Chicken Heat Shock Protein 70 Is an Essential Host Protein for Infectious Bursal Disease Virus Infection In Vitro
Abstract
1. Introduction
2. Results
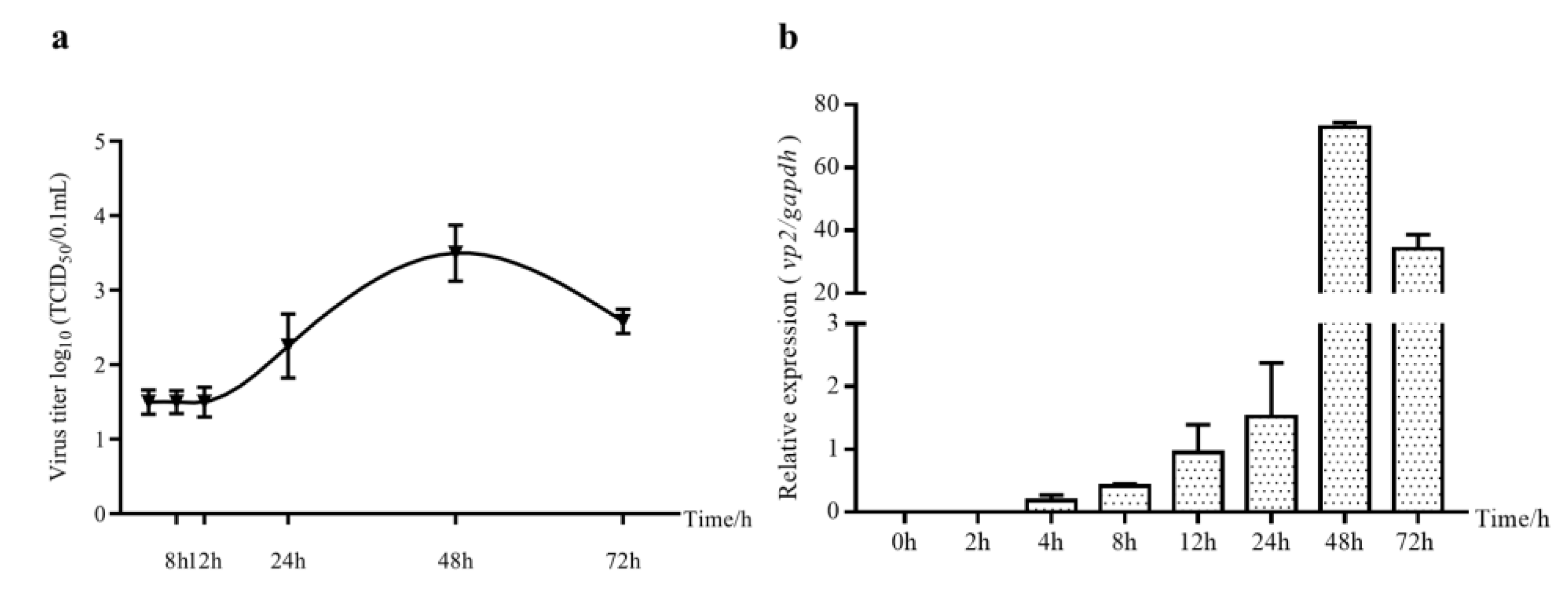
2.1. Viral Load Changes in IBDV-Infected DF-1 Cells
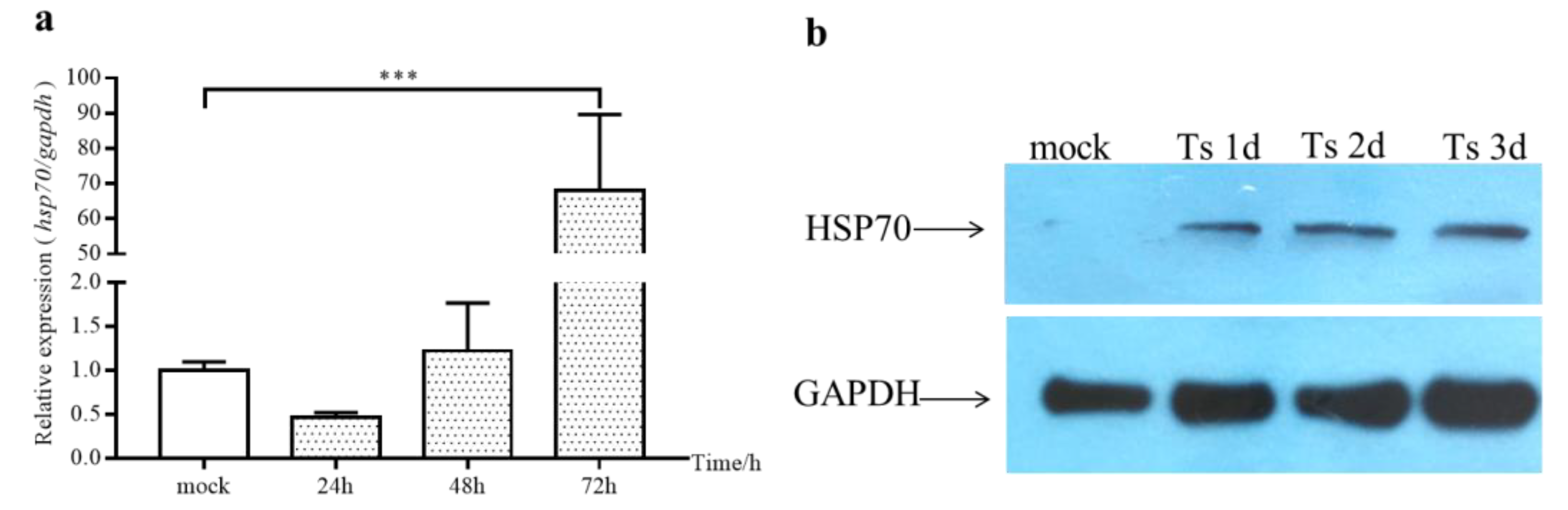
2.2. The Expression of cHsp70 Gradually Increased during IBDV Infection
2.3. cHsp70 has Direct Interaction with IBDV in DF-1 Cells
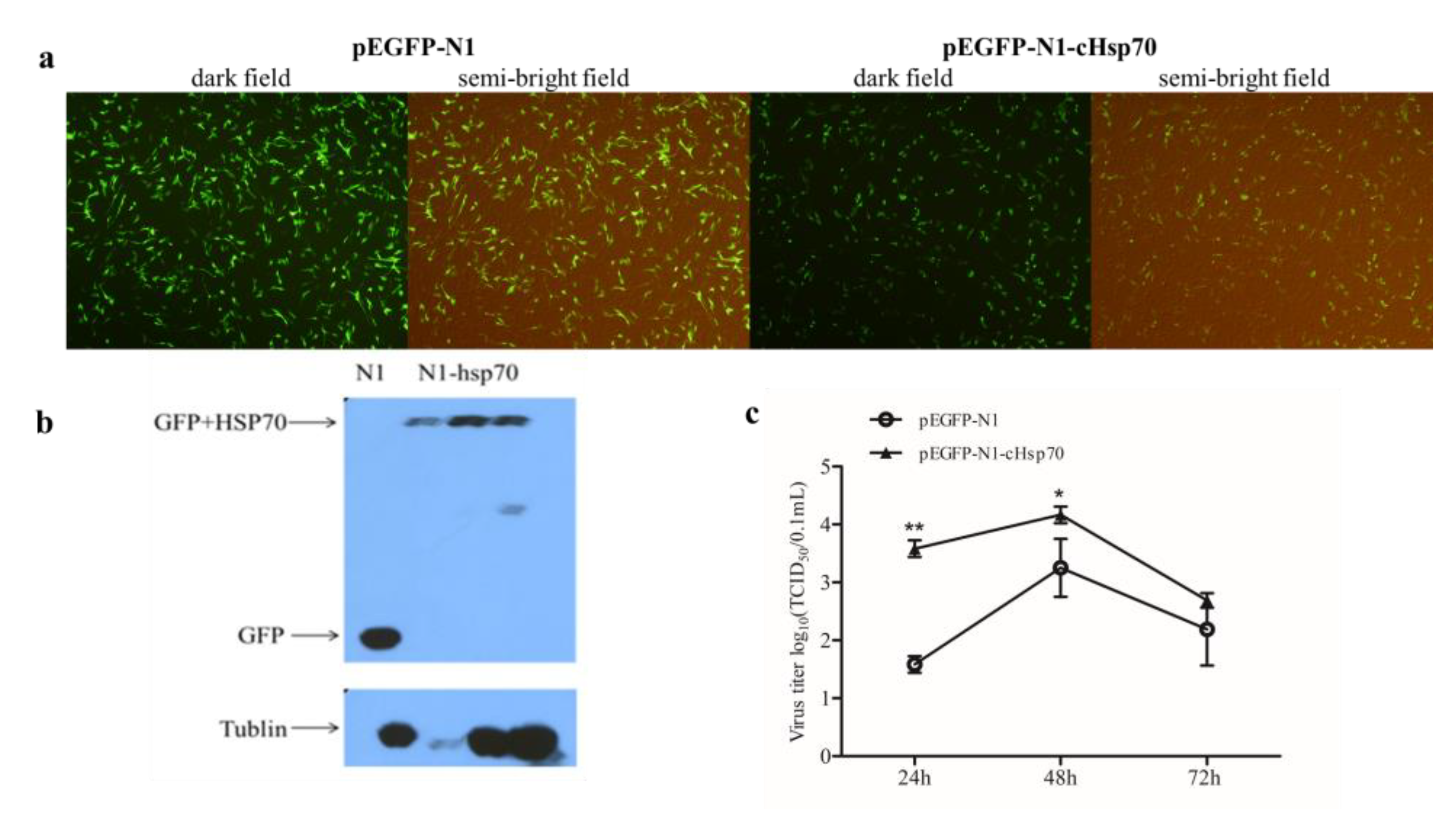
2.4. Overexpression of cHsp70 Promotes the Production of IBDV
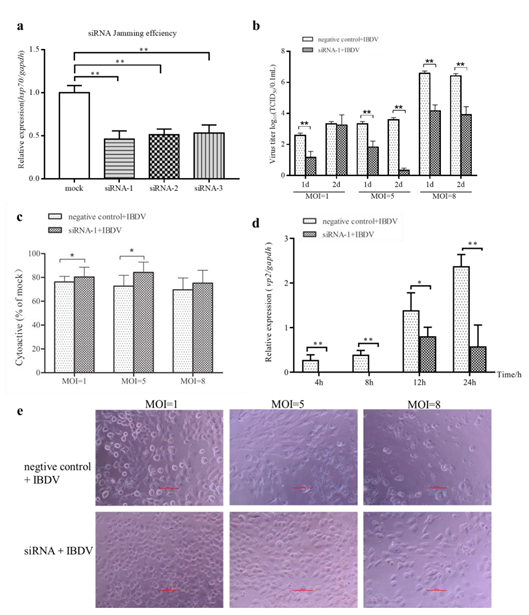
2.5. Inhibition of cHsp70 by siRNA Attenuates the Production of IBDV
3. Discussion
4. Materials and Methods
4.1. Cells and Viruses
4.2. Chemicals, Reagents, Antibodies and Kits
4.3. Determination of 50% Tissue Culture Infectious Dose (TCID50) by IFA
4.4. Location Detection by IFA
4.5. Co-Immunoprecipitation (Co-IP) Assay
4.6. Western Blot
4.7. Overexpression of cHsp70 in DF-1 Cells
4.8. siRNA Interference
4.9. Cell Activity Detection by CCK8 Assay
4.10. qRT-PCR Analysis of Gene Expression
4.11. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luque, D.; Rivas, G.; Alfonso, C.; Carrascosa, J.L.; Rodríguez, J.F.; Castón, J.R. Infectious bursal disease virus is an icosahedral polyploid dsRNA virus. Proc. Natl. Acad. Sci. USA 2009, 106, 2148–2152. [Google Scholar] [CrossRef] [PubMed]
- McFerran, J.B.; McNulty, M.S.; McKillop, E.R.; Connor, T.J.; McCracken, R.M.; Collins, D.S.; Allan, G.M. Isolation and serological studies with infectious bursal disease viruses from fowl, turkeys and ducks: Demonstration of a second serotype. Avian Pathol. 1980, 9, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, D.J.; Saif, Y.M.; Hughes, J.H. Characteristics and serologic studies of two serotypes of infectious bursal disease virus in turkeys. Avian Dis. 1982, 26, 871–882. [Google Scholar] [CrossRef]
- McNulty, M.S.; Saif, Y.M. Antigenic relationship of non-serotype 1 turkey infectious bursal disease viruses from the United States and United Kingdom. Avian Dis. 1988, 32, 374–375. [Google Scholar] [CrossRef]
- Laura, B.; Nicolás, R.; Fernando, M.; Elisabet, D.-B.; Oscar, C.-R.; Daniel, F.; Liliana, L.C.-G.; Céline, C.; Nicolas, E.; Sébastien, M.S.; et al. Type I interferon acts as a major barrier to the establishment of persistent infectious bursal disease virus infections. J. Virol. 2021, 95, e2017–e2020. [Google Scholar]
- Van den Berg, T.P. Acute infectious bursal disease in poultry: A review. Avian Pathol. 2000, 29, 175–194. [Google Scholar] [CrossRef]
- Eleuterio, L.; Antonio, M.; José, R.C.; José, R.; Armando, F.-A.; Antonio, S.; José, L.C.; José, F.R. VP1, the putative RNA-dependent RNA polymerase of infectious bursal disease virus, forms complexes with the capsid protein VP3, leading to efficient encapsidation into virus-like particles. J. Virol. 1999, 73, 6973–6983. [Google Scholar]
- Noman, A.; Aqeel, M.; Khalid, N.; Hashem, M.; Alamari, S.; Zafar, S.; Qasim, M.; Irshad, M.K.; Qari, S.H. Spike glycoproteins: Their significance for corona viruses and receptor binding activities for pathogenesis and viral survival. Microb. Pathog. 2021, 150, 104719. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.L.; Zhou, M.; Arvin, A.M. Varicella-zoster virus: Molecular controls of cell fusion-dependent pathogenesis. Biochem. Soc. Trans. 2020, 48, 2415–2434. [Google Scholar] [CrossRef]
- Lin, T.W.; Lo, C.W.; Lai, S.Y.; Fan, R.J.; Lo, C.J.; Chou, Y.M.; Thiruvengadam, R.; Wang, A.H.J.; Wang, M.Y. Chicken heat shock protein 90 is a component of the putative cellular receptor complex of infectious bursal disease virus. J. Virol. 2007, 81, 8730–8741. [Google Scholar] [CrossRef]
- Hung, J.-J.; Chung, C.-S.; Chang, W. Molecular chaperone Hsp90 is important for vaccinia virus growth in cells. J. Virol. 2002, 76, 1379–1390. [Google Scholar] [CrossRef]
- María, C.G.; Mariam, I.; Javal, S.; María, I.C.; Mauricio, R.T.; Laura, R.D. Phosphatidylinositol 3-phosphate mediates the establishment of infectious bursal disease virus replication complexes in association with early endosomes. J. Virol. 2021, 95, e2313–e2320. [Google Scholar]
- Yu, Y.; Xu, Z.; Liu, Y.; Zhang, H.; Ou, C.; Zhang, Y.; Liu, T.; Wang, Q.; Ma, J. Effects of infectious bursal disease virus infection on interferon and antiviral gene expression in layer chicken bursa. Microb. Pathog. 2020, 144, 104182. [Google Scholar] [CrossRef]
- Manzoor, R.; Kuroda, K.; Yoshida, R.; Tsuda, Y.; Fujikura, D.; Miyamoto, H.; Kajihara, M.; Kida, H.; Takada, A. Heat shock protein 70 modulates influenza A virus polymerase activity. J. Biol. Chem. 2014, 289, 7599–7614. [Google Scholar] [CrossRef]
- Janewanthanakul, S.; Supungul, P.; Tang, S.; Tassanakajon, A. Heat shock protein 70 from Litopenaeus vannamei (LvHSP70) is involved in the innate immune response against white spot syndrome virus (WSSV) infection. Dev. Comp. Immunol. 2020, 102, 103476. [Google Scholar] [CrossRef]
- Gao, J.; Xiao, S.; Liu, X.; Wang, L.; Ji, Q.; Mo, D.; Chen, Y. Inhibition of HSP70 reduces porcine reproductive and respiratory syndrome virus replication in vitro. BMC Microbiol. 2014, 14, 64. [Google Scholar] [CrossRef]
- Wang, H.; Bu, L.; Wang, C.; Zhang, Y.; Zhou, H.; Zhang, X.; Guo, W.; Long, C.; Guo, D.; Sun, X. The Hsp70 inhibitor 2-phenylethynesulfonamide inhibits replication and carcinogenicity of Epstein–Barr virus by inhibiting the molecular chaperone function of Hsp70. Cell Death Dis. 2018, 9, 734. [Google Scholar] [CrossRef]
- Khachatoorian, R.; Cohn, W.; Buzzanco, A.; Riahi, R.; Arumugaswami, V.; Dasgupta, A.; Whitelegge, J.P.; French, S.W. HSP70 copurifies with Zika virus particles. Virology 2018, 522, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Taguwa, S.; Yeh, M.T.; Rainbolt, T.K.; Nayak, A.; Shao, H.; Gestwicki, J.E.; Andino, R.; Frydman, J. Zika virus dependence on host Hsp70 provides a protective strategy against infection and disease. Cell Rep. 2019, 26, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Khachatoorian, R.; Riahi, R.; Ganapathy, E.; Shao, H.; Wheatley, N.M.; Sundberg, C.; Jung, C.L.; Ruchala, P.; Dasgupta, A.; Arumugaswami, V.; et al. Allosteric heat shock protein 70 inhibitors block hepatitis C virus assembly. Int. J. Antimicrob. Agents 2016, 47, 289–296. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, X.; Xia, X.; Sun, H. Inhibition of infectious bursal disease virus infection by artificial microRNAs targeting chicken heat-shock protein 90. J. Gen. Virol. 2012, 93, 876–879. [Google Scholar] [CrossRef]
- Liew, P.K.; Zulkifli, I.; Hair-Bejo, M.; Omar, A.R.; Israf, D.A. Effects of early age feed restriction and heat conditioning on heat shock protein 70 expression, resistance to infectious bursal disease, and growth in male broiler chickens subjected to heat stress. Poult. Sci. 2003, 82, 1879–1885. [Google Scholar] [CrossRef]
- Hemanta, K.M.; Sohini, D.; Madhan, C.M.; Sagar, A.K.; Dinesh, C.P.; Vikram, N.V. Protective efficacy of a DNA vaccine construct encoding the VP2 gene of infectious bursal disease and a truncated Hsp70 of Mycobacterium tuberculosis in chickens. Vaccine 2015, 33, 1033–1039. [Google Scholar]
- Chen, C.; Qin, Y.; Qian, K.; Shao, H.; Ye, J.; Qin, A. HSC70 is required for infectious bursal disease virus (IBDV) infection in DF-1 cells. Virol. J. 2020, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Himly, M.; Foster, D.N.; Bottoli, I.; Iacovoni, J.S.; Vogt, P.K. The DF-1 chicken fibroblast cell line: Transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology 1998, 248, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Rui, L.; Shao, Q.; Liu, H.; Lu, Y.; Zhang, Y.; Li, Z. Changes of CD4+CD25+ cells ratio in immune organs from chickens challenged with infectious bursal disease virus strains with varying virulences. Viruses 2015, 7, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Lahaye, X.; Vidy, A.; Fouquet, B.; Blondel, D. Hsp70 protein positively regulates rabies virus infection. J. Virol. 2012, 86, 4743–4751. [Google Scholar] [CrossRef]
- Liu, J.; Bai, J.; Zhang, L.; Jiang, Z.; Wang, X.; Li, Y.; Jiang, P. Hsp70 positively regulates porcine circovirus type 2 replication in vitro. Virology 2013, 447, 52–62. [Google Scholar] [CrossRef]
- Broquet, A.H.; Lenoir, C.; Gardet, A.; Sapin, C.; Chwetzoff, S.; Jouniaux, A.M.; Lopez, S.; Trugnan, G.; Bachelet, M.; Thomas, G. Hsp70 negatively controls rotavirus protein bioavailability in Caco-2 cells infected by the rotavirus RF strain. J. Virol. 2007, 81, 1297–1304. [Google Scholar] [CrossRef]
- Rodenberg, J.; Sharma, J.M.; Belzer, S.W.; Nordgren, R.M.; Naqi, S. Flow cytometric analysis of B cell and T cell subpopulations in specific-pathogen-free chickens infected with infectious bursal disease virus. Avian Dis. 1994, 38, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Westaway, E.G.; Khromykh, A.A.; Mackenzie, J.M. Nascent flavivirus RNA colocalized in situ with double-stranded RNA in stable replication complexes. Virology 1999, 258, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Targett-Adams, P.; Boulant, S.; McLauchlan, J. Visualization of doublestranded RNA in cells supporting hepatitis C virus RNA replication. J. Virol. 2008, 82, 2182–2195. [Google Scholar] [CrossRef] [PubMed]
- Beura, L.K.; Dinh, P.X.; Osorio, F.A.; Pattnaik, A.K. Cellular poly (c) binding proteins 1 and 2 interact with porcine reproductive and respiratory syndrome virus nonstructural protein 1β and support viral replication. J. Virol. 2011, 85, 12939–12949. [Google Scholar] [CrossRef] [PubMed]

| Name. | Direction | Sequence |
|---|---|---|
| pEGFP-N1-cHsp70 | Forward | ATT CTG CAG TCG ACG GTA C ATGTCTGGCAAAGGGCCG |
| Reverse | GAC CGG TGG ATC CCG GGCATCTACTTCTTCAATGGTTG |
| Name | Direction | Sequence |
|---|---|---|
| siRNA-1 | Forward | CCCGCUUACUUCAACGACUTT |
| Reverse | AGUCGUUGAAGUAAGCGGGTT | |
| siRNA-2 | Forward | GCGUGACAAUGCUGGCAAUTT |
| Reverse | AUUGCCAGCAUUGUCACGCTT | |
| siRNA-3 | Forward | GCAAGCCAGCAUUGAGAUUTT |
| Reverse | AAUCUCAAUGCUGGCUUGCTT |
| Gene | Direction | Sequence |
|---|---|---|
| cHsp70 | Forward | TGTTATCACAGTGCCCGCTTAC |
| Reverse | CACGTTAAGGCCAGTGATGG | |
| cGAPDH a | Forward | CTCTGCCCCCTCTGCTGAT |
| Reverse | CAGGAGGCATTGCTGATGATC | |
| TS b | Forward | ACCGGCACCGACAACCTTA |
| Reverse | CCCTGCCTGACCACCACTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Yu, X.; You, C.; Zhang, W.; Sun, Y.; Li, L.; Jin, T.; Pan, P.; Xie, A. Chicken Heat Shock Protein 70 Is an Essential Host Protein for Infectious Bursal Disease Virus Infection In Vitro. Pathogens 2021, 10, 664. https://doi.org/10.3390/pathogens10060664
Meng Y, Yu X, You C, Zhang W, Sun Y, Li L, Jin T, Pan P, Xie A. Chicken Heat Shock Protein 70 Is an Essential Host Protein for Infectious Bursal Disease Virus Infection In Vitro. Pathogens. 2021; 10(6):664. https://doi.org/10.3390/pathogens10060664
Chicago/Turabian StyleMeng, Yufang, Xiaoxue Yu, Chunxue You, Wenjuan Zhang, Yingfeng Sun, Liuan Li, Tianming Jin, Pengyu Pan, and Ailing Xie. 2021. "Chicken Heat Shock Protein 70 Is an Essential Host Protein for Infectious Bursal Disease Virus Infection In Vitro" Pathogens 10, no. 6: 664. https://doi.org/10.3390/pathogens10060664
APA StyleMeng, Y., Yu, X., You, C., Zhang, W., Sun, Y., Li, L., Jin, T., Pan, P., & Xie, A. (2021). Chicken Heat Shock Protein 70 Is an Essential Host Protein for Infectious Bursal Disease Virus Infection In Vitro. Pathogens, 10(6), 664. https://doi.org/10.3390/pathogens10060664






