Molecular and Serological Detection of Piroplasms in Horses from Nigeria
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
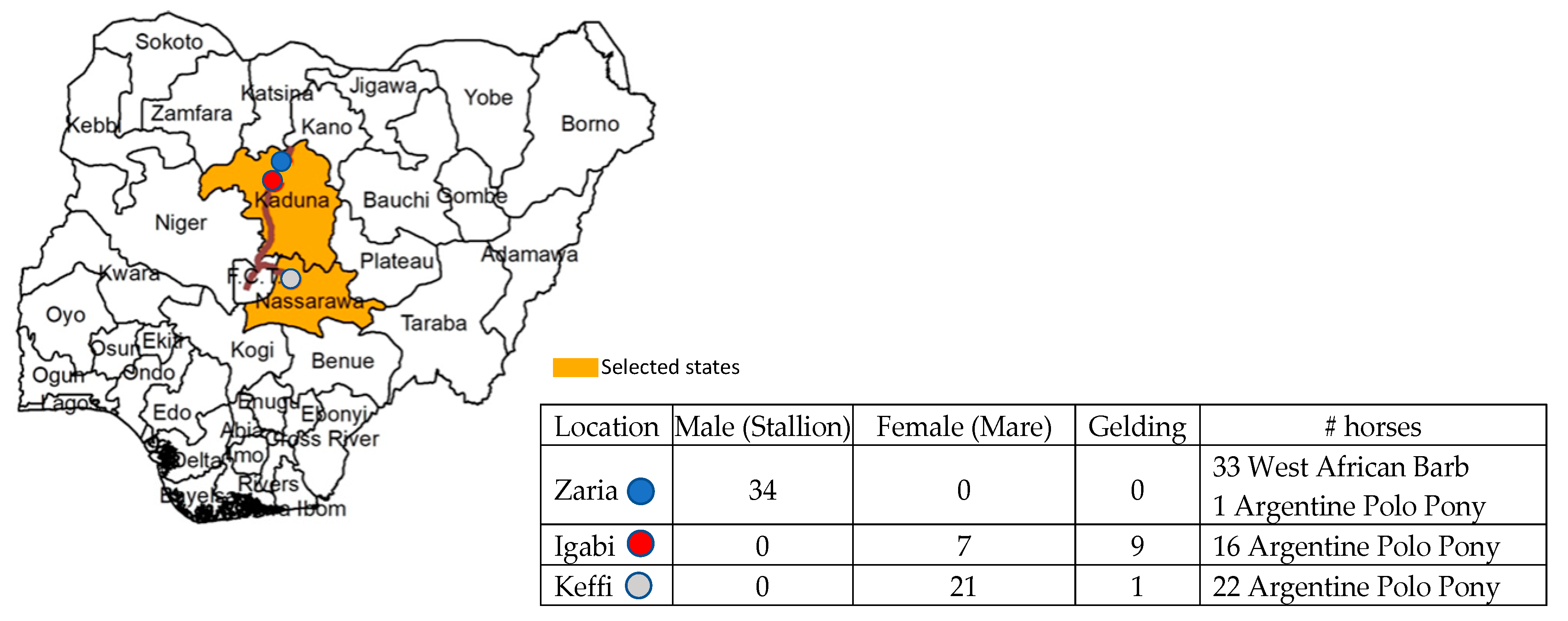
4.1. Study Population
4.2. Polymerase Chain Reaction (PCR)
4.3. Detection of T. equi- and B. caballi-Specific Antibodies
4.4. Morphological Identification of Ticks
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wise, L.N.; Kappmeyer, L.S.; Silva, M.G.; White, S.N.; Grause, J.F.; Knowles, D.P. Verification of post-chemotherapeutic clearance of Theileria equi through concordance of nested PCR and immunoblot. Ticks Tick Borne Dis. 2018, 9, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Knowles, D.P.; Kappmeyer, L.S.; Haney, D.; Herndon, D.R.; Fry, L.M.; Munro, J.B.; Sears, K.; Ueti, M.W.; Wise, L.N.; Silva, M.; et al. Discovery of a novel species, Theileria haneyi n. sp., infective to equids, highlights exceptional genomic diversity within the genus Theileria: Implications for apicomplexan parasite surveillance. Int. J. Parasitol. 2018, 48, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Ehizibolo, D.O.; Kamani, J.; Ehizibolo, P.O.; Egwu, K.O.; Dogo, G.I.; Salami-Shinaba, J.O. Prevalence and Significance of Parasites of Horses in Some States of Northern Nigeria. J. Equine Sci. 2012, 23, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Onyiche, T.E.; Taioe, M.O.; Ogo, N.I.; Sivakumar, T.; Biu, A.A.; Mbaya, A.W.; Xuan, X.; Yokoyama, N.; Thekisoe, O. Molecular evidence of Babesia caballi and Theileria equi in equines and ticks in Nigeria: Prevalence and risk factors analysis. Parasitology 2020, 147, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Scoles, G.A.; Ueti, M.W. Vector Ecology of Equine Piroplasmosis. Annu. Rev. Èntomol. 2015, 60, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Ueti, M.W.; Palmer, G.H.; Kappmeyer, L.S.; Scoles, G.A.; Knowles, D.P. Expression of Equi Merozoite Antigen 2 during Development of Babesia equi in the Midgut and Salivary Gland of the Vector Tick Boophilus microplus. J. Clin. Microbiol. 2003, 41, 5803–5809. [Google Scholar] [CrossRef] [PubMed]
- Ueti, M.W.; Palmer, G.H.; Kappmeyer, L.S.; Statdfield, M.; Scoles, G.A.; Knowles, D.P. Ability of the Vector Tick Boophilus microplus to Acquire and Transmit Babesia equi following Feeding on Chronically Infected Horses with Low-Level Parasitemia. J. Clin. Microbiol. 2005, 43, 3755–3759. [Google Scholar] [CrossRef] [PubMed]
- Schwint, O.N.; Knowles, D.P.; Ueti, M.W.; Kappmeyer, L.S.; Scoles, G.A. Transmission of Babesia caballi by Dermacentor nitens (Acari: Ixodidae) is restricted to one generation in the absence of alimentary reinfection on a susceptible equine host. J. Med. Entomol. 2008, 45, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Scoles, G.A.; Hutcheson, H.J.; Schlater, J.L.; Hennager, S.G.; Pelzel, A.M.; Knowles, D.P. Equine Piroplasmosis Associated with Amblyomma cajennense Ticks, Texas, USA. Emerg. Infect. Dis. 2011, 17, 1903–1905. [Google Scholar] [CrossRef] [PubMed]
- Ikadai, H.; Matsuu, A.; Sasaki, M.; Ishida, H.; Fujisaki, K.; Oyamada, T.; Igarashi, I. Molecular evidence of Babesia equi transmission in Haemaphysalis longicornis. Am. J. Trop. Med. Hyg. 2007, 76, 694–697. [Google Scholar] [CrossRef]
- Zapf, F.; Schein, E. The development of Babesia (Theileria) equi (Laveran, 1901) in the gut and the haemolymph of the vector ticks, Hyalomma species. Parasitol. Res. 1994, 80, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Ueti, M.W.; Palmer, G.H.; Scoles, G.A.; Kappmeyer, L.S.; Knowles, D.P. Persistently Infected Horses Are Reservoirs for Intrastadial Tick-Borne Transmission of the Apicomplexan Parasite Babesia equi. Infect. Immun. 2008, 76, 3525–3529. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Ranjan, R.; Uppal, S.K.; Singla, L.D. Transplacental transmission of Babesia equi (Theileria equi) from carrier mares to foals. J. Parasit. Dis. 2011, 36, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Georges, K.C.; Ezeokoli, C.D.; Sparagano, O.; Pargass, I.; Campbell, M.; D’Abadie, R.; Yabsley, M.J. A case of transplacental transmission of Theileria equi in a foal in Trinidad. Vet. Parasitol. 2011, 175, 363–366. [Google Scholar] [CrossRef]
- Tirosh-Levy, S.; Gottlieb, Y.; Mimoun, L.; Mazuz, M.L.; Steinman, A. Transplacental Transmission of Theileria equi Is Not a Common Cause of Abortions and Infection of Foals in Israel. Animals 2020, 10, 341. [Google Scholar] [CrossRef]
- Short, M.A.; Clark, C.K.; Harvey, J.W.; Wenzlow, N.; Hawkins, I.K.; Allred, D.R.; Knowles, D.P.; Corn, J.L.; Grause, J.F.; Hennager, S.G.; et al. Outbreak of equine piroplasmosis in Florida. J. Am. Vet. Med. Assoc. 2012, 240, 588–595. [Google Scholar] [CrossRef]
- Wise, L.; Kappmeyer, L.; Mealey, R.; Knowles, D. Review of Equine Piroplasmosis. J. Vet. Intern. Med. 2013, 27, 1334–1346. [Google Scholar] [CrossRef]
- Grause, J.F.; Ueti, M.W.; Nelson, J.T.; Knowles, D.P.; Kappmeyer, L.S.; Bunn, T.O. Efficacy of imidocarb dipropionate in eliminating Theileria equi from experimentally infected horses. Vet. J. 2013, 196, 541–546. [Google Scholar] [CrossRef]
- Ueti, M.W.; Mealey, R.H.; Kappmeyer, L.S.; White, S.N.; Kumpula-McWhirter, N.; Pelzel, A.M.; Grause, J.F.; Bunn, T.O.; Schwartz, A.; Traub-Dargatz, J.L.; et al. Re-Emergence of the Apicomplexan Theileria equi in the United States: Elimination of Persistent Infection and Transmission Risk. PLoS ONE 2012, 7, e44713. [Google Scholar] [CrossRef]
- Onyiche, T.E.; Suganuma, K.; Igarashi, I.; Yokoyama, N.; Xuan, X.; Thekisoe, O. A Review on Equine Piroplasmosis: Epidemiology, Vector Ecology, Risk Factors, Host Immunity, Diagnosis and Control. Int. J. Environ. Res. Public Health 2019, 16, 1736. [Google Scholar] [CrossRef]
- Mshelia, P.W.; Kappmeyer, L.; Johnson, W.C.; Kudi, C.A.; Oluyinka, O.O.; Balogun, E.O.; Richard, E.E.; Onoja, E.; Sears, K.P.; Ueti, M.W. Molecular detection of Theileria species and Babesia caballi from horses in Nigeria. Parasitol. Res. 2020, 119, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Mshelia, W.P.; Sambo, K.W.; Adamu, S.; Edeh, E.R.; Onoja, I.I. Persistence of equine piroplasmosis in horses in Nigeria. J. Equine Vet. Sci. 2016, 39, S98–S107. Available online: https://core.ac.uk/download/pdf/82099107.pdf (accessed on 7 March 2021). [CrossRef]
- World Organization for Animal Health (OIE). Laboratory Criteria: OIE Manual of Standards for Diagnostic Tests and Vaccines for Terrestrial Animals; Chapter 2.5.8; World Organization for Animal Health (OIE): Paris, France, 2005. [Google Scholar]
- Harvey, J.W. Normal hematologic values. In Equine Clinical Neonatology; Koterba, A.M., Drummond, W.H., Kosch, P.C., Eds.; Lea and Febiger: Philadelphia, PA, USA, 1990; pp. 561–570. [Google Scholar]
- Harvey, J.W.; Asquith, R.L.; McNulty, P.K.; Kivipelto, J.; Bauer, J.E. Haematology of foals up to one year old. Equine Vet. J. 1984, 16, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Bishop, R.P.; Kappmeyer, L.S.; Onzere, C.K.; Odongo, D.O.; Githaka, N.; Sears, K.P.; Knowles, D.P.; Fry, L.M. Equid infective Theileria cluster in distinct 18S rRNA gene clades comprising multiple taxa with unusually broad mammalian host ranges. Parasites Vectors 2020, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Motloang, M.Y.; Thekisoe, O.M.; Alhassan, A.; Bakheit, M.; Motheo, M.P.; Masangane, F.E.; Thibedi, M.L.; Inoue, N.; Igarashi, I.; Sugimoto, C.; et al. Prevalence of Theileria equi and Babesia caballi infections in horses belonging to resource-poor farmers in the north-eastern Free State Province, South Africa. Onderstepoort J. Vet. Res. 2008, 75, 141–146. [Google Scholar] [CrossRef]
- Moloi, T.P. Serology, Molecular Epidemiology and Strain Diversity of Equine Piroplasms in South Africa. Master’s Thesis, University of the Free State Qwaqwa Campus, Phuthaditjhaba, South Africa, February 2010. [Google Scholar]
- Nagore, D.; García-Sanmartín, J.; García-Pérez, A.L.; Juste, R.A.; Hurtado, A. Detection and identification of equine Theileria and Babesia species by reverse line blotting: Epidemiological survey and phylogenetic analysis. Vet. Parasitol. 2004, 123, 41–54. [Google Scholar] [CrossRef]
- Qablan, M.A.; Oborník, M.; Petrželková, K.J.; Sloboda, M.; Shudiefat, M.F.; Hořín, P.; Lukeš, J.; Modrý, D. Infections by Babesia caballi and Theileria equi in Jordanian equids: Epidemiology and genetic diversity. Parasitology 2013, 140, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Knowles, D.P.; E Perryman, L.; Goff, W.L.; Miller, C.D.; Harrington, R.D.; Gorham, J.R. A monoclonal antibody defines a geographically conserved surface protein epitope of Babesia equi merozoites. Infect. Immun. 1991, 59, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.K.; Gamil, I.S.; Abd-El Baky, A.A.; Hussein, M.M.; Tohamy, A.A. Comparative molecular and conventional detection methods of Babesia equi (B. Equi) in Egyptian Equine. Glob. Vet. 2011, 7, 201–210. [Google Scholar]
- Kamyingkird, K.; Yangtara, S.; Desquesnes, M.; Cao, S.; Adjou Moumouni, P.K.; Jittapalapong, S.; Nimsupan, B.; Terkawi, M.A.; Masatani, T.; Nishikawa, Y.; et al. Seroprevalence of Babesia caballi and Theileria equi in horses and mules from Northern Thailand. J. Protozool. Res. 2014, 24, 11–17. [Google Scholar]
- Turaki, U.A.; Kumsha, H.A.; Biu, A.A.; Bokko, P.B. Prevalence of Piroplasmosis amongst local horses in Northeastern Nigeria. IOSR J. Agric. Vet. Sci. 2014, 7, 4–7. [Google Scholar]
- Mahmoud, M.S.; Abu El-Ezz, N.T.; Abdel-Shafy, S.; Nassar, S.A.; El Namaky, A.H.; Khalil, W.K.B.; Knowles, D.; Kappmeyer, L.; Silva, M.G.; Suarez, C.E. Assessment of Theileria equi and Babesia caballi infections in equine populations in Egypt by molecular, serological and hematological approaches. Parasites Vectors 2016, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A.; Aharonson-Raz, K.; Berlin, D.; Tal, S.; Gottlieb, Y.; Klement, E.; Steinman, A. Molecular characterization of the Babesia caballi rap-1 gene and epidemiological survey in horses in Israel. Infect. Genet. Evol. 2014, 23, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bhoora, R.; Quan, M.; Zweygarth, E.; Guthrie, A.J.; Prinsloo, S.A.; Collins, N.E. Sequence heterogeneity in the gene encoding the rhoptry-associated protein-1 (RAP-1) of Babesia caballi isolates from South Africa. Vet. Parasitol. 2010, 169, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Cortés, M.G.M.; Fernández-García, J.L.; Martínez-Estéllez, M. Ángel H. Seroprevalence of Theileria equi and Babesia caballi in horses in Spain. Parasite 2017, 24, 14. [Google Scholar] [CrossRef]
- Kappmeyer, L.S.; Perryman, L.E.; Hines, S.A.; Baszler, T.V.; Katz, J.B.; Hennager, S.G.; Knowles, D.P. Detection of Equine Antibodies to Babesia caballi by Recombinant B. caballi Rhoptry-Associated Protein 1 in a Competitive-Inhibition Enzyme-Linked Immunosorbent Assay. J. Clin. Microbiol. 1999, 37, 2285–2290. [Google Scholar] [CrossRef]
- Bastos, R.; Sears, K.; Dinkel, K.; Kappmeyer, L.; Ueti, M.; Knowles, D.; Fry, L. Development of an Indirect ELISA to Detect Equine Antibodies to Theileria haneyi. Pathogens 2021, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Idoko, I.S.; Tirosh-Levy, S.; Mazuz, M.L.; Adam, B.M.; Garba, B.S.; Nafarnda, D.W.; Steinman, A. Genetic Characterization of Piroplasms in Donkeys and Horses from Nigeria. Animals 2020, 10, 324. [Google Scholar] [CrossRef]
- de Waal, D.T. The transovarial transmission of Babesia caballi by Hyalomma truncatum. Onderstepoort J. Vet. Res. 1990, 57, 99–100. [Google Scholar]
- Walker, A.R.; Bouattour, A.; Camicas, J.-L.; Estrada-Pena, A.; Horak, I.; Latif, A.A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2014. [Google Scholar]
| Piroplasm | |||||||
|---|---|---|---|---|---|---|---|
| T. equi | B. caballi | T. haneyi | |||||
| Hematology | Horses tested | nPCR | cELISA | nPCR | cELISA | nPCR | cELISA |
| Abnormal | 41 | 15 | 37 | 1 | 0 | 1 | NA |
| Normal | 31 | 5 | 24 | 0 | 0 | 2 | NA |
| Total | 72 | 20 | 61 | 1 | 0 | 3 | NA |
| Piroplasm | ||
|---|---|---|
| nPCR/cELISA | T. equi | B. caballi |
| +/− | 2 | 1 |
| −/+ | 43 | 0 |
| +/+ | 18 | 0 |
| Total | 63 | 1 |
| Tick Species | Male | Females | Total |
|---|---|---|---|
| Rhipicephalus evertsi evertsi | 164 (50.8%) | 159 (49.2%) | 323 (69.6%) |
| Rhipicephalus sanguineus | 4 (40.0%) | 6 (60.0%) | 10 (2.2%) |
| Amblyomma variegatum | 37 (55.2%) | 30 (44.8%) | 67 (14.4%) |
| Hyalomma dromedarii | 22 (68.8%) | 10 (31.2%) | 32 (6.9%) |
| Hyalomma impeltatum | 0 | 4 (100%) | 4 (0.9%) |
| Hyalomma truncatum | 0 | 1 (100%) | 1 (0.2%) |
| Boophilus decoloratus | 0 | 7 (100%) | 7 (1.5%) |
| Total | 227 (51.1%) | 217 (48.9%) | 444 (100%) |
| Primer | Sequence (5’-3’) | Expected Product Size | Target Gene |
|---|---|---|---|
| T. equi external-forward | GAGGAGGAGAAACCCAAG | 549 bp | ema1 |
| T. equi external-reverse | GCCATCGCCCTTGTAGAG | ||
| T. equi internal-forward | TCAAGGACAACAAGCCATAC | 229 bp | |
| T. equi internal-reverse | TTGCCTGGAGCCTTGAAG | ||
| B. caballi external-forward | GATTACTTGTCGGCTGTGTCT | 374 bp | rap1 |
| B. caballi external-reverse | CGCAAAGTTCTCAATGTCAG | ||
| B. caballi internal-forward | GCTAAGTACCAACCGCTGA | 221 bp | |
| B. caballi internal-reverse | CGCAAAGTTCTCAATGTCAG | ||
| T. haneyi external-forward | CCATACAACCCACTAGAG | 381 bp | hypothetical single copy gene |
| T. haneyi external-reverse | CTGTCATTTGGGTTTGATAG | ||
| T. haneyi internal-forward | GACAACAGAGAGGTGATT | 238 bp | |
| T. haneyi internal-reverse | CGTTGAATGTAATGGGAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idoko, I.S.; Edeh, R.E.; Adamu, A.M.; Machunga-Mambula, S.; Okubanjo, O.O.; Balogun, E.O.; Adamu, S.; Johnson, W.; Kappmeyer, L.; Mousel, M.; et al. Molecular and Serological Detection of Piroplasms in Horses from Nigeria. Pathogens 2021, 10, 508. https://doi.org/10.3390/pathogens10050508
Idoko IS, Edeh RE, Adamu AM, Machunga-Mambula S, Okubanjo OO, Balogun EO, Adamu S, Johnson W, Kappmeyer L, Mousel M, et al. Molecular and Serological Detection of Piroplasms in Horses from Nigeria. Pathogens. 2021; 10(5):508. https://doi.org/10.3390/pathogens10050508
Chicago/Turabian StyleIdoko, Idoko S., Richard E. Edeh, Andrew M. Adamu, Salamatu Machunga-Mambula, Oluyinka O. Okubanjo, Emmanuel O. Balogun, Sani Adamu, Wendell Johnson, Lowell Kappmeyer, Michelle Mousel, and et al. 2021. "Molecular and Serological Detection of Piroplasms in Horses from Nigeria" Pathogens 10, no. 5: 508. https://doi.org/10.3390/pathogens10050508
APA StyleIdoko, I. S., Edeh, R. E., Adamu, A. M., Machunga-Mambula, S., Okubanjo, O. O., Balogun, E. O., Adamu, S., Johnson, W., Kappmeyer, L., Mousel, M., & Ueti, M. W. (2021). Molecular and Serological Detection of Piroplasms in Horses from Nigeria. Pathogens, 10(5), 508. https://doi.org/10.3390/pathogens10050508






