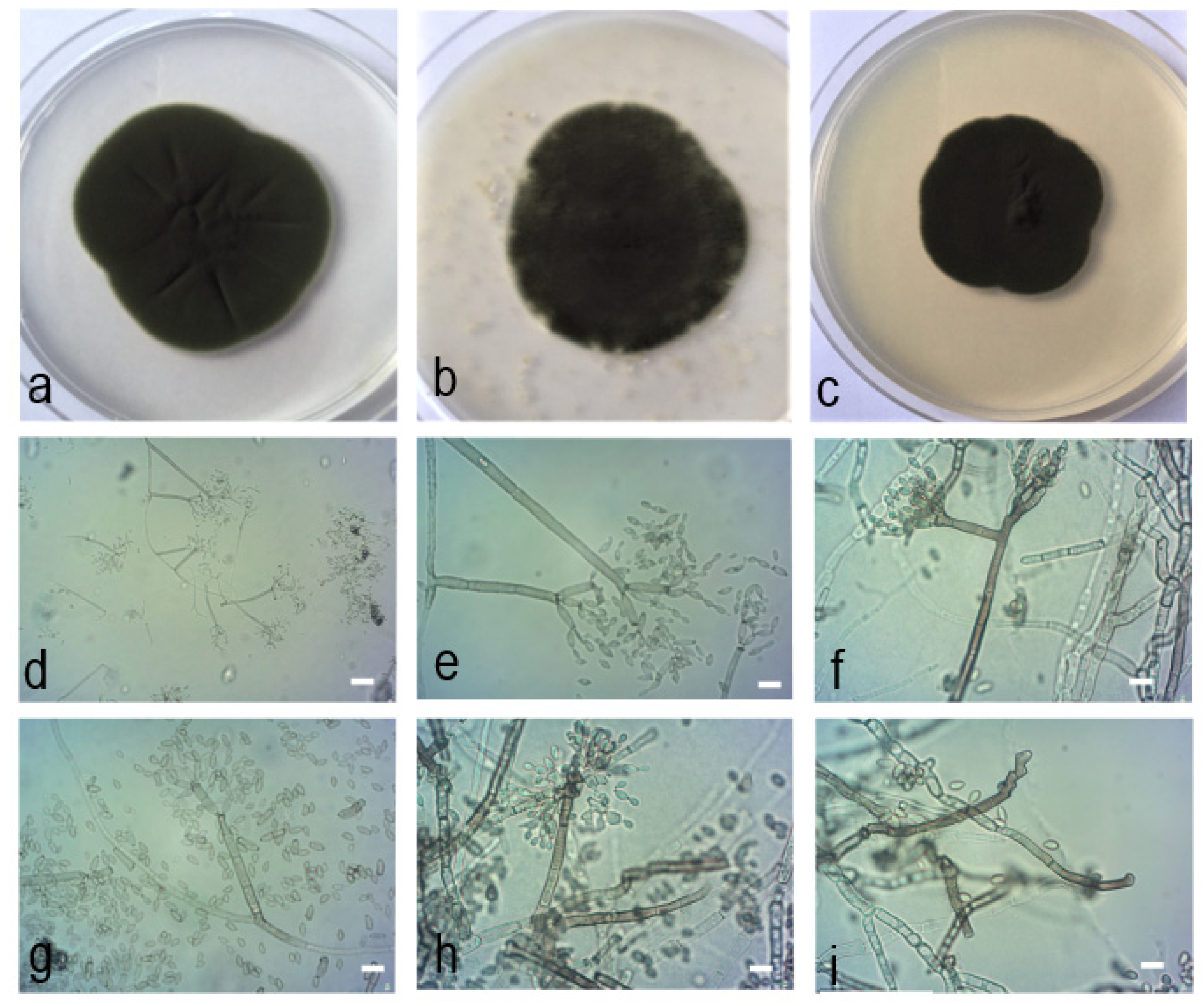
2.4. Morphological Characteristics
2.5. Taxonomy
3. Discussion
4. Materials and Methods
4.1. Isolates Collection
4.2. DNA Isolation, Amplification and Sequencing
4.3. Phylogenetic Analyses
4.4. DNA-Based Species Delimitation
4.5. Morphological Observations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bensch, K.; Groenewald, J.Z.; Braun, U.; Dijksterhuis, J.; de Jesús Yáñez-Morales, M.; Crous, P.W. Common but different: The expanding realm of Cladosporium. Stud. Mycol. 2015, 82, 23–74. [Google Scholar] [CrossRef]
- Schubert, K. Morphotaxonomic Revision of Foliicolous Cladosporium Species (Hyphomycetes). Ph.D. Thesis, Mathematisch-Naturwissenschaftlich-Technischen Fakultät, Martin-Luther-University Halle, Wittenberg, Germany, 2005. [Google Scholar]
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The genus Cladosporium. Stud. Mycol. 2012, 72, 1–401. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Barnes, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef] [PubMed]
- Rosado, A.W.C.; Custódio, F.A.; Pinho, D.B.; Ferreira, A.P.S.; Pereira, O.L. Cladosporium species associated with disease symptoms on Passiflora edulis and other crops in Brazil, with descriptions of two new species. Phytotaxa 2019, 409, 239–260. [Google Scholar] [CrossRef]
- Velázquez-Jiménez, Y.; Hernández-Castro, R.; Romero-Romero, L.; Salas-Garrido, C.G.; Martínez-Chavarría, L.C. Feline phaeohyphomycotic cerebellitis caused by Cladosporium cladosporioides-complex: Case report and review of literature. J. Comp. Pathol. 2019, 170, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Denis, M.; Sutton, D.A.; Martin-Vicente, A.; Cano-Lira, J.F.; Wiederhold, N.; Guarro, J.; Gené, J. Cladosporium species recovered from clinical samples in the United States. J. Clin. Microbiol. 2015, 53, 2990–3000. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Denis, M.; Gené, J.; Sutton, D.A.; Wiederhold, N.; Cano-Lira, J.F.; Guarro, J. New species of Cladosporium associated with human and animal infections. Persoonia 2016, 36, 281. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Chen, P.; Sun, T.T.; Wang, X.J.; Li, D.M. Acne-like subcutaneous phaeohyphomycosis caused by Cladosporium cladosporioides: A rare case report and review of published literatures. Mycopathologia 2016, 181, 567–573. [Google Scholar] [CrossRef]
- Bensch, K.; Groenewald, J.Z.; Meijer, M.; Dijksterhuis, J.; Jurjević, Ž.; Andersen, B.; Houbraken, J.; Crous, P.W.; Samson, R.A. Cladosporium species in indoor environments. Stud. Mycol. 2018, 89, 177–301. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Chen, Q.; Fan, Y.L.; Wang, Q.; Chen, S.F.; Liu, X.Z.; Cai, L.; Yao, B. Six new soil-inhabiting Cladosporium species from plateaus in China. Mycologia 2017, 109, 244–260. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.E.St.J.; Gené, J.; Guarro, J.; Baseia, I.G.; García, D.; Gusmão, L.F.P.; Souza-Motta, C.M.; et al. Fungal planet description sheets: 716–784. Persoonia 2018, 40, 240–393. [Google Scholar] [CrossRef] [PubMed]
- Tibpromma, S.; Hyde, K.D.; Bhat, J.D.; Mortimer, P.E.; Xu, J.; Promputtha, I.; Doilom, M.; Yang, J.-B.; Tang, A.M.C.; Karunarathna, S.C. Identification of endophytic fungi from leaves of Pandanaceae based on their morphotypes and DNA sequence data from southern Thailand. MycoKeys 2018, 33, 25–67. [Google Scholar] [CrossRef] [PubMed]
- Iturrieta-González, I.; García, D.; Gené, J. Novel species of Cladosporium from environmental sources in Spain. MycoKeys 2021, 77, 1–25. [Google Scholar] [CrossRef]
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzón, A.; Ownley, B.H.; et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Baccarini, P. Sopra un curioso cecidio della Capparis spinosa L. Malpighia 1893, 7, 405–414. [Google Scholar]
- Docters van Leeuwen, W.M. Ueber eine galle auf Symploccus fasiculata Zoll., verursacht durch eine gallmucke: Asphondylia bursaria Felt, die mit einem fungus zusammen lebt. Marcellia 1929, 25, 61–66. [Google Scholar]
- Adair, R.J.; Burgess, T.; Serdani, M.; Barber, P. Fungal associations in Asphondylia (Diptera: Cecidomyiidae) galls from Australia and South Africa: Implications for biological control of invasive acacias. Fungal Ecol. 2009, 2, 121–134. [Google Scholar] [CrossRef]
- Lebel, T.; Peele, C.; Veenstra, A. Fungi associated with Asphondylia (Diptera: Cecidomyiidae) galls on Sarcocornia quinqueflora and Tecticornia arbuscula (Chenopodiaceae). Fungal Divers. 2012, 55, 143–154. [Google Scholar] [CrossRef]
- Kobune, S.; Kajimura, H.; Masuya, H.; Kubono, T. Symbiotic fungal flora in leaf galls induced by Illiciomyia yukawai (Diptera: Cecidomyiidae) and in its mycangia. Microbial Ecol. 2012, 63, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Borkent, A.; Bissett, J. Gall midges (Diptera: Cecidomyiidae) are vectors for their fungal symbionts. Symbiosis 1985, 1, 185–194. [Google Scholar]
- Zimowska, B.; Viggiani, G.; Nicoletti, R.; Furmanczyk, A.; Becchimanzi, A.; Kot, I. First report of the gall midge Asphondylia serpylli on thyme (Thymus vulgaris), and identification of the associated fungal symbiont. Ann. Appl. Biol. 2017, 171, 89–94. [Google Scholar] [CrossRef]
- Bernardo, U.; Nugnes, F.; Gualtieri, L.; Nicoletti, R.; Varricchio, P.; Sasso, R.; Viggiani, G. A new gall midge species of Asphondylia (Diptera: Cecidomyiidae) inducing flower galls on Clinopodium nepeta (Lamiaceae) from Europe, its phenology, and associated fungi. Environ. Entomol. 2018, 47, 609–622. [Google Scholar] [CrossRef]
- Zimowska, B.; Okoń, S.; Becchimanzi, A.; Krol, E.D.; Nicoletti, R. Phylogenetic characterization of Botryosphaeria strains associated with Asphondylia galls on species of Lamiaceae. Diversity 2020, 12, 41. [Google Scholar] [CrossRef]
- Park, I.; Sanogo, S.; Hanson, S.F.; Thompson, D.C. Molecular identification of Botryosphaeria dothidea as a fungal associate of the gall midge Asphondylia prosopidis on mesquite in the United States. BioControl 2019, 64, 209–219. [Google Scholar] [CrossRef]
- Bensch, K.; Groenewald, J.Z.; Dijksterhuis, J.; Starink-Willemse, M.; Andersen, B.; Summerell, B.A. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Stud. Mycol. 2010, 67, 1–94. [Google Scholar] [CrossRef] [PubMed]
- Patyshakuliyeva, A.; Falkoski, D.L.; Wiebenga, A.; Timmermans, K.; de Vries, R.P. Macroalgae derived fungi have high abilities to degrade algal polymers. Microorganisms 2020, 8, 52. [Google Scholar] [CrossRef]
- Wang, X.; Radwan, M.M.; Taráwneh, A.H.; Gao, J.; Wedge, D.E.; Rosa, L.H.; Cutler, H.G.; Cutler, S.J. Antifungal activity against plant pathogens of metabolites from the endophytic fungus Cladosporium cladosporioides. J. Agric. Food Chem. 2013, 61, 4551–4555. [Google Scholar] [CrossRef]
- Nicoletti, R.; Fiorentino, A. Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta. Agriculture 2015, 5, 918–970. [Google Scholar] [CrossRef]
- Katoch, M.; Pull, S. Endophytic fungi associated with Monarda citriodora, an aromatic and medicinal plant and their biocontrol potential. Pharm. Biol. 2017, 55, 1528–1535. [Google Scholar] [CrossRef]
- Karpiński, T.M. Essential oils of Lamiaceae family plants as antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Wilgenbusch, J.C.; Swofford, D. Inferring evolutionary trees with PAUP*. Curr. Prot. Bioinform. 2003, 00, 6.4.1–6.4.28. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, A.J.; Bhunjun, C.S.; Maharachchikumbura, S.S.N.; Liu, J.K. Applied aspects of methods to infer phylogenetic relationships amongst fungi. Mycosphere 2020, 11, 2652–2676. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D.J. jModelTest 2: More models, new heuristics and high-performance computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Pons, J.; Barraclough, T.G.; Gomez-Zurita, J.; Cardoso, A.; Duran, D.P.; Hazell, S.; Kamoun, S.; Sumlin, W.D.; Vogler, A.P. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst. Biol. 2006, 55, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, D.E.; Oliva, M.; Leiva, S.; Mendoza, J.E.; Bobadilla, L.; Angulo, G.; Calderon, M.S. Phylogeny and species delimitations in the entomopathogenic genus Beauveria (Hypocreales, Ascomycota), including the description of B. peruviensis sp. nov. MycoKeys 2019, 58, 47. [Google Scholar] [CrossRef] [PubMed]
- Haelewaters, D.; De Kesel, A.; Pfister, D.H. Integrative taxonomy reveals hidden species within a common fungal parasite of ladybirds. Sci. Rep. 2018, 8, 15966. [Google Scholar] [CrossRef] [PubMed]
- Parnmen, S.; Rangsiruji, A.; Mongkolsuk, P.; Boonpragob, K.; Nutakki, A.; Lumbsch, H.T. Using phylogenetic and coalescent methods to understand the species diversity in the Cladia aggregata complex (Ascomycota, Lecanorales). PLoS ONE 2012, 7, e52245. [Google Scholar] [CrossRef]
- Rayner, R.W. A Mycological Colour Chart; Commonwealth Mycological Institute; British Mycological Society: Kew, UK, 1970; 34p. [Google Scholar]




| Species | Code | Host | Country | ITS | TEF1 | ACT |
|---|---|---|---|---|---|---|
| C. acalyphae | CBS 125982 | Acalypha australis | South Korea | HM147994 | HM148235 | HM148481 |
| C. alboflavescens | CBS 140690 | bronchoalveolar lavage fluid | United States | LN834420 | LN834516 | LN834604 |
| C. angulosum | CPC 22271 | indoor air | United States | MF472918 | MF473345 | MF473768 |
| C. angulosum | CBS 140692 | bronchoalveolar lavage fluid | United States | LN834425 | LN834521 | LN834609 |
| C. angustisporum | CBS 125983 | Alloxylon wickhamii | Australia | HM147995 | HM148236 | HM148482 |
| C. angustisporum | DTO-127-E6 | air in bakery | United States | KP701935 | KP701812 | KP702057 |
| C. angustiterminale | CBS 140480 | Banksia grandis | Australia | KT600379 | KT600476 | KT600575 |
| C. anthropophilum | CBS 117483 | - | United States | HM148007 | HM148248 | HM148494 |
| C. anthropophilum | CPC 22393 | indoor air | United States | MF472922 | MF473349 | MF473772 |
| C. arenosum | CHFC-EA 566 | marine sediment | Antarctica | MN879328 | MN890011 | MN890008 |
| C. asperulatum | CBS 126340 | Protea susannae | Portugal | HM147998 | HM148239 | HM148485 |
| C. asperulatum | CBS 126339 | leaf litter | India | HM147997 | HM148238 | HM148484 |
| C. australiense | DTO-255-F3 | bathroom | Netherlands | KP701978 | KP701855 | KP702100 |
| C. australiense | CBS 125984 | Eucalyptus moluccana | Australia | HM147999 | HM148240 | HM148486 |
| C. austroafricanum | CBS 140481 | leaf litter | South Africa | KT600381 | KT600478 | KT600577 |
| C. chalastosporoides | CBS 125985 | Teratosphaeria proteae-arboreae on Protea arborea | South Africa | HM148001 | HM148242 | HM148488 |
| C. chasmanthicola | CBS 142612 | Chasmanthe aethiopica | South Africa | KY646221 | KY646227 | KY646224 |
| C. chubutense | CBS 124457 | Pinus ponderosa | Argentina | FJ936158 | FJ936161 | FJ936165 |
| C. cladosporioides | CBS 113739 | crested wheat grass | United States | HM148005 | HM148246 | HM148492 |
| C. cladosporioides | CBS 145.35 | Pisum sativum | Germany | HM148013 | HM148254 | HM148500 |
| C. cladosporioides | CBS 101367 | soil | Brazil | HM148002 | HM148243 | HM148489 |
| C. cladosporioides | CBS 112388 | indoor air | Germany | HM148003 | HM148244 | HM148490 |
| C. cladosporioides | CPC 15615 | wild tree | Mexico | KT600386 | KT600483 | KT600581 |
| C. cladosporioides | CPC 22367 | indoor air | United States | MF472941 | MF473368 | MF473791 |
| C. cladosporioides | CPC 14271 | unidentified tree | France | HM148045 | HM148286 | HM148532 |
| C. cladosporioides | CPC 15626 | wild plant | Mexico | KT600387 | KT600484 | KT600582 |
| C. colocasiae | CBS 386.64 | Colocasia esculenta | Taiwan | HM148067 | HM148310 | HM148555 |
| C. colocasiae | CBS 119542 | Colocasia esculenta | Japan | HM148066 | HM148309 | HM148554 |
| C. colombiae | CBS 274.80B | Cortaderia sp. | Colombia | FJ936159 | FJ936163 | FJ936166 |
| C. crousii | CBS 140686 | bronchoalveolar lavage fluid | United States | LN834431 | LN834527 | LN834615 |
| C. cucumerinum | CBS 174.62 | painted floor | United States | HM148076 | HM148320 | HM148565 |
| C. cucumerinum | CBS 174.54 | Cucumis sativus | Netherlands | HM148075 | HM148319 | HM148564 |
| C. delicatulum | CBS 126342 | indoor air | Denmark | HM148079 | HM148323 | HM148568 |
| C. delicatulum | CBS 126344 | Tilia cordata | Germany | HM148081 | HM148325 | HM148570 |
| C. europaeum | CBS 134914 | building material | Denmark | HM148056 | HM148298 | HM148543 |
| C. europaeum | CPC 14238 | fruit of Sambucus nigra | Netherlands | HM148055 | HM148297 | HM148542 |
| C. exasperatum | CBS 125986 | Eucalyptus tintinnans | Australia | HM148090 | HM148334 | HM148579 |
| C. exile | CBS 125987 | Phyllactinia guttata on leaf of Corylus sp. | United States | HM148091 | HM148335 | HM148580 |
| C. flabelliforme | CBS 126345 | Melaleuca cajuputi | Australia | HM148092 | HM148336 | HM148581 |
| C. flavovirens | CBS 140462 | toe nail | United States | LN834440 | LN834536 | LN834624 |
| C. funiculosum | CBS 122129 | Vigna umbellata | Japan | HM148094 | HM148338 | HM148583 |
| C. funiculosum | CBS 122128 | Ficus carica | Japan | HM148093 | HM148337 | HM148582 |
| C. gamsianum | CBS 125989 | Strelitzia sp. | South Africa | HM148095 | HM148339 | HM148584 |
| C. gamsianum | CPC 15617 | seeds of Glycine max | Mexico | KT600392 | KT600489 | KT600587 |
| C. globisporum | CBS 812.96 | meat stamp | Sweden | HM148096 | HM148340 | HM148585 |
| C. globisporum | DTO-220-D4 | indoor environment | Netherlands | KP701967 | KP701844 | KP702089 |
| C. grevilleae | CBS 114271 | leaf of Grevillea sp. | Australia | JF770450 | JF770472 | JF770473 |
| C. hillianum | CBS 125988 | leaf of Typha orientalis | New Zealand | HM148097 | HM148341 | HM148586 |
| C. hillianum | CPC 15458 | leaf of Typha orientalis | New Zealand | HM148098 | HM148342 | HM148587 |
| C. inversicolor | CBS 401.80 | Triticum aestivum | Netherlands | HM148101 | HM148345 | HM148590 |
| C. inversicolor | DTO-108-F8 | indoor environment | France | KP701908 | KP701785 | KP702031 |
| C. ipereniae | CBS 140483 | Puya sp. | Chile | KT600394 | KT600491 | KT600589 |
| C. ipereniae | CPC 16855 | Arctostaphylos pallida | United States | KT600395 | KT600492 | KT600590 |
| C. iranicum | CBS 126346 | leaf of Citrus sinensis | Iran | HM148110 | HM148354 | HM148599 |
| C. kenpeggii | CBS 142613 | leaf of Passiflora edulis | Australia | KY646222 | KY646228 | KY646225 |
| C. licheniphilum | CBS 125990 | Physcia sp. | Germany | HM148111 | HM148355 | HM148600 |
| C. longicatenatum | CBS 140485 | unknown plant | Australia | KT600403 | KT600500 | KT600598 |
| C. lycoperdinum | CBS 274.80C | Puya sp. | Colombia | HM148114 | HM148358 | HM148603 |
| C. lycoperdinum | CBS 126347 | gall of Apiosporina morbosa on Prunus sp. | Canada | HM148112 | HM148601 | HM148601 |
| C. montecillanum | CBS 140486 | pine needles | Mexico | KT600406 | KT600504 | KT600602 |
| C. montecillanum | CPC 15605 | Taraxacum sp. | Mexico | KT600407 | KT600505 | KT600603 |
| C. myrtacaearum | CBS 126350 | Corymbia foelscheana | Australia | HM148117 | HM148361 | HM148606 |
| C. myrtacaearum | CBS 126349 | Eucalyptus placita | Australia | MH863925 | HM148360 | HM148605 |
| C. needhamense | CBS 143359 | indoor air sample | United States | MF473142 | MF473570 | MF473991 |
| C. neerlandicum | CBS 143360 | archive dust | Netherlands | KP701887 | KP701764 | KP702010 |
| C. neopsychrotolerans | CGMCC3.18031 | rhizosphere of Saussurea involucrata | China | KX938383 | KX938400 | KX938366 |
| C. neopsychrotolerans | CGMCC3.18032 | rhizosphere of Saussurea involucrata | China | KX938384 | KX938401 | KX938367 |
| C. oxysporum | CBS 125991 | soil | China | HM148118 | HM148362 | HM148607 |
| C. oxysporum | CBS 126351 | indoor air | Venezuela | HM148119 | HM148363 | HM148608 |
| C. paracladosporioides | CBS 171.54 | - | - | HM148120 | HM148364 | HM148609 |
| C. parapenidielloides | CBS 140487 | Eucalyptus sp. | Australia | KT600410 | KT600508 | KT600606 |
| C. perangustum | CBS 125996 | Cussonia sp. | South Africa | HM148121 | HM148365 | HM148610 |
| C. perangustum | CBS 126365 | Phyllactinia guttata on leaf of Corylus sp. | United States | MH863940 | HM148367 | HM148612 |
| C. phaenocomae | CBS 128769 | Phaenocoma prolifera | South Africa | JF499837 | JF499875 | JF499881 |
| C. phaenocomae | CPC 18221 | Phaenocoma prolifera | South Africa | JF499838 | JF499876 | JF499882 |
| C. phyllactiniicola | CBS 126354 | Phyllactinia guttata on leaf of Corylus sp. | United States | MH863930 | HM148396 | HM148641 |
| C. phyllactiniicola | CBS 126355 | Phyllactinia guttata on leaf of Corylus sp. | United States | HM148153 | HM148397 | HM148642 |
| C. phyllophilum | CBS 125992 | Taphrina sp. on Prunus cerasus | Germany | HM148154 | HM148398 | HM148643 |
| C. phyllophilum | CPC 13873 | Teratosphaeria proteae-arboreae on Protea arborea | South Africa | HM148155 | HM148399 | HM148644 |
| C. pini-ponderosae | CBS 124456 | Pinus ponderosa | Argentina | FJ936160 | FJ936164 | FJ936167 |
| C. pseudochalastosporoides | CBS 140490 | pine needles | Mexico | KT600415 | KT600513 | KT600611 |
| C. pseudocladosporioides | CBS 125993 | air | Netherlands | HM148158 | HM148402 | HM148647 |
| C. pseudocladosporioides | CBS 117153 | leaf of Paeonia sp. | Germany | HM148157 | HM148401 | HM148646 |
| C. ramotenellum | CPC 14300 | building material | Denmark | KT600438 | KT600537 | KT600635 |
| C. rectoides | CBS 125994 | Vitis flexuosa | South Korea | HM148193 | HM148438 | HM148683 |
| C. rectoides | CBS 126357 | Plectranthus sp. | South Korea | MH863933 | HM148439 | HM148684 |
| C. rugulovarians | CBS 140495 | unidentified Poaceae | Brazil | KT600459 | KT600558 | KT600656 |
| C. scabrellum | CBS 126358 | Ruscus hypoglossum | Slovenia | HM148195 | HM148440 | HM148685 |
| C. silenes | CBS 109082 | Silene uniflora | United Kingdom | EF679354 | EF679429 | EF679506 |
| C. silenes | MFLUCC 17-0195 | Vitis vinifera | China | MG938717 | MG938830 | MG938682 |
| C. sinuatum | CGMCC3.18096 | soil | China | KX938385 | KX938402 | KX938368 |
| C. sinuatum | CGMCC3.18097 | soil | China | KX938386 | KX938403 | KX938369 |
| Cladosporium sp. | UTHSC DI-13-227 | human sputum | United States | LN834422 | LN834518 | LN834606 |
| Cladosporium sp. | UTHSC DI-13-245 | toe | United States | LN834429 | LN834525 | LN834613 |
| Cladosporium sp. | UTHSC DI-13-265 | bronchoalveolar lavage fluid | United States | LN834435 | LN834531 | LN834619 |
| Cladosporium sp. | UTHSC DI-13-218 | bronchoalveolar lavage fluid | United States | LN834418 | LN834514 | LN834602 |
| Cladosporium sp. | UTHSC DI-13-210 | human skin | United States | LN834414 | LN834510 | LN834598 |
| C. subuliforme | CBS 126500 | Chamaedorea metallica | Thailand | HM148196 | HM148441 | HM148686 |
| C. subuliforme | DTO-130-H8 | indoor environment | Thailand | KP701938 | KP701815 | KP702060 |
| C. tenuissimum | XCSY3 | Coriandrum sativum | China | MG873079 | MT154184 | MT154174 |
| C. tenuissimum | CBS 125995 | Lagerstoemia sp. | United States | HM148197 | HM148442 | HM148687 |
| C. tianshanense | CGMCC3.18033 | rhizosphere of Saussurea involucrata | China | KX938381 | KX938398 | KX938364 |
| C. tianshanense | CGMCC3.18034 | rhizosphere of Saussurea involucrata | China | KX938382 | KX938399 | KX938365 |
| C. uredinicola | CPC 5390 | Cronartium fusiforme on Quercus nigra | United States | AY251071 | HM148467 | HM148712 |
| C. uwebraunianum | CBS 143365 | indoor air | Netherlands | MF473306 | MF473729 | MF474156 |
| C. uwebraunianum | DTO-305-H9 | house dust | New Zealand | MF473307 | MF473730 | MF474157 |
| C. varians | CBS 126361 | leaf debris | India | MH863937 | HM148469 | HM148714 |
| C. varians | CBS 126362 | Catalpa bungei | Russia | HM148224 | HM148470 | HM148715 |
| C. verrucocladosporioides | CBS 126363 | Rhus chinensis | South Korea | HM148226 | HM148472 | HM148717 |
| C. vicinum | CBS 143366 | indoor air | United States | MF473311 | MF473734 | MF474161 |
| C. vicinum | CBS 306.84 | uredospore of Puccinia allii | United Kingdom | HM148057 | HM148299 | HM148544 |
| C. vignae | CBS 121.25 | Vigna unguiculata | United States | HM148227 | HM148473 | HM148718 |
| C. welwitschiicola | CPC 18648 | Welwitschia mirabilis | Namibia | KY646223 | KY646229 | KY646226 |
| C. westerdijkiae | CPC 10150 | Fatoua villosa | South Korea | HM148062 | HM148304 | HM148549 |
| C. westerdijkiae | CPC 14284 | Triticum sp. | Germany | HM148065 | HM148307 | HM148552 |
| C. xanthocromaticum | CBS 126364 | Erythrophleum chlorostachys | Australia | HM148122 | HM148366 | HM148611 |
| C. xanthocromaticum | CPC 22239 | indoor air | United States | MF473316 | MF473739 | MF474166 |
| C. xylophilum | CBS 125997 | dead wood of Picea abies | Russia | HM148230 | HM148476 | HM148721 |
| C. xylophilum | CBS 113749 | Prunus avium | United States | HM148228 | HM148474 | HM148719 |
| Strain | Source | Location | ITS | TEF1 | ACT |
|---|---|---|---|---|---|
| AjNa1 | Ajuga reptans—receptacle | Napoli | MK387884 | MK416088 | MK416045 |
| AcAv2 | Clinopodium nepeta—achene | Averno | MK387911 | MK416115 | MK416072 |
| AcAv4 | Clinopodium nepeta—larva of A. nepetae | Averno | MK387888 | MK416092 | MK416049 |
| AcAv16 | Clinopodium nepeta—larva of parasitoid | Averno | MK387905 | MK416109 | MK416066 |
| AcBa1 | Clinopodium nepeta—larva of A. nepetae | Napoli | MK387916 | MK416120 | MK416077 |
| AcBa2 | Clinopodium nepeta—gall wall | Napoli | MK387899 | MK416103 | MK416060 |
| AcBa3 | Clinopodium nepeta—gall wall | Napoli | MK387917 | MK416121 | MK416078 |
| AcBa8 | Clinopodium nepeta—larva of parasitoid | Napoli | MK387906 | MK416110 | MK416067 |
| AcCe1 | Clinopodium nepeta—gall wall | Caserta | MK387910 | MK416114 | MK416071 |
| AcMn6 | Clinopodium nepeta—gall wall | Montenuovo | MK387914 | MK416118 | MK416075 |
| AcMt5 | Clinopodium nepeta—gall wall | Matera | MK387880 | MK416084 | MK416041 |
| AcMt6 | Clinopodium nepeta—larva of A. nepetae | Matera | MK387883 | MK416087 | MK416044 |
| AcNa1 | Clinopodium nepeta—gall wall | Astroni | MK387881 | MK416085 | MK416042 |
| AcPp1 | Clinopodium nepeta—gall wall | Pietrapertosa | MK387900 | MK416104 | MK416061 |
| AcPp2 | Clinopodium nepeta—receptacle | Pietrapertosa | MK387885 | MK416089 | MK416046 |
| AcRi7 | Clinopodium nepeta—receptacle | Rivello | MK387886 | MK416090 | MK416047 |
| SG8 | Clinopodium nepeta—gall wall | San Giorgio a Cremano | MK387907 | MK416111 | MK416068 |
| CL1 | Clinopodium vulgare—gall wall | Rivello | MK387908 | MK416112 | MK416069 |
| CL3 | Clinopodium vulgare—gall wall | Rivello | MK387898 | MK416102 | MK416059 |
| CL4 | Clinopodium vulgare—achene | Rivello | MK387904 | MK416108 | MK416065 |
| S1 | Clinopodium vulgare—achene | Grunau im Almtal | MK387902 | MK416106 | MK416063 |
| LaPo1 | Lamiastrum sp.—receptacle | Pontone | MK387878 | MK416082 | MK416039 |
| LaNa1 | Lamium album—receptacle | Napoli | MK387903 | MK416107 | MK416064 |
| LaVe1 | Lamium bifidum—receptacle | Ottaviano | MK387879 | MK416083 | MK416040 |
| LaPo2 | Lamium purpureum—receptacle | Portici | MK387877 | MK416081 | MK416038 |
| MfCa2 | Micromeria fruticulosa—gall wall | Capri | MK387882 | MK416086 | MK416043 |
| MgPo1 | Micromeria graeca—receptacle | Pontone | MK387890 | MK416094 | MK416051 |
| MgLu1 | Micromeria graeca—ovary | Lucrino | MK387918 | MK416122 | MK416079 |
| MgLu2 | Micromeria graeca—receptacle | Lucrino | MK387901 | MK416105 | MK416062 |
| MgVi1 | Micromeria graeca—gall wall | Vivara | MK387893 | MK416097 | MK416054 |
| MgVi2 | Micromeria graeca—larva of Asphondylia sp. | Vivara | MK387887 | MK416091 | MK416048 |
| MgVi3 | Micromeria graeca—receptacle | Vivara | MK387892 | MK416096 | MK416053 |
| Nc/f17 | Nepeta cataria—receptacle | Konopnica | MK387896 | MK416100 | MK416057 |
| SpCa1 | Salvia sp.—receptacle | Capri | MK387891 | MK416095 | MK416052 |
| ThSC1 | Thymus sp.—receptacle | Monte Santa Croce | MK387909 | MK416113 | MK416070 |
| Th/S345 | Thymus vulgaris—achene | Fajsławice | MK387889 | MK416093 | MK416050 |
| Th/lg/2015 | Thymus vulgaris—gall wall | Fajsławice | MK387912 | MK416116 | MK416073 |
| Th/lg/2031 | Thymus vulgaris—gall wall | Fajsławice | MK387897 | MK416101 | MK416058 |
| Th/lg/2334 | Thymus vulgaris—gall wall | Fajsławice | MK387894 | MK416098 | MK416055 |
| Th/k/258 | Thymus vulgaris—receptacle | Fajsławice | MK387895 | MK416099 | MK416056 |
| Strains | Conidiophores (µm) | Ramoconidia (µm) | Secondary 1 Ramoconidia (µm) | Intercalary Conidia 1 (µm) | Conidia 1 (µm) | Colony Diameter 2 after 14 Days (mm) |
|---|---|---|---|---|---|---|
| Th/lg/2334 | (22−)70−130 × 2−3.6 | 12.9−36 × 2.5−4.1, 0−1 septate | 9.5−17.1 × 2.4−4 | 6.4−11 × 2−3.5 | 3−5.8(−6) × (1.5−)2−2.8 | PDA: 63 Malt-extract agar (MEA): 58 Oatmeal agar (OA): 53 |
| Th/k/258 | (25−)42.7−151 × 2.4−5.1 | 14.3−39.8 × 2.4−5.2, 0−1 septate | 7.9−23.2 × 2.6−4 | 5.6−8.8 × 2−3.9 | 3.8−5.6 × (1.5−)2−3 | PDA: 70 MEA: 53 OA: 60 |
| Cladosporium pseudocladosporioides [3] | 15−155 × 2−4 | 19−48 × 3−4, 0−2(−3) septate | 16.1 × 2.9 | 8.8 × 2.6 | 4.1 × 2.1 | PDA: 65−78 MEA: 52−75 OA: 55−73 |
| MgPo1 | (28.1−)44.4−142.5 × (2.1−)2.5−3.9(−4.5) | 10.1−20.1 × 2.2−3.7 (−4.3) | (7.1−)8.3−14.6 × 2.1−3.1, 2−4 apical hila | 6.1−9.5 × 2−2.9 | (2.1−)2.4−4.9(−5.1) × (1.7−)2.1−2.5(−2.8) | PDA: 47 MEA: 37 OA: 46 |
| MgVi3 | (57−)68−126.5 × (1.9−)2.4−4.2 | 11.5−22.2 × 2.4−3.5(−3.9) | (5.7−)7.4−16.6 × 1.8−2.7 2−4 apical hila | 5.8−10.5 × 2−3.3 | (1.4−)2.2−4.3 × (1.3−)1.6−2.5(−2.8) | PDA: 47 MEA: 40 OA: 45 |
| Cladosporium xylophilum [3] | 155(−190) × 2−4(−5) | 19-35 × 2.5-3 | 14.5(±5.1) × 3.1(±0.5), up to 6(−9) apical hila | 7.7(±2.2) × 2.6(±0.3) | 3.9(±0.9) × 2.3(±0.3) | PDA: 52−74 MEA: 47−74 OA: 47−58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimowska, B.; Becchimanzi, A.; Krol, E.D.; Furmanczyk, A.; Bensch, K.; Nicoletti, R. New Cladosporium Species from Normal and Galled Flowers of Lamiaceae. Pathogens 2021, 10, 369. https://doi.org/10.3390/pathogens10030369
Zimowska B, Becchimanzi A, Krol ED, Furmanczyk A, Bensch K, Nicoletti R. New Cladosporium Species from Normal and Galled Flowers of Lamiaceae. Pathogens. 2021; 10(3):369. https://doi.org/10.3390/pathogens10030369
Chicago/Turabian StyleZimowska, Beata, Andrea Becchimanzi, Ewa Dorota Krol, Agnieszka Furmanczyk, Konstanze Bensch, and Rosario Nicoletti. 2021. "New Cladosporium Species from Normal and Galled Flowers of Lamiaceae" Pathogens 10, no. 3: 369. https://doi.org/10.3390/pathogens10030369
APA StyleZimowska, B., Becchimanzi, A., Krol, E. D., Furmanczyk, A., Bensch, K., & Nicoletti, R. (2021). New Cladosporium Species from Normal and Galled Flowers of Lamiaceae. Pathogens, 10(3), 369. https://doi.org/10.3390/pathogens10030369









