Antimalarial Effect of the Total Glycosides of the Medicinal Plant, Ranunculus japonicus
Abstract
:1. Introduction
2. Results
2.1. Antimalarial Impact and Cytotoxicity of the Ranunculus japonicus Extract in Studies Performed In Vitro
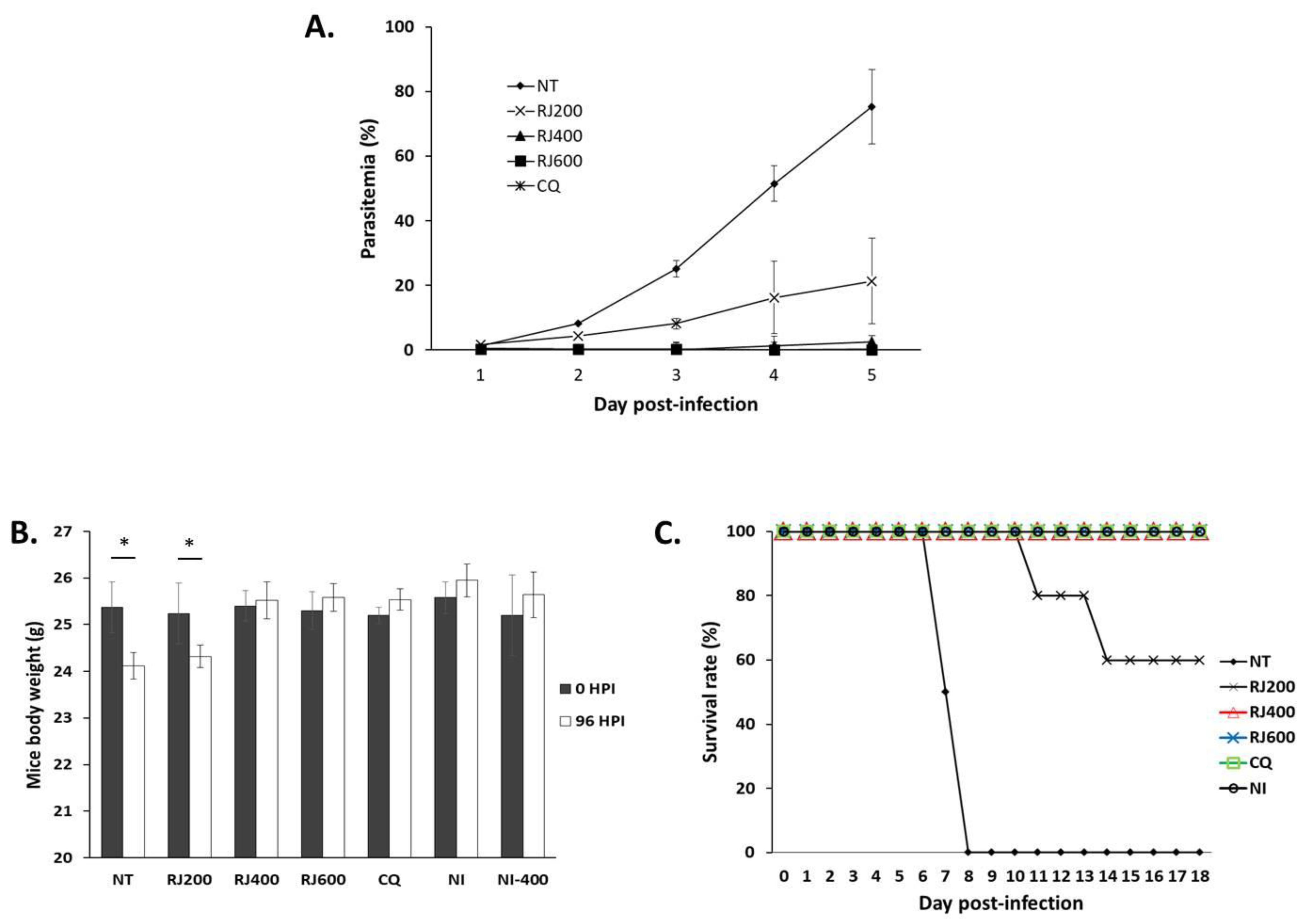
2.2. Antimalarial Impact of the R. japonicus Extract Used to Treat Mice Infected with Plasmodium berghei
2.3. Impact of R. japonicus Extract on Hepatic Function of P. berghei-Infected Mice
2.4. Impact of the R. japonicus Extract on Renal Function of P. berghei-Infected Mice
2.5. Impact of R. japonicus Extract on Hematologic Parameters of P. berghei-Infected Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of Herb Extracts
4.2. Cell Cytotoxicity Assay
4.3. In Vitro Testing Against Plasmodium falciparum 3D7 and Dd2 Strains
4.4. Selective Index
4.5. In Vivo Testing Against the Plasmodium berghei ANKA Strain in Infected Mice
4.6. Blood Sample Preparation
4.7. Liver and Kidney Toxicity
4.8. Hematological Parameters
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, Z.W. Compilation of the National Chinese Herbal Medicine; People’s Health Publishing House: Beijing, China, 1996; p. 201. [Google Scholar]
- Wang, R.L.; Tan, Y.Z. Effects of ranunculin on angiotensin II-induced myocardial hypertrophy. J. Guangdong Coll. Pharm. 2008, 24, 154–156. [Google Scholar]
- Wang, R.L.; Tan, Y.Z.; Luo, S.B. Anti-inflammatory and Analgesic Effect of Total Glycosides of Ranunculus japonicus. Lishizhen Med. Mater. Med. Res. 2009, 20, 290–292. [Google Scholar]
- Centers for Disease Control and Prevention: CDC’s Malaria Program. 2021. Available online: https://www.cdc.gov/malaria/resources/pdf/fsp/cdc_malaria_program_2021.pdf (accessed on 3 March 2021).
- World Health Organization. World Malaria Report 2020. 2020. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020 (accessed on 10 January 2021).
- Autino, B.; Noris, A.; Russo, R.; Castelli, F. Epidemiology of malaria in endemic areas. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012060. [Google Scholar] [CrossRef] [PubMed]
- Tse, E.G.; Korsik, M.; Todd, M.H. The past, present and future of anti-malarial medicines. Malar. J. 2019, 18, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arya, A.; Foko, L.P.K.; Chaudhry, S.; Sharma, A.; Singh, V. Artemisinin-based combination therapy (ACT) and drug resistance molecular markers: A systemic review of clinical studies from two malaria endemic regions–India and Sub-Saharan Africa. Int. J. Parasitol. Drug Resist 2020, 15, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Singh, S. A brief History of Quinoline as Antimalarial Agents. Int. J. Pharm. Sci. Rev. Res. 2014, 50, 295–302. [Google Scholar]
- O’Neill, P.M.; Barton, V.E.; Ward, S.A.; Chadwick, J. Treatment and Prevention of Malaria: Antimalarial Drug Chemistry, Action and Use; Springer Science and Business Media: Basel, Switzerland, 2012. [Google Scholar]
- Hailemeskel, E.; Kassa, M.; Taddesse, G.; Mohammed, H.; Woyessa, A.; Tasew, G.; Sleshi, M.; Kebede, A.; Petros, B. Prevalence of sulfadoxine-pyrimethamine resistance-associated mutations in dhfr and dhps genes of Plasmodium falciparum three years after SP withdrawal in Bahir Dar, Northwest Ethiopia. Acta Trop. 2013, 128, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Vanshika, L.; Manoj, K.D.; Neeru, S.; Vas, D.; Wajihullah, K.; Yagya, D.S. Multiple Origins of Plasmodium falciparum Dihydropteroate Synthetase Mutant Alleles Associated with Sulfadoxine Resistance in India. Antimicrob. Agents Chemother. 2011, 55, 2813–2817. [Google Scholar]
- Adan, I.; Ibrahim, Y.; Gasim, G.I. Efficacy and safety of artemisinin-based combination therapy for umcomplicated Plasmodium falciparum malaria in Sudan: A systematic review and meta-analysis. Malar. J. 2018, 17, 110. [Google Scholar] [CrossRef]
- World Health Organization. Status Report on Artemisinin and ACT Resistance. 2017. Available online: https://www.who.int/malaria/publications/atoz/artemisinin-resistance-april2017/en/ (accessed on 4 January 2021).
- Noronha, M.; Pawar, V.; Prajapati, A.; Subramanian, R.B. A literature review on traditional herbal medicines for malaria. S. Afr. J. Bot. 2020, 128, 292–303. [Google Scholar] [CrossRef]
- David, A.F.; Philip, J.R.; Simon, L.C.; Reto, B.; Solomon, N. Antimalarial drug discovery: Efficiency models for compound screening. Nat. Rev. 2004, 3, 509–520. [Google Scholar]
- Krafts, K.; Hempelmann, E.; Skórska-Stania, A. From methylene blue to chloroquine: A brief review of the development of an antimalarial therapy. Parasitol. Res. 2012, 111, 1–6. [Google Scholar] [CrossRef]
- Cui, L.; Su, X. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev. Anti Infect. Ther. 2007, 7, 999–1013. [Google Scholar] [CrossRef]
- Carvalho, L.H.; Brandão, M.G.; Santos-Filho, D.; Lopes, J.L.; Krettli, A.U. Antimalarial activity of crude extracts from Brazilian plants studied in vivo in Plasmodium berghei-infected mice and in vitro against Plasmodium falciparum in culture. Braz. J. Med. Biol. Res. 1991, 24, 1113–1123. [Google Scholar]
- Willcox, M.; Bodeker, G.; Rasoanaivo, P.; Addae-Kyereme, J. Traditional Medicinal Plants and Malaria, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 255–270. [Google Scholar]
- Ramazani, A.; Zakeri, S.; Sardari, S.; Khodakarim, N.; Djadidt, N.D. In vitro and in vivo anti-malarial activity of Boerhavia elegans and Solanum surattense. Malar. J. 2010, 9, 124. [Google Scholar] [CrossRef] [Green Version]
- Woodford, J.; Shanks, G.D.; Griffin, P.; Chalon, S.; McCarthy, J.S. The Dynamics of liver function test abnormalities after malaria infection: A retrospective observation study. Am. J. Trop. Med. Hyg. 2018, 98, 1113–1119. [Google Scholar] [CrossRef]
- Brown, D.D.; Solomon, S.; Lerner, D.; Rio, M.D. Malaria and acute kidney injury. Pediatr. Nephrol. 2020, 35, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Bantie, L.; Assefa, S.; Teklehaimanot, T.; Engidawork, E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hocsht. (Euphorbiaceae) against Plasmodium berghei in mice. BMC Complement Altern. Med. 2014, 14, 79. [Google Scholar] [CrossRef]
- Viriyavejakul, P.; Khachonsaksumet, V.; Punsawad, C. Liver changes in severe Plasmodium falciparum malaria: Histopathology, apoptosis and nuclear factor kappa B expression. Malar. J. 2014, 13, 106. [Google Scholar] [CrossRef] [Green Version]
- Das, B.S. Renal failure in malaria. J. Vector Borne Dis. 2008, 45, 83–97. [Google Scholar]
- Abro, A.H.; Ustadi, A.M.; Younis, N.J.; Abdou, A.S.; Hamed, D.A.; Saleh, A.A. Malaria and hematological changes. Pak. J. Med. Sci. 2008, 24, 287–291. [Google Scholar]
- Sirak, S.; Fola, A.A.; Worku, L.; Biadgo, B. Malaria parasitemia and its association with lipid and hematological parameters among malaria-infected patients attending at Metema Hospital, Northwest Ethiopia. Pathol. Lab. Med. Int. 2016, 8, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Pittman, R.N. Regulation of Tissue Oxygenation: Chapter 4 Oxygen Transport San Rafael (CA). Morgan Claypool Life Sci. 2011, 3, 1–100. [Google Scholar]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.H.; Su, X. Artemisinin: Discovery from the Chinese Herbal Garden. Cell 2011, 146, 855–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.P.S.; Molino, S.; Sikora, J.; Rasmussen, S.; Rybova, J.; Tate, E.; Geurts, A.M.; Turner, P.V.; Mckillop, W.M.; Medin, J.A. Hepatic pathology and altered gene transcription in a murine model of acid ceramidase deficiency. Lab. Investig. 2019, 99, 1572–1592. [Google Scholar] [CrossRef]
| Compound | IC50 in Pf3D7 (μg/mL) | IC50 in PfDd2 (μg/mL) | Cytotoxicity in HFF (μg/mL) | SI (HFF/Pf3D7) | SI (HFF/PfDd2) |
|---|---|---|---|---|---|
| Ranunculus japonicus | 6.29 ± 2.78 | 5.36 ± 4.93 | 845.89 ± 13.28 | 134.48 | 157.82 |
| Chloroquine | 0.005 ± 0.001 | 0.40 ± 0.02 | >45 | ||
| Artemisinin | 0.002 ± 0.0009 | >70 |
| AST (U/L) | ALT (U/L) | Total Bilirubin (mg/dL) | Indirect Bilirubin (mg/dL) | Direct Bilirubin (mg/dL) | |
|---|---|---|---|---|---|
| NT | 61.28 ± 2.70 | 35.89 ± 2.81 | 1.42 ± 0.48 | 0.78 ± 0.23 | 0.19 ± 0.02 |
| RJ200 | 54.42 ± 3.12 | 28.56 ± 2.85 | 0.91 ± 0.05 | 0.31 ± 0.09 * | 0.42 ± 0.08 |
| RJ400 | 50.12 ± 3.77 * | 25.23 ± 1.91 * | 0.82 ± 0.01 * | 0.19 ± 0.02 * | 0.74 ± 0.05 * |
| RJ600 | 50.13 ± 2.56 * | 25.31 ± 3.20 | 0.87 ± 0.01 * | 0.19 ± 0.08 * | 0.75 ± 0.04 * |
| CQ | 50.18 ± 2.06 * | 23.13 ± 2.53 * | 0.89 ± 0.45 * | 0.19 ± 0.09 * | 0.77 ± 0.01 * |
| NI | 50.13 ± 1.78 * | 23.01 ± 1.42 * | 0.77 ± 0.13 * | 0.16 ± 0.07 * | 0.68 ± 0.03 * |
| NI400 | 50.31 ± 2.24 * | 23.21 ± 0.87 * | 0.75 ± 0.15 * | 0.17 ± 0.01 * | 0.66 ± 0.02 * |
| Urea (mg/dL) | Creatinine (mg/dL) | |
|---|---|---|
| NT | 68.87 ± 5.24 | 3.47 ± 0.55 |
| RJ200 | 41.13 ± 4.25 * | 1.53 ± 0.16 * |
| RJ400 | 33.45 ± 4.24 * | 1.21 ± 0.12 * |
| RJ600 | 33.63 ± 2.13 * | 1.21 ± 0.08 * |
| CQ | 33.24 ± 2.25 * | 1.18 ± 0.21 * |
| NI | 31.348 ± 1.87 * | 1.29 ± 0.19 * |
| NI400 | 33.16 ± 2.84 * | 1.27 ± 0.32 * |
| Number of Erythrocytes (106/µL) | Hematocrit (%) | Hemoglobin (g/dL) | Platelets (103/µL) | |
|---|---|---|---|---|
| NT | 3.19 ± 0.83 | 28.89 ± 5.42 | 7.45 ± 1.98 | 320.24 ± 27.24 |
| RJ200 | 4.89 ± 2.83 | 33.98 ± 10.35 | 11.24 ± 1.35 | 543.13 ± 89.13 |
| RJ400 | 9.45 ± 0.89 * | 43.13 ± 5.98 * | 14.23 ± 1.93 * | 730.55 ± 32.13 * |
| RJ600 | 9.55 ± 1.42 * | 43.13 ± 1.46 * | 14.45 ± 0.98 * | 763.15 ± 29.23 * |
| CQ | 9.79 ± 0.21 * | 47.22 ± 0.53 * | 14.98 ± 0.35 * | 793.53 ± 24.88 * |
| NI | 9.29 ± 0.83 * | 45.76 ± 3.13 * | 14.66 ± 0.13 * | 734.13 ± 19.29 * |
| NI400 | 9.32 ± 0.99 * | 48.13 ± 2.13 * | 14.77 ± 0.01 * | 766.75 ± 24.21 * |
| Number of Leukocytes (103/µL) | Lymphocytes (103/µL) | Monocytes (103/µL) | Segmented Neutrophils (103/µL) | |
|---|---|---|---|---|
| NT | 130.24 ± 14.12 | 84.13 ± 6.29 | 4.13 ± 0.23 | 20.99 ± 7.13 |
| RJ200 | 43.13 ± 13.83 | 33.12 ± 8.13 | 2.12 ± 2.21 | 14.13 ± 3.23 |
| RJ400 | 5.24 ± 0.48 * | 3.24 ± 0.13 * | 0.29 ± 0.11 * | 1.39 ± 0.47 * |
| RJ600 | 5.35 ± 0.97 * | 3.21 ± 0.11 * | 0.25 ± 0.27 * | 1.49 ± 0.14 * |
| CQ | 5.21 ± 0.84 * | 3.11 ± 0.83 * | 0.25 ± 0.03 * | 1.52 ± 0.89 * |
| NI | 5.42 ± 0.71 * | 3.33 ± 0.28 * | 0.34 ± 0.01 *. | 1.74 ± 0.88 * |
| NI400 | 5.27 ± 0.21 * | 3.46 ± 0.12 * | 0.34 ± 0.01 * | 1.71 ± 0.09 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.-S.; Dinzouna-Boutamba, S.-D.; Lee, S.; Moon, Z.; Kwak, D.; Rhee, M.-H.; Chung, D.-I.; Hong, Y.; Goo, Y.-K. Antimalarial Effect of the Total Glycosides of the Medicinal Plant, Ranunculus japonicus. Pathogens 2021, 10, 532. https://doi.org/10.3390/pathogens10050532
Yun H-S, Dinzouna-Boutamba S-D, Lee S, Moon Z, Kwak D, Rhee M-H, Chung D-I, Hong Y, Goo Y-K. Antimalarial Effect of the Total Glycosides of the Medicinal Plant, Ranunculus japonicus. Pathogens. 2021; 10(5):532. https://doi.org/10.3390/pathogens10050532
Chicago/Turabian StyleYun, Hae-Soo, Sylvatrie-Danne Dinzouna-Boutamba, Sanghyun Lee, Zin Moon, Dongmi Kwak, Man-Hee Rhee, Dong-Il Chung, Yeonchul Hong, and Youn-Kyoung Goo. 2021. "Antimalarial Effect of the Total Glycosides of the Medicinal Plant, Ranunculus japonicus" Pathogens 10, no. 5: 532. https://doi.org/10.3390/pathogens10050532







