Cytokine Biosignature of Active and Latent Mycobacterium Tuberculosis Infection in Children
Abstract
1. Introduction
2. Results
2.1. Basic Characteristics of the Study Group
2.2. Identification of Differentially Expressed Th17-Related Cytokines and Inflammatory Mediators in the Study Groups
2.3. Discriminative Biomarker Potential by Receiver Operating Characteristic (ROC) Analysis
2.4. Identification of the Cytokine Biosignature for Discriminating between Different Status of M.tb Infection
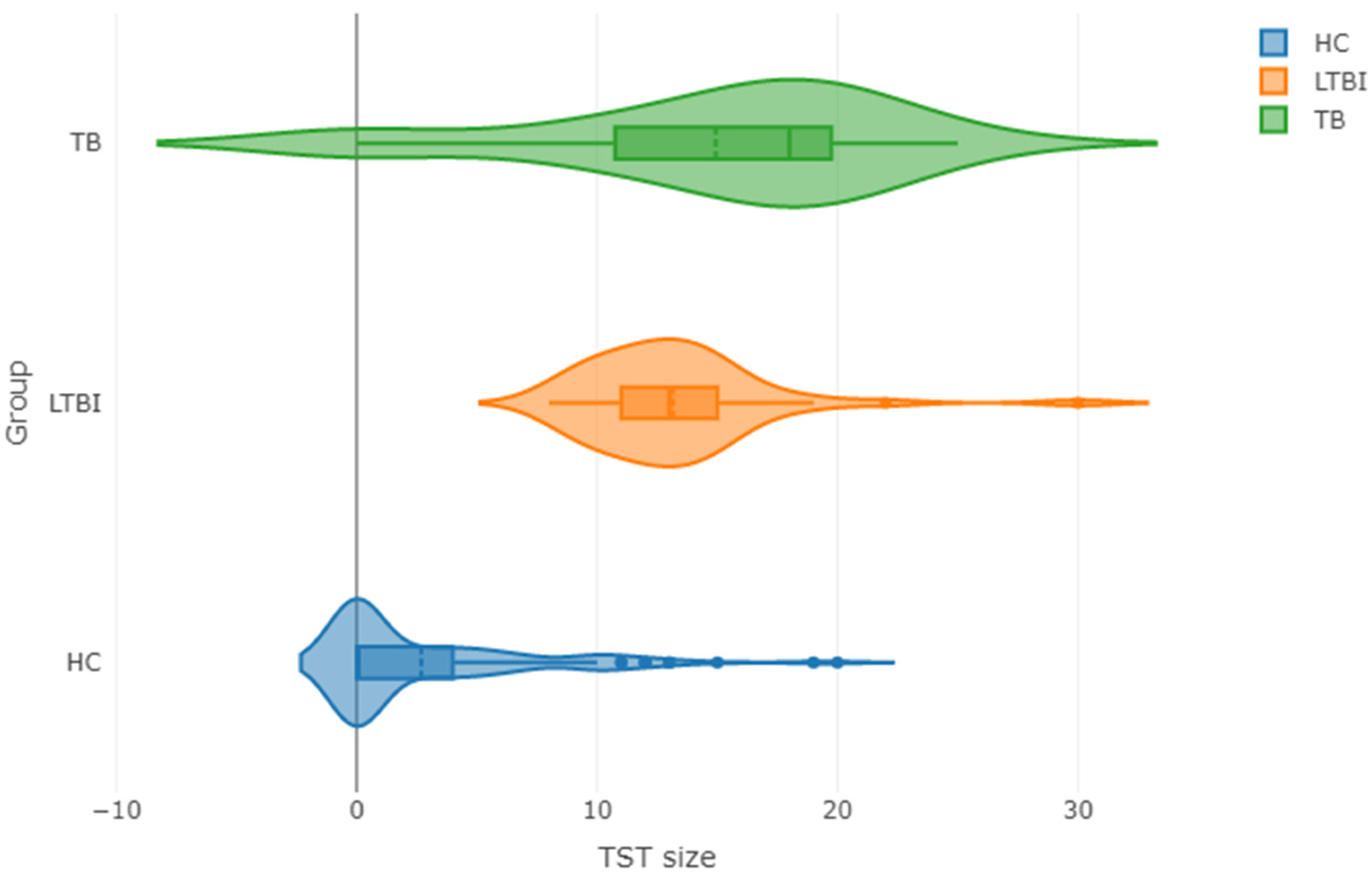
2.5. Discriminative Biomarker Profiles in Children with a Positive (TST-Positive) or Negative (TST-Negative) Skin Test Reaction to Tuberculin
3. Discussion
4. Materials and Methods
4.1. Children Characteristics
4.2. Blood Specimens
4.3. Tuberculin Skin Testing
4.4. Multiplex Bead Assays
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swaminathan, S.; Rekha, B. Pediatric tuberculosis: Global overview and challenges. Clin. Infect. Dis. 2010, 50 (Suppl. S3), S184–S194. [Google Scholar] [CrossRef]
- Bakir, M.; Millington, K.A.; Soysal, A.; Deeks, J.J.; Efee, S.; Aslan, Y.; Dosanjh, D.P.; Lalvani, A. Prognostic value of a T-cell-based, interferon-γ biomarker in children with tuberculosis contact. Ann. Intern. Med. 2008, 149, 777–786. [Google Scholar] [CrossRef]
- Cobat, A.; Poirier, C.; Hoal, E.; Boland-Auge, A.; de La Rocque, F.; Corrard, F.; Grange, G.; Migaud, M.; Bustamante, J.; Boisson-Dupuis, S.; et al. Tuberculin skin test negativity is under tight genetic control of chromosomal region 11p14-15 in settings with different tuberculosis endemicities. J. Infect. Dis. 2015, 211, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Angulo, J.M.; Arriaga, M.B.; Melo, M.; Silva, E.C.; Alvarado-Arnez, L.E.; de Almeida, A.S.; Moraes, M.O.; Moreira, A.; Lapa E Silva, J.R.; Fukutani, K.F.; et al. Polymorphisms in interferon pathway genes and risk of Mycobacterium tuberculosis infection in contacts of tuberculosis cases in Brazil. Int. J. Infect. Dis. 2020, 92, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Detjen, A.K.; Keil, T.; Roll, S.; Hauer, B.; Mauch, H.; Wahn, U.; Magdorf, K. Interferon-γ release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin. Infect. Dis. 2007, 45, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Mao, T.E.; Mori, T.; Miura, T.; Sugiyama, T.; Yoshiyama, T.; Mitarai, S.; Onozaki, I.; Harada, N.; Saint, S.; et al. Performance of an interferon-gamma release assay for diagnosing latent tuberculosis infection in children. Epidemiol. Infect. 2008, 136, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Lighter, J.; Rigaud, M. Diagnosing childhood tuberculosis: Traditional and innovative modalities. Curr. Probl. Pediatr. Adolesc. Health Care 2009, 39, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, H.; Lawson, L.; Squire, S.B.; Coulter, B.; Ravn, P.; Brock, I.; Hart, C.A.; Cuevas, L.E. Risk for tuberculosis among children. Emerg. Infect. Dis. 2006, 12, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Haustein, T.; Ridout, D.A.; Hartley, J.C.; Thaker, U.; Shingadia, D.; Klein, N.J.; Novelli, V.; Dixon, G.L.J. The likelihood of an indeterminate test result from a whole-blood interferon-γ release assay for the diagnosis of mycobacterium tuberculosis infection in children correlates with age and immune status. Pediatr. Infect. Dis. J. 2009, 28, 669–673. [Google Scholar] [CrossRef]
- Hesseling, A.C.; Schaaf, H.S.; Gie, R.P.; Starke, J.R.; Beyers, N. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis. Int. J. Tuberc. Lung Dis. 2002, 6, 1038–1045. [Google Scholar] [PubMed]
- Jacobs, R.; Malherbe, S.; Loxton, A.G.; Stanley, K.; van der Spuy, G.; Walzl, G.; Chegou, N.N. Identification of novel host biomarkers in plasma as candidates for the immunodiagnosis of tuberculosis disease and monitoring of tuberculosis treatment response. Oncotarget 2016, 7, 57581–57592. [Google Scholar] [CrossRef] [PubMed]
- Hand, D.J.; Till, R.J. A simple generalisation of the area Under the ROC curve for multiple class classification problems. Mach. Learn. 2001, 45, 171–186. [Google Scholar] [CrossRef]
- Spolski, R.; Wang, L.; Wan, C.-K.; Bonville, C.A.; Domachowske, J.B.; Kim, H.-P.; Yu, Z.; Leonard, W.J. IL-21 Promotes the pathologic immune response to pneumovirus infection. J. Immunol. 2012, 188, 1924–1932. [Google Scholar] [CrossRef]
- Leonard, W.J.; Wan, C.K. IL-21 Signaling in Immunity. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Lindner, S.; Dahlke, K.; Sontheimer, K.; Hagn, M.; Kaltenmeier, C.; Barth, T.F.E.; Beyer, T.; Reister, F.; Fabricius, D.; Lotfi, R.; et al. Interleukin 21-induced granzyme b-expressing b cells infiltrate tumors and regulate t cells. Cancer Res. 2013, 73, 2468–2479. [Google Scholar] [CrossRef] [PubMed]
- Jenabian, M.A.; Patel, M.; Kema, I.; Vyboh, K.; Kanagaratham, C.; Radzioch, D.; Thébault, P.; Lapointe, R.; Gilmore, N.; Ancuta, P.; et al. Soluble CD40-ligand (sCD40L, sCD154) plays an immunosuppressive role via regulatory T cell expansion in HIV infection. Clin. Exp. Immunol. 2014, 178, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.W.; Lim, J.B. Clinical significance of elevated serum soluble CD40 ligand levels as a diagnostic and prognostic tumor marker for pancreatic ductal adenocarcinoma. J. Transl. Med. 2014, 12, 102. [Google Scholar] [CrossRef]
- Djoba Siawaya, J.F.; Chegou, N.N.; van Den Heuvel, M.M.; Diacon, A.H.; Beyers, N.; van Helden, P.; Walzl, G. Differential cytokine/chemokines and KL-6 profiles in patients with different forms of tuberculosis. Cytokine 2009, 47, 132–136. [Google Scholar] [CrossRef]
- Shah, K.; Lee, W.W.; Lee, S.H.; Kim, S.H.; Kang, S.W.; Craft, J.; Kang, I. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res. Ther. 2010, 12, 1–10. [Google Scholar] [CrossRef]
- Kononova, T.E.; Urazova, O.I.; Novitskii, V.V.; Churina, E.G.; Kolobovnikova, Y.V.; Ignatov, M.V.; Zakharova, P.A.; Pechenova, O.V. Functional activity of Th-17 lymphocytes in pulmonary tuberculosis. Bull. Exp. Biol. Med. 2014, 156, 743–745. [Google Scholar] [CrossRef]
- Pappu, R.; Ramirez-Carrozzi, V.; Sambandam, A. The interleukin-17 cytokine family: Critical players in host defence and inflammatory diseases. Immunology 2011, 134, 8–16. [Google Scholar] [CrossRef]
- Acosta-Rodriguez, E.V.; Rivino, L.; Geginat, J.; Jarrossay, D.; Gattorno, M.; Lanzavecchia, A.; Sallusto, F.; Napolitani, G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007, 8, 639–646. [Google Scholar] [CrossRef]
- Aujla, S.J.; Chan, Y.R.; Zheng, M.; Fei, M.; Askew, D.J.; Pociask, D.A.; Reinhart, T.A.; McAllister, F.; Edeal, J.; Gaus, K.; et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008, 14, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, J.; Tian, J.; Zhu, B.; Zhang, Y.; Yang, K.; Ling, Y.; Hu, Y. IL-17 and IFN-γ production in peripheral blood following BCG vaccination and Mycobacterium tuberculosis infection in human. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 2029–2036. [Google Scholar] [PubMed]
- Kumar, N.P.; Anuradha, R.; Suresh, R.; Ganesh, R.; Shankar, J.; Kumaraswami, V.; Nutman, T.B.; Babu, S. Suppressed type 1, type 2, and type 17 cytokine responses in active tuberculosis in children. Clin. Vaccine Immunol. 2011, 18, 1856–1864. [Google Scholar] [CrossRef]
- Scriba, T.J.; Kalsdorf, B.; Abrahams, D.-A.; Isaacs, F.; Hofmeister, J.; Black, G.; Hassan, H.Y.; Wilkinson, R.J.; Walzl, G.; Gelderbloem, S.J.; et al. Distinct, specific IL-17- and IL-22-producing CD4 + T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 2008, 180, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chin, C.H.; Liu, S.F.; Wu, C.C.; Tsen, C.C.; Wang, Y.H.; Chao, T.Y.; Lie, C.H.; Chen, C.J.; Wang, C.C.; et al. Prognostic values of serum IP-10 and IL-17 in patients with pulmonary tuberculosis. Dis. Markers 2011, 31, 101–110. [Google Scholar] [CrossRef]
- Heidarnezhad, F.; Asnaashari, A.; Rezaee, S.A.; Ghezelsofla, R.; Ghazvini, K.; Valizadeh, N.; Basiri, R.; Ziaeemehr, A.; Sobhani, S.; Rafatpanah, H. Evaluation of interleukin 17 and interleukin 23 expression in patients with active and latent tuberculosis infection. Iran. J. Basic Med. Sci. 2016, 19, 844–850. [Google Scholar]
- Kumar, N.P.; Anuradha, R.; Andrade, B.B.; Suresh, N.; Ganesh, R.; Shankar, J.; Kumaraswami, V.; Nutman, T.B.; Babu, S. Circulating biomarkers of pulmonary and extrapulmonary tuberculosis in children. Clin. Vaccine Immunol. 2013, 20, 704–711. [Google Scholar] [CrossRef]
- Marín, N.D.; París, S.C.; Rojas, M.; García, L.F. Functional profile of CD4+ and CD8+ T cells in latently infected individuals and patients with active TB. Tuberculosis 2013, 93, 155–166. [Google Scholar] [CrossRef]
- Dheda, K.; Chang, J.-S.; Lala, S.; Huggett, J.F.; Zumla, A.; Rook, G.A.W. Gene expression of IL17 and IL23 in the lungs of patients with active tuberculosis. Thorax 2008, 63, 566–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, L.; Cui, G.; Jia, H.; Zhu, Y.; Ding, Y.; Chen, J.; Lu, C.; Ye, P.; Gao, H.; Li, L.; et al. Decreased IL-17 during treatment of sputum smear-positive pulmonary tuberculosis due to increased regulatory T cells and IL-10. J. Transl. Med. 2016, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Chegou, N.N.; Sutherland, J.S.; Malherbe, S.; Crampin, A.C.; Corstjens, P.L.A.M.; Geluk, A.; Mayanja-Kizza, H.; Loxton, A.G.; Van Der Spuy, G.; Stanley, K.; et al. Diagnostic performance of a seven-marker serum protein biosignature for the diagnosis of active TB disease in African primary healthcare clinic attendees with signs and symptoms suggestive of TB. Thorax 2016, 71, 785–794. [Google Scholar] [CrossRef]
- Chegou, N.N.; Black, G.F.; Kidd, M.; van Helden, P.D.; Walzl, G. Host markers in Quantiferon supernatants differentiate active TB from latent TB infection: Preliminary report. BMC Pulm. Med. 2009, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rohlwink, U.K.; Mauff, K.; Wilkinson, K.A.; Enslin, N.; Wegoye, E.; Wilkinson, R.J.; Figaji, A.A. Biomarkers of cerebral injury and infammation in pediatric tuberculous meningitis. Clin. Infect. Dis. 2017, 65, 1298–1307. [Google Scholar] [CrossRef]
- Ong, C.W.M.; Elkington, P.T.; Friedland, J.S. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am. J. Respir. Crit. Care Med. 2014, 190, 9–18. [Google Scholar] [CrossRef]
- Green, J.A.; Dholakia, S.; Janczar, K.; Ong, C.W.M.; Moores, R.; Fry, J.; Elkington, P.T.; Roncaroli, F.; Friedland, J.S. Mycobacterium tuberculosis-infected human monocytes down-regulate microglial MMP-2 secretion in CNS tuberculosis via TNFα, NFκB, p38 and caspase 8 dependent pathways. J. Neuroinflamm. 2011, 8, 1–12. [Google Scholar] [CrossRef]
- Chang, J.C.; Wysocki, A.; Tchou-Wong, K.M.; Moskowitz, N.; Zhang, Y.; Rom, W.N. Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax 1996, 51, 306–311. [Google Scholar] [CrossRef]
- Matsuura, E.; Umehara, F.; Hashiguchi, T.; Fujimoto, N.; Okada, Y.; Osame, M. Marked increase of matrix metalloproteinase 9 in cerebrospinal fluid of patients with fungal or tuberculous meningoencephalitis. J. Neurol. Sci. 2000, 173, 45–52. [Google Scholar] [CrossRef]
- Thuong, N.T.T.; Dunstan, S.J.; Chau, T.T.H.; Thorsson, V.; Simmons, C.P.; Quyen, N.T.H.; Thwaites, G.E.; Thi Ngoc Lan, N.; Hibberd, M.; Teo, Y.Y.; et al. Identification of tuberculosis susceptibility genes with human macrophage gene expression profiles. PLoS Pathog. 2008, 4, e1000229. [Google Scholar] [CrossRef]
- Subbian, S.; Tsenova, L.; O’Brien, P.; Yang, G.; Koo, M.S.; Peixoto, B.; Fallows, D.; Zeldis, J.B.; Muller, G.; Kaplan, G. Phosphodiesterase-4 inhibition combined with isoniazid treatment of rabbits with pulmonary tuberculosis reduces macrophage activation and lung pathology. Am. J. Pathol. 2011, 179, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Mehra, S.; Pahar, B.; Dutta, N.K.; Conerly, C.N.; Philippi-Falkenstein, K.; Alvarez, X.; Kaushal, D. Transcriptional reprogramming in nonhuman primate (Rhesus Macaque) tuberculosis granulomas. PLoS ONE 2010, 5, e12266. [Google Scholar] [CrossRef]
- Hsieh, W.Y.; Kuan, T.C.; Cheng, K.S.; Liao, Y.C.; Chen, M.Y.; Lin, P.H.; Hsu, Y.C.; Huang, C.Y.; Hsu, W.H.; Yu, S.Y.; et al. ACE/ACE2 ratio and MMP-9 activity as potential biomarkers in tuberculous pleural effusions. Int. J. Biol. Sci. 2012, 8, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Kathamuthu, G.R.; Kumar, N.P.; Moideen, K.; Nair, D.; Banurekha, V.V.; Sridhar, R.; Baskaran, D.; Babu, S. Matrix metalloproteinases and tissue inhibitors of metalloproteinases are potential biomarkers of pulmonary and extra-pulmonary tuberculosis. Front. Immunol. 2020, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Ugarte-Gil, C.A.; Elkington, P.; Gilman, R.H.; Coronel, J.; Tezera, L.B.; Bernabe-Ortiz, A.; Gotuzzo, E.; Friedland, J.S.; Moore, D.A.J. Induced sputum MMP-1, -3 & -8 concentrations during treatment of tuberculosis. PLoS ONE 2013, 8, e61333. [Google Scholar] [CrossRef]
- Rohlwink, U.K.; Walker, N.F.; Ordonez, A.A.; Li, Y.J.; Tucker, E.W.; Elkington, P.T.; Wilkinson, R.J.; Wilkinson, K.A. Matrix metalloproteinases in pulmonary and central nervous system tuberculosis—A review. Int. J. Mol. Sci. 2019, 20, 1350. [Google Scholar] [CrossRef]
- Stek, C.; Allwood, B.; Walker, N.F.; Wilkinson, R.J.; Lynen, L.; Meintjes, G. The immune mechanisms of lung parenchymal damage in tuberculosis and the role of host-directed therapy. Front. Microbiol. 2018, 9, 2603. [Google Scholar] [CrossRef]
- Manyelo, C.M.; Solomons, R.S.; Snyders, C.I.; Mutavhatsindi, H.; Manngo, P.M.; Stanley, K.; Walzl, G.; Chegou, N.N. Potential of host serum protein biomarkers in the diagnosis of tuberculous meningitis in children. Front. Pediatr. 2019, 7, 376. [Google Scholar] [CrossRef]
- Chen, T.; Li, Z.; Yu, L.; Li, H.; Lin, J.; Guo, H.; Wang, W.; Chen, L.; Zhang, X.; Wang, Y.; et al. Profiling the human immune response to Mycobacterium tuberculosis by human cytokine array. Tuberculosis 2016, 97, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Chegou, N.N.; Detjen, A.K.; Thiart, L.; Walters, E.; Mandalakas, A.M.; Hesseling, A.C.; Walzl, G. Utility of host markers detected in Quantiferon supernatants for the diagnosis of tuberculosis in children in a high-burden setting. PLoS ONE 2013, 8, e64226. [Google Scholar] [CrossRef] [PubMed]
- Comella-Del-Barrio, P.; Abellana, R.; Villar-Hernández, R.; Jean Coute, M.D.; Sallés Mingels, B.; Canales Aliaga, L.; Narcisse, M.; Gautier, J.; Ascaso, C.; Latorre, I.; et al. A Model based on the combination of IFN-γ, IP-10, Ferritin and 25-Hydroxyvitamin D for discriminating latent from active tuberculosis in children. Front. Microbiol. 2019, 10, 1855. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Shen, Y.; Wu, J.; Gao, Y.; Zhang, S.; Shao, L.; Jin, J.; Zhang, Y.; Zhang, W. Screening and identification of a six-cytokine biosignature for detecting TB infection and discriminating active from latent TB. J. Transl. Med. 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Zambuzi, F.A.; Cardoso-Silva, P.M.; Espindola, M.S.; Soares, L.S.; Galvão-Lima, L.J.; Brauer, V.S.; Gomes, M.S.; Amaral, L.R.; Schaller, M.; Bollela, V.R.; et al. Identification of promising plasma immune biomarkers to differentiate active pulmonary tuberculosis. Cytokine 2016, 88, 99–107. [Google Scholar] [CrossRef]
- Clifford, V.; Tebruegge, M.; Zufferey, C.; Germano, S.; Forbes, B.; Cosentino, L.; Matchett, E.; McBryde, E.; Eisen, D.; Robins-Browne, R.; et al. Cytokine biomarkers for the diagnosis of tuberculosis infection and disease in adults in a low prevalence setting. Tuberculosis 2019, 114, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Sudbury, E.L.; Otero, L.; Tebruegge, M.; Messina, N.L.; Seas, C.; Montes, M.; Rìos, J.; Germano, S.; Gardiner, K.; Clifford, V.; et al. Mycobacterium tuberculosis-specific cytokine biomarkers for the diagnosis of childhood TB in a TB-endemic setting. J. Clin. Tuberc. Other Mycobact. Dis. 2019, 16, 100102. [Google Scholar] [CrossRef]
- Sigal, G.B.; Segal, M.R.; Mathew, A.; Jarlsberg, L.; Wang, M.; Barbero, S.; Small, N.; Haynesworth, K.; Davis, J.L.; Weiner, M.; et al. Biomarkers of tuberculosis severity and treatment effect: A directed screen of 70 host markers in a randomized clinical trial. EBioMedicine 2017, 25, 112–121. [Google Scholar] [CrossRef]
- Won, E.J.; Choi, J.H.; Cho, Y.N.; Jin, H.M.; Kee, H.J.; Park, Y.W.; Kwon, Y.S.; Kee, S.J. Biomarkers for discrimination between latent tuberculosis infection and active tuberculosis disease. J. Infect. 2017, 74, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Suzukawa, M.; Akashi, S.; Nagai, H.; Nagase, H.; Nakamura, H.; Matsui, H.; Hebisawa, A.; Ohta, K. Combined analysis of IFN-γ, IL-2, IL-5, IL-10, IL-1RA and MCP-1 in QFT supernatant is useful for distinguishing active tuberculosis from latent infection. PLoS ONE 2016, 11, e0152483. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, M.; Rudnicka, W.; Janiszewska-Drobinska, B.; Kielnierowski, G.; Kowalewicz-Kulbat, M.; Fol, M.; Druszczynska, M. Interferon-gamma assay in combination with tuberculin skin test are insufficient for the diagnosis of culture-negative pulmonary tuberculosis. PLoS ONE 2014, 9, e107208. [Google Scholar] [CrossRef] [PubMed]
- Bapat, P.R.; Husain, A.A.; Daginawala, H.F.; Agrawal, N.P.; Panchbhai, M.S.; Satav, A.R.; Taori, G.M.; Kashyap, R.S. The assessment of cytokines in Quantiferon supernatants for the diagnosis of latent TB infection in a tribal population of Melghat, India. J. Infect. Public Health 2015, 8, 329–340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Radhakrishna, S.; Frieden, T.R.; Subramani, R. Association of initial tuberculin sensitivity, age and sex with the incidence of tuberculosis in south India: A 15-year follow-up. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2003, 7, 1083–1091. [Google Scholar] [CrossRef]
- Chu, C.Q.; Field, M.; Andrew, E.; Haskard, D.; Feldmann, M.; Maini, R.N. Detection of cytokines at the site of tuberculin-induced delayed-type hypersensitivity in man. Clin. Exp. Immunol. 1992, 90, 522–529. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Groups | ||
|---|---|---|---|
| TB | LTBI | HC | |
| N | 15 | 50 | 151 |
| Sex M/F | 5/10 | 24/26 | 89/62 |
| Ethnicity | Caucasian | Caucasian | Caucasian |
| Age | |||
| median | 15 | 8 | 7 |
| range | 1–17 | 1–17 | 1–17 |
| years (IQR) | 11–16 | 5–12 | 3–11 |
| BCG vaccination | 100% | 100% | 100% |
| QFT result, N (%) | |||
| positive | 12 (80%) | 50 (100%) | 0 (0%) |
| negative | 3 (20%) | 0 (0%) | 151 (100%) |
| TST result, N (%) | |||
| positive | 13 (87%) | 44 (88%) | 18 (12%) |
| negative | 2 (13%) | 6 (12%) | 133 (88%) |
| WBC, Counts/mm3 | 9770 | 8130 | 8550 |
| Neutrophils (%) | 60.7 | 43.8 | 40.1 |
| Lymphocytes (%) | 26.8 | 42.8 | 47.4 |
| Monocytes (%) | 9.2 | 7.8 | 8.1 |
| Eosinophils (%) | 2.6 | 4.9 | 3.8 |
| Basophils (%) | 0.5 | 0.5 | 0.6 |
| RBC, Counts/mm3 | 4,630,000 | 4,730,000 | 4,790,000 |
| HGB, g/dL | 13.1 | 13.3 | 13.2 |
| HCT, % | 38.9 | 39.5 | 39.2 |
| MCHC, g/dL | 33.6 | 33.8 | 33.7 |
| PLT, Counts/mm3 | 348,300 | 307,800 | 309,800 |
| Bilirubin (mg/dL) | 0.3 | 0.4 | 0.3 |
| ALT (U/L) | 12.1 | 19.7 | 15.6 |
| AST (U/L) | 18.9 | 25.7 | 27.5 |
| CRP (mg/L) | 41.9 | 0.7 | 0.8 |
| Proteins | Coefficients | p Value | Adjusted p Value | |
|---|---|---|---|---|
| HC vs. TB and LTBI (Jointly) | TB vs. LTBI | |||
| Th-17-Related Cytokines | ||||
| IL-21 | −1.83 × 102 | −1.09 × 102 | <0.001 | <0.001 |
| sCD40L | −3.33 × 103 | 6.92 × 102 | 0.003 | 0.025 |
| IL-10 | 38.3 | −8.04 | 0.034 | 0.173 |
| IL-6 | −2.67 × 102 | −1.07 × 102 | 0.052 | 0.196 |
| IL-31 | 1.09 × 103 | 5.58 × 102 | 0.148 | 0.444 |
| IL-17A | 60.6 | −4.14 | 0.177 | 0.444 |
| IL-23 | 1.15 × 103 | −7.61 × 102 | 0.253 | 0.543 |
| TNF-α | 3.59 × 102 | −42.5 | 0.349 | 0.640 |
| IL-33 | 3.67 × 102 | −30.0 | 0.427 | 0.640 |
| Inflammatory markers | ||||
| MMP-2 | 4.42 × 103 | −1.31 × 103 | <0.001 | 0.003 |
| IL-8 | −1.31 × 103 | 9.64 × 102 | 0.003 | 0.057 |
| sCD163 | −4.48 × 1010 | 4.48 × 1010 | 0.012 | 0.151 |
| LIGHT/TNFSF14 | −33.7 | 28.8 | 0.028 | 0.261 |
| IL-34 | −3.35 | −0.513 | 0.044 | 0.326 |
| chitinase 3-like 1 | −1.37 × 103 | 3.57 × 102 | 0.056 | 0.350 |
| sTNF-R1 | −4.84 × 102 | 1.06 × 102 | 0.075 | 0.401 |
| IL-12 (p70) | −1.93 | 1.87 | 0.111 | 0.455 |
| IFN-γ | 14.5 | −5.05 | 0.117 | 0.455 |
| OPN | −94.5 | −2.49 × 103 | 0.123 | 0.455 |
| Protein | Comparisons | |||
|---|---|---|---|---|
| 3-Class AUC | 2-Class AUC | |||
| HC vs. TB | HC vs. LTBI | LTBI vs. TB | ||
| Th-17-related cytokines | ||||
| IL-1β | 0.554 | 0.562 | 0.570 | 0.530 |
| IL-4 | 0.570 | 0.524 | 0.603 | 0.583 |
| IL-6 | 0.556 | 0.594 | 0.530 | 0.544 |
| IL-10 | 0.614 | 0.668 | 0.479 | 0.694 |
| IL-17A | 0.611 | 0.647 | 0.567 | 0.620 |
| IL-17F | 0.517 | 0.453 | 0.564 | 0.534 |
| IL-21 | 0.580 | 0.632 | 0.655 | 0.453 |
| IL-22 | 0.502 | 0.484 | 0.510 | 0.512 |
| IL-23 | 0.608 | 0.658 | 0.649 | 0.518 |
| IL-25 | 0.530 | 0.422 | 0.531 | 0.638 |
| IL-31 | 0.536 | 0.545 | 0.447 | 0.618 |
| IL-33 | 0.524 | 0.538 | 0.548 | 0.486 |
| IFN-γ | 0.559 | 0.584 | 0.534 | 0.558 |
| sCD40L | 0.615 | 0.676 | 0.605 | 0.565 |
| TNF-α | 0.563 | 0.587 | 0.505 | 0.598 |
| Inflammatory mediators | ||||
| APRIL/TNFSF13 | 0.519 | 0.529 | 0.516 | 0.512 |
| BAFF/TNFSF13B | 0.700 | 0.809 | 0.562 | 0.727 |
| sCD30/TNFRSF8 | 0.526 | 0.609 | 0.421 | 0.546 |
| sCD163 | 0.580 | 0.555 | 0.563 | 0.620 |
| chitinase 3-like 1 | 0.661 | 0.722 | 0.637 | 0.623 |
| gp130/sIL-6Rβ | 0.369 | 0.298 | 0.419 | 0.388 |
| IFN-α2 | 0.511 | 0.523 | 0.451 | 0.556 |
| IFN-β | 0.541 | 0.536 | 0.526 | 0.559 |
| IFN-γ | 0.580 | 0.654 | 0.621 | 0.463 |
| IL-2 | 0.518 | 0.522 | 0.536 | 0.494 |
| sIL-6Rα | 0.454 | 0.429 | 0.506 | 0.424 |
| IL-8 | 0.661 | 0.761 | 0.521 | 0.700 |
| IL-10 | 0.524 | 0.471 | 0.540 | 0.559 |
| IL-11 | 0.383 | 0.320 | 0.442 | 0.387 |
| IL-12 (p40) | 0.506 | 0.505 | 0.529 | 0.483 |
| IL-12 (p70) | 0.567 | 0.530 | 0.577 | 0.591 |
| IL-19 | 0.473 | 0.458 | 0.482 | 0.479 |
| IL-20 | 0.563 | 0.603 | 0.509 | 0.576 |
| IL-22 | 0.522 | 0.530 | 0.531 | 0.504 |
| IL-26 | 0.505 | 0.507 | 0.489 | 0.519 |
| IL-27 | 0.558 | 0.578 | 0.513 | 0.582 |
| IL-28A/IFN-λ2 | 0.530 | 0.546 | 0.497 | 0.544 |
| IL-29/IFN-λ1 | 0.529 | 0.470 | 0.590 | 0.526 |
| IL-32 | 0.581 | 0.552 | 0.577 | 0.613 |
| IL-34 | 0.636 | 0.580 | 0.722 | 0.603 |
| IL-35 | 0.570 | 0.570 | 0.600 | 0.539 |
| LIGHT/TNFSF14 | 0.674 | 0.736 | 0.661 | 0.626 |
| MMP-1 | 0.527 | 0.541 | 0.516 | 0.522 |
| MMP-2 | 0.758 | 0.848 | 0.721 | 0.701 |
| MMP-3 | 0.532 | 0.558 | 0.466 | 0.570 |
| Osteocalcin | 0.404 | 0.355 | 0.444 | 0.412 |
| OPN | 0.675 | 0.738 | 0.543 | 0.744 |
| PTX-3 | 0.539 | 0.664 | 0.565 | 0.387 |
| sTNF-R1 | 0.588 | 0.619 | 0.620 | 0.524 |
| sTNF-R2 | 0.606 | 0.692 | 0.480 | 0.645 |
| TSLP | 0.484 | 0.472 | 0.483 | 0.495 |
| TWEAK/TNFSF12 | 0.536 | 0.685 | 0.544 | 0.378 |
| Proteins | HC vs. LTBI | HC vs. TB | TB vs. LTBI |
|---|---|---|---|
| Th17-related cytokines | |||
| IL-1β | - | - | - |
| IL-4 | - | - | - |
| IL-6 | 3.87 × 10−4 | - | - |
| IL-10 | - | - | - |
| IL-17A | - | - | - |
| IL-17F | - | - | - |
| IL-21 | - | - | - |
| IL-22 | - | - | - |
| IL-23 | - | - | - |
| IL-25 | - | - | - |
| IL-31 | - | - | - |
| IL-33 | - | - | - |
| IFN-γ | - | - | - |
| sCD40L | - | - | - |
| TNF-α | - | - | - |
| Inflammatory mediators | |||
| April/TNFSF13 | 9.43 × 10−7 | 5.90 × 10−6 | 1.81 × 10−6 |
| BAFF/TNFSF13B | - | - | - |
| sCD30/TNFRSF8 | −7.60 × 10−4 | −1.36 × 10−3 | −1.07 × 10−3 |
| sCD163 | - | - | - |
| chitinase 3-like 1 | - | 5.41 × 10−4 | - |
| Gp130/sIL-6Rbeta | 3.67 × 10−5 | - | - |
| IFN-α2 | - | - | −1.45 × 10−1 |
| IFN-γ | - | - | −9.30 × 10−3 |
| IL-2 | −3.24 × 10−4 | - | 1.52 × 10−4 |
| sIL-6Rα | 4.41 × 10−5 | −4.18 × 10−4 | −3.25 × 10−4 |
| IL-8 | 2.94 × 10−4 | 1.60 × 10−4 | 2.41 × 10−4 |
| IL-10 | - | - | - |
| IL-11 | - | −1.13 × 10−2 | −3.68 × 10−3 |
| IL-12 (p40) | - | - | - |
| IL-12 (p70) | - | −4.71 | - |
| IL-19 | - | −1.05 × 10−1 | - |
| IL-27 | - | - | - |
| IL-28A/IFNλ2 | - | −1.45 × 10−4 | - |
| IL-29/IFNλ1 | 2.18 × 10−2 | - | −3.50 × 10−2 |
| IL-32 | - | - | - |
| IL-34 | - | - | - |
| IL-35 | 4.26 × 10−3 | - | - |
| LIGHT/TNFSF14 | - | 6.50 × 10−2 | 7.13 × 10−3 |
| MMP-1 | - | 7.36 × 10−5 | 6.44 × 10−5 |
| MMP-2 | −1.51 × 10−4 | −4.09 × 10−4 | −3.55 × 10−4 |
| MMP-3 | −8.31 × 10−5 | 3.72 × 10−3 | 4.46 × 10−3 |
| osteocalcin | - | - | 6.23 × 10−7 |
| OPN | 6.75 × 10−5 | −5.10 × 10−4 | −5.81 × 10−5 |
| PTX-3 | −2.44 × 10−4 | −1.44 × 10−4 | - |
| sTNFR1 | - | - | - |
| sTNFR2 | −1.70 × 10−4 | - | - |
| TSLP | - | - | −1.28 × 10−2 |
| TWEAK/TNFSF12 | −1.93 × 10−3 | −1.37 × 10−2 | −6.15 × 10−3 |
| Th17-Related Cytokines | Coefficient | Inflammatory Mediators | Coefficient |
|---|---|---|---|
| IL-1β | - | April/TNFSF13 | 1.37 × 10−7 |
| IL-4 | −3.21 × 10−5 | BAFF/TNFSF13B | - |
| IL-6 | 2.68 × 10−4 | sCD30/TNFRSF8 | −6.09 × 10−4 |
| IL-10 | - | sCD163 | - |
| IL-17A | - | chitinase 3-like 1 | - |
| IL-17F | - | gp130/sIL-6Rβ | −3.57 × 10−6 |
| IL-21 | - | IFN-α2 | - |
| IL-22 | - | IFN-γ | - |
| IL-23 | - | IL-2 | - |
| IL-25 | - | sIL-6Rα | - |
| IL-31 | −1.57 × 10−5 | IL-8 | 3.22 × 10−5 |
| IL-33 | - | IL-10 | 1.02 × 10−3 |
| IFN-γ | - | IL-11 | - |
| sCD40L | 3.11 × 10−6 | IL-12 (p40) | - |
| TNF-α | −9.67 × 10−5 | IL-12 (p70) | - |
| IL-19 | - | ||
| IL-27 | - | ||
| IL-28A/IFNλ2 | - | ||
| IL-29/IFNλ1 | 6.86 × 10−4 | ||
| IL-32 | - | ||
| IL-34 | - | ||
| IL-35 | 1.68 × 10−3 | ||
| LIGHT/TNFSF14 | 1.29 × 10−4 | ||
| MMP-1 | - | ||
| MMP-2 | −7.76 × 10−5 | ||
| MMP-3 | - | ||
| osteocalcin | - | ||
| OPN | 7.65 × 10−6 | ||
| PTX-3 | - | ||
| sTNFR1 | - | ||
| sTNFR2 | −1.77 × 10−4 | ||
| TSLP | - | ||
| TWEAK/TNFSF12 | −6.58 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Druszczynska, M.; Seweryn, M.; Wawrocki, S.; Kowalewska-Pietrzak, M.; Pankowska, A.; Rudnicka, W. Cytokine Biosignature of Active and Latent Mycobacterium Tuberculosis Infection in Children. Pathogens 2021, 10, 517. https://doi.org/10.3390/pathogens10050517
Druszczynska M, Seweryn M, Wawrocki S, Kowalewska-Pietrzak M, Pankowska A, Rudnicka W. Cytokine Biosignature of Active and Latent Mycobacterium Tuberculosis Infection in Children. Pathogens. 2021; 10(5):517. https://doi.org/10.3390/pathogens10050517
Chicago/Turabian StyleDruszczynska, Magdalena, Michal Seweryn, Sebastian Wawrocki, Magdalena Kowalewska-Pietrzak, Anna Pankowska, and Wieslawa Rudnicka. 2021. "Cytokine Biosignature of Active and Latent Mycobacterium Tuberculosis Infection in Children" Pathogens 10, no. 5: 517. https://doi.org/10.3390/pathogens10050517
APA StyleDruszczynska, M., Seweryn, M., Wawrocki, S., Kowalewska-Pietrzak, M., Pankowska, A., & Rudnicka, W. (2021). Cytokine Biosignature of Active and Latent Mycobacterium Tuberculosis Infection in Children. Pathogens, 10(5), 517. https://doi.org/10.3390/pathogens10050517








