Abstract
Exercise-induced immune perturbations have been proposed to increase susceptibility to viral infections. We investigated the replication of persisting viruses as indicators of immune function in elite cross-country skiers after ten months of sustained high-performance exercise. The viruses evaluated, nine human herpesviruses (HHVs) and torque teno virus (TTV), are typically restrained in health but replicate actively in immunosuppressed individuals. We collected sera from 27 Finnish elite cross-country skiers at the end of the competition’s season and 27 matched controls who perform moderate exercise. We quantified all the HHVs and—TTV via highly sensitive qPCRs. To verify equal past exposures between the groups, we assessed the IgG antibody prevalences toward HHV-4 (Epstein–Barr virus, EBV) and HHV-5 (human cytomegalovirus, HCMV). We found equal TTV DNA prevalences in athletes (63%) and controls (63%) and loads with respective geometric means of 1.7 × 103 and 1.2 × 103 copies/mL of serum. Overall, the copy numbers were low and consistent with those of healthy individuals. Neither of the groups presented with herpesvirus viremia despite similar past exposures to HHVs (seroprevalences of EBV 70% vs. 78% and HCMV 52% vs. 44% in athletes and controls, respectively). We found no evidence of increased replication of persistent viruses in elite athletes, arguing against impaired viral immunity due to high-performance exercise.
1. Introduction
Transient and cumulative perturbations in immune function following strenuous exercise have been proposed as the underlying cause for an increased risk of infection in elite athletes [1,2]. Indeed, higher frequencies of respiratory symptoms have been reported during and after competitions, although only a handful of studies have provided laboratory confirmation supporting the clinical assessment [3,4,5,6,7]. We have previously observed up to a sevenfold increase in the relative risk to viral respiratory infections in elite cross-country skiers compared to healthy controls [7,8], with long-haul travel, social housing, and mass-gatherings as important risk factors.
To circumvent confounding epidemiological variables, in the present study we examined reactivations of pre-acquired viral infections. The aim was to investigate whether sustained strenuous exercise would affect immune function and result in the replication of persistent viruses.
Many viruses that infect us during childhood remain latent in the tissues, their replication being repressed through continuous surveillance by both innate and adaptive arms of the immune system [9,10,11]. This is the case for the nine human herpesviruses (HHVs) and torque teno virus (TTV)that can reactivate upon immunosuppression or specific environmental triggers. HHV reactivations can be asymptomatic or present with clinical manifestations ranging from local (e.g., HSV-1&2 and VZV mucocutaneous lesions) to life-threatening conditions (e.g., HCMV disease or EBV post-transplantation lymphoproliferative disorder). While local bursts of replication may be common even in healthy individuals, the presence of HHV DNA in sera is a strong indicator of immunosuppression [12,13,14,15,16,17]. TTV infections, on the other hand, result in asymptomatic, lifelong low-level viremia that can be significantly increased in immunosuppression [18,19] and immunosenescence [20,21].
We evaluated the genomic frequencies and copy numbers of these ten viruses in the sera of elite cross-country skiers from the national ski team of Finland, participating in the 2019 National Championships in Äänekoski, Finland, an event held at the end of the skiers’ competition season (5 months). As controls, we included age and gender-matched individuals who perform moderate exercise. Moreover, we determined the serological status toward HHV-4 (Epstein–Barr virus, EBV) and HHV-5 (human cytomegalovirus, HCMV) to verify equal past exposures to these viruses in the groups.
2. Results
2.1. Exercise Load
The participants were selected based on similar high training loads. The mean yearly training volume of the 26 elite cross-country skiers was 766 h (range 580–902), i.e., on average 15 h per week. The training consisted typically of 90% endurance (low, moderate, or high intensity), 8% strength, and 2% speed. The modes of training included running, cycling, and skiing/roller skiing. During the 5-month competition season, the athletes participated in 30–60 (median 35) events. In the matched control group, the exercise load was less than 6 h per week.
2.2. Detection of Viral DNAs in Sera
We quantified TTV loads in sera via a highly sensitive qPCR that recognizes all known genogroups of TT viruses.
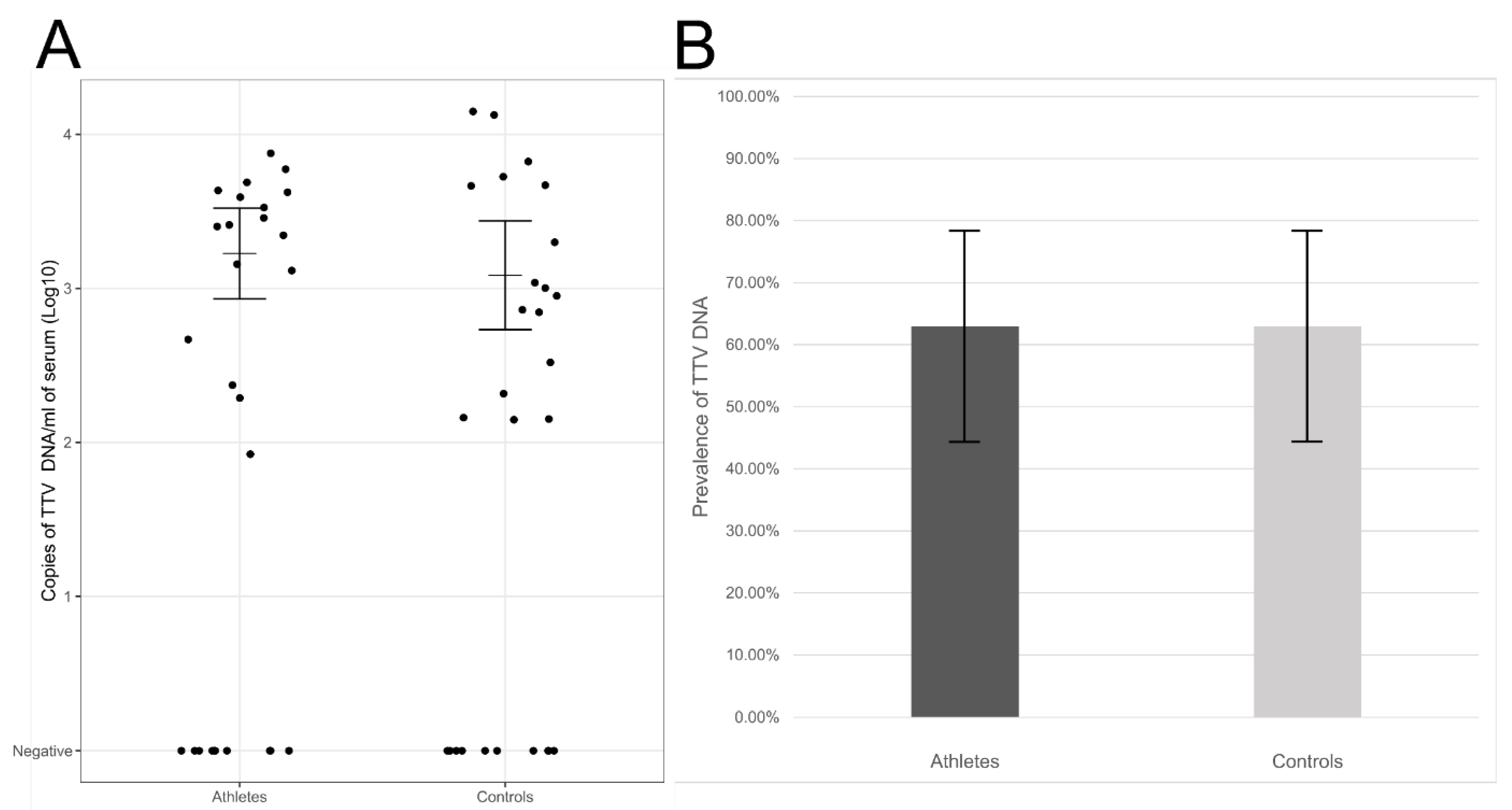
We detected TTV DNA in 63% of elite athletes (17/27) and 63% of controls (17/27) (95% CI ranges 44–78%). The viral loads, calculated from individuals with TTV viremia, were on average 1.7 × 103 copies/mL of serum in athletes and 1.2 × 103 copies/mL of serum in the control group (geometric mean, 95% CI ranges 9.0 × 102–3.1 × 103 and 5.6 × 102–2.6 × 103 copies/mL of serum, respectively). The differences in the viral DNA prevalences or loads lacked statistical significance (p = 1.00 and p = 0.519, respectively; Figure 1).
Figure 1.
(A) TTV DNA viral loads in sera of elite athletes and controls. Represented are in the y-axis, the log10 copies of TTV DNA/mL of serum in athletes (left) and controls (right). Horizontal lines represent the geometric means and whiskers the 95% confidence interval of the viral loads calculated from the TTV DNA-positive individuals. (B) TTV DNA prevalences in sera of athletes (dark grey) or controls (light grey). The differences were not statistically significant (p > 0.05).
We detected none of the HHV DNAs in the sera of either the elite athletes or the controls, ruling against reactivation.
2.3. Past Immunity against Herpesviruses
Given the ubiquitous nature of the viruses studied and the homogeneous demographics of the target and control groups, similar past exposures were to be expected. Nevertheless, to verify this, we examined the IgG antibody prevalences toward EBV and HCMV.
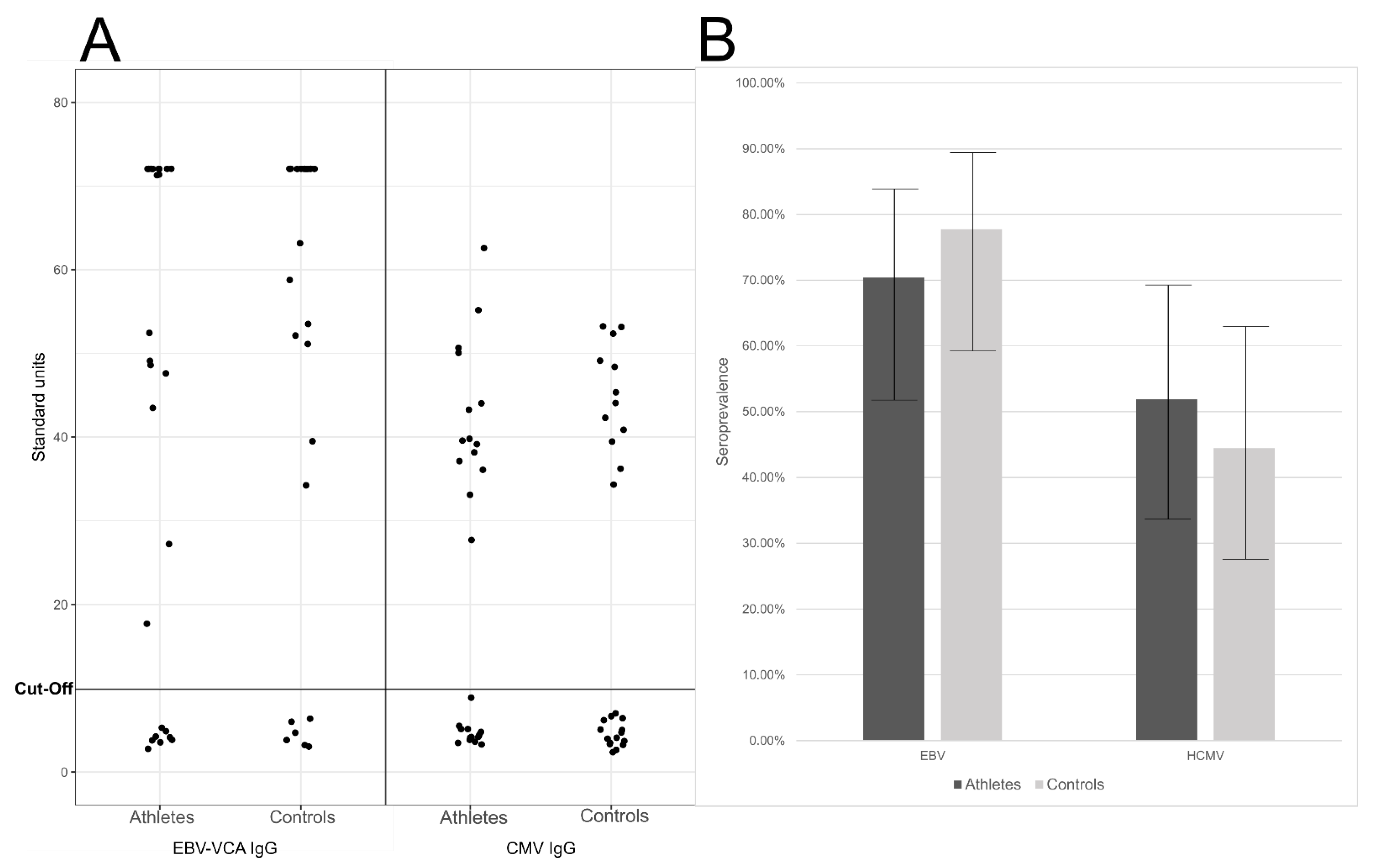
We found similar seroprevalences in the elite athletes and controls (Figure 2), being respectively the EBV IgG positivity 70% (19/27) and 78% (21/27), (95% CI ranges of 52–84%, and 59–89%, respectively), and the HCMV IgG positivity 52% (14/27) and 44% (12/27), (95% CI ranges of 34–69% and 28–63%, respectively; Figure 2).
Figure 2.
(A) EBV VCA (viral capsid antigen) IgG and HCMV IgG levels. Represented are the calculated standard units (y-axis) for each of theathletes (left) and controls (right). (B) Seroprevalences of EBV and HCMV. Represented are the percentage of IgG positive individuals (y-axis) in athletes (dark grey) and controls (light grey). The whiskers represent the 95% confidence interval of the prevalences. The differences were not statistically significant (p > 0.05).
There were no statistically significant differences in the serological findings (p = 0.757 for EBV and p = 0.786 for HCMV).
3. Discussion
Recurrent flares of immunosuppression following strenuous exercise may cumulatively increase the susceptibility to infection in elite athletes [2]. We investigated latent viruses as integral markers of immune function since all components of the immune system are required to contain reactivation [10,22].
We examined the viral loads of TTV and the HHVs in the sera of elite cross-country skiers after 10 months of heavy training and competitions, following probably the highest training volume of all athletes [23]. The aim was to evaluate whether sustained high-performance exercise could compromise the control of these otherwise normally suppressed viruses [9].
TTV infections are acquired soon after birth and result in chronic asymptomatic viremia [24] that can be significantly elevated in immunosuppression [18,25] and immune senescence [20,21,26]. Indeed, in immunocompromised individuals, such as transplant recipients [17], the average copy numbers can be as high as 1010/mL of plasma [18]. For this reason, the kinetics of this virus in the blood continue to be extensively evaluated as a diagnostic and prognostic marker of immune function in different conditions [21,27,28,29,30,31,32], and in the monitoring of immunosuppressive treatments [17,19,22,33].
In the present study, the TTV DNA prevalences found were similar in both elite athletes and control subjects, and the viral loads compatible with those of healthy individuals [25,26].
Herpesviruses establish latency with occasional cycles of active replication being triggered, among others, by physical and emotional stress. In athletes, EBV DNA has been previously investigated in peripheral blood leukocytes [34] and saliva [35,36,37]. However, these sample types are more likely to reflect latency or intermittent stages of replication [38,39,40,41], and positivity is common in the general population. Gleeson et al. [35], for example, found a correlation between EBV DNA shedding in the saliva of elite swimmers and upper respiratory symptoms; however, low-level replication of EBV also occurs in the saliva of healthy individuals [40] and this was not accounted for in the study by inclusion of a control group.
In contrast, the finding of HHV DNAs in cell-free fluids, such as serum, is uncommon in healthy individuals and consequently is a specific indicator of immune deficiency [12,42,43].
We found no cases of HHV DNA in the sera of either the elite athletes or controls. In blood cells, Hoffmann et al. [34] detected statistically significantly higher loads of EBV DNA in athletes compared to healthy controls, albeit in both groups the copy numbers were low. The differences reported may mirror ongoing cellular processes, favoring low-level replication that may not be reflected in sera, if not resulting in lysis of the host cell. Thus, follow-up studies evaluating sequential viral loads in both the cell-free and cellular fractions of blood in larger cohorts are warranted [44].
Given the high seroprevalences of most of the viruses studied here [9,45,46] and the analogous demographics of the target and control groups, it is feasible to assume corresponding past exposures to these viral infections. Nevertheless, we verified similar IgG seroprevalences toward EBV and HCMV, which were in line with those of the general population of similar ages (78–95% for EBV and 34–72% for HCMV in Western countries) and other athletes (78% and 36% for EBV and HCMV, respectively) [34,45,47,48,49,50].
In the present study, we investigated the reactivation of common pre-acquired viral infections at the end of the competition’s season, thus accounting for the long-term sequelae on immune competence. Taken together, we found no evidence of viral reactivation in connection to sustained high-performance exercise.
4. Materials and Methods
The study included 27 athletes belonging to the Finnish cross-country ski team participating in the 2019 National Championships in Äänekoski, Finland. The participants were selected based on their similar training loads. The training season of the athletes started on 1 May and the competition season on 1 November, accounting for 10 months of heavy physical stress.
The training load of the athletes was collected from the day-to-day training diary data of the previous 10 months. The information of one athlete could not be obtained. For every other athlete, one healthy, moderately physically active (<6 h/week) control subject was recruited among the students and staff of Turku University Hospital and Turku University. The controls (n = 27) were matched for age (±2 years), gender, and the number of children younger than 5 years of age in the household (Table 1). The serum samples and health-related information were collected by a study nurse from the athletes before the competition (28 March 28 and 1 April 2019), and from the controls between 2 and 11 April 2019. The serum samples were immediately frozen upon collection.

Table 1.
Clinical characteristics of the study population.
The DNA was extracted from 200 µL of serum using the QIAamp DNA Blood Mini Kit (Qiagen) following the manufacturer protocol. The final elution volume was in 80 µL AE buffer.
The nine HHVs (human simplex viruses 1 and 2, varicella-zoster virus, EBV, HCMV, human herpesviruses 6A-B and 7, Kaposi’s sarcoma-associated herpesvirus) were analyzed via quantitative multiplex PCRs [51] and TTV DNA via qPCR as described [52].
The EBV and HCMV IgG analyses were performed with the human anti-Epstein–Barr viral capsid antigen (VCA) IgG ELISA Kit and anti-cytomegalovirus IgG ELISA kit (Abcam) according to the manufacturer protocols. The standard units were calculated as specified by the manufacturer.
Fisher’s exact test was used to compare the TTV DNA prevalences and the EBV and HCMV IgG seroprevalences between the groups. Wilson’s score interval was used to calculate the 95% confidence interval for binomial proportions. The viral loads from TTV DNA-positive individuals were log10-transformed for statistical analyses and compared via Student’s t-test. The analyses were done with SPSS (V.23) and p > 0.05 interpreted as not statistically significant. R-studio (v.1.2.5033) and Excel (v.2002) were used to create the figures.
Author Contributions
Conceptualization, M.V., R.L., O.J.H., O.R. and M.F.P.; methodology, L.P., W.G. and M.W.; software, L.P.; validation, L.P., O.R. and M.F.P.; formal analysis, L.P. and M.F.P.; investigation, L.P., M.V., R.L.,W.G., M.W., O.J.H., O.R. and M.F.P.; resources, O.R. and M.F.P.; data curation, L.P.; writing—original draft preparation, L.P. and M.F.P.; writing—review and editing, all authors; visualization, L.P.; supervision, M.F.P.; project administration, O.R. and M.F.P.; funding acquisition, L.P., O.J.H. and M.F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by grants from the Finnish Medical Society (LP, MFP), Instrumentarium Science Foundation (LP), Biomedicum Helsinki Foundation (LP), Finska Läkaresällskapet (MFP), the Finnish Cultural Foundation (MFP), the Life and Health Medical Foundation (MFP) and the Jenny and Antti Wihuri Foundation (MV, OJH). Open access funding provided by University of Helsinki.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Hospital District of Southwest Finland (ETMK Dnro: 5/1801/2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Additional information will be available from the corresponding author upon reasonable request.
Acknowledgments
We thank Klaus Hedman (Department of Virology, University of Helsinki) for his help in revising the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Gleeson, M. Immune Function in Sport and Exercise. J. Appl. Physiol. 2007, 103, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W.; Davison, G. Exercise, Immunity, and Illness. In Muscle and Exercise Physiology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 317–344. [Google Scholar]
- Nieman, D.C.; Johanssen, L.M.; Lee, J.W.; Arabatzis, K. Infectious Episodes in Runners before and after the Los Angeles Marathon. J. Sports Med. Phys. Fit. 1990, 30, 316–328. [Google Scholar]
- Peters, E.M.; Goetzsche, J.M.; Grobbelaar, B.; Noakes, T.D. Vitamin C Supplementation Reduces the Incidence of Postrace Symptoms of Upper-Respiratory-Tract Infection in Ultramarathon Runners. Am. J. Clin. Nutr. 1993, 57, 170–174. [Google Scholar] [CrossRef]
- Cox, A.J.; Gleeson, M.; Pyne, D.B.; Callister, R.; Hopkins, W.G.; Fricker, P.A. Clinical and Laboratory Evaluation of Upper Respiratory Symptoms in Elite Athletes. Clin. J. Sport Med. 2008, 18, 438–445. [Google Scholar] [CrossRef]
- Svendsen, I.S.; Gleeson, M.; Haugen, T.A.; Tønnessen, E. Effect of an Intense Period of Competition on Race Performance and Self-Reported Illness in Elite Cross-Country Skiers. Scand. J. Med. Sci. Sports 2015, 25, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Valtonen, M.; Waris, M.; Vuorinen, T.; Eerola, E.; Hakanen, A.J.; Mjosund, K.; Grönroos, W.; Heinonen, O.J.; Ruuskanen, O. Common Cold in Team Finland during 2018 Winter Olympic Games (PyeongChang): Epidemiology, Diagnosis Including Molecular Point-of-Care Testing (POCT) and Treatment. Br. J. Sports Med. 2019, 53, 1093–1098. [Google Scholar] [CrossRef]
- Valtonen, M.; Grönroos, W.; Luoto, R.; Waris, M.; Uhari, M.; Heinonen, O.J.; Ruuskanen, O. Increased Risk of Respiratory Viral Infections in Elite Athletes: A Controlled Study. PLoS ONE 2021, 16, e0250907. [Google Scholar] [CrossRef]
- Virgin, H.W.; Wherry, E.J.; Ahmed, R. Redefining Chronic Viral Infection. Cell 2009, 138, 30–50. [Google Scholar] [CrossRef]
- Roberts, M.B.; Fishman, J.A. Immunosuppressive Agents and Infectious Risk in Transplantation: Managing the “Net State of Immunosuppression”. Clin. Infect. Dis. 2020, ciaa1189. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I. Herpesvirus Latency. J. Clin. Investig. 2020, 130, 3361–3369. [Google Scholar] [CrossRef]
- Ward, K.N. The Natural History and Laboratory Diagnosis of Human Herpesviruses-6 and -7 Infections in the Immunocompetent. J. Clin. Virol. 2005, 32, 183–193. [Google Scholar] [CrossRef]
- Miller, G.G.; Dummer, J.S. Herpes Simplex and Varicella Zoster Viruses: Forgotten but Not Gone. Am. J. Transpl. 2007, 7, 741–747. [Google Scholar] [CrossRef]
- Fishman, J.A. Overview: Cytomegalovirus and the Herpesviruses in Transplantation. Am. J. Transpl. 2013, 13, 1–8. [Google Scholar] [CrossRef]
- Lee, D.H.; Zuckerman, R.A. Herpes Simplex Virus Infections in Solid Organ Transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transpl. 2019, 33, e13526. [Google Scholar] [CrossRef]
- Focosi, D.; Spezia, P.G.; Macera, L.; Salvadori, S.; Navarro, D.; Lanza, M.; Antonelli, G.; Pistello, M.; Maggi, F. Assessment of Prevalence and Load of Torquetenovirus Viraemia in a Large Cohort of Healthy Blood Donors. Clin. Microbiol. Infect. 2020, 26, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Heilos, A.; Bond, G.; Meyer, E.; Böhm, M.; Puchhammer-Stöckl, E.; Arbeiter, K.; Müller-Sacherer, T.; Csaicsich, D.; Aufricht, C.; et al. Torque Teno Viral Load Reflects Immunosuppression in Paediatric Kidney-Transplanted Patients—A Pilot Study. Pediatr. Nephrol. 2021, 36, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Görzer, I.; Haloschan, M.; Jaksch, P.; Klepetko, W.; Puchhammer-Stöckl, E. Plasma DNA Levels of Torque Teno Virus and Immunosuppression after Lung Transplantation. J. Hear. Lung Transpl. 2014, 33, 320–323. [Google Scholar] [CrossRef]
- Jaksch, P.; Kundi, M.; Görzer, I.; Muraközy, G.; Lambers, C.; Benazzo, A.; Hoetzenecker, K.; Klepetko, W.; Puchhammer-Stöckl, E. Torque Teno Virus as a Novel Biomarker Targeting the Efficacy of Immunosuppression After Lung Transplantation. J. Infect. Dis. 2018, 218, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Giacconi, R.; Maggi, F.; Macera, L.; Spezia, P.G.; Pistello, M.; Provinciali, M.; Piacenza, F.; Basso, A.; Bürkle, A.; Moreno-Villanueva, M.; et al. Prevalence and Loads of Torquetenovirus in the European Mark-Age Study Population. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 1838–1845. [Google Scholar] [CrossRef]
- Westman, G.; Schoofs, C.; Ingelsson, M.; Järhult, J.D.; Muradrasoli, S. Torque Teno Virus Viral Load Is Related to Age, CMV Infection and HLA Type but Not to Alzheimer’s Disease. PLoS ONE 2020, 15, e0227670. [Google Scholar] [CrossRef]
- De Vlaminck, I.; Khush, K.K.; Strehl, C.; Kohli, B.; Luikart, H.; Neff, N.F.; Okamoto, J.; Snyder, T.M.; Cornfield, D.N.; Nicolls, M.R.; et al. Temporal Response of the Human Virome to Immunosuppression and Antiviral Therapy. Cell 2013, 155, 1178. [Google Scholar] [CrossRef]
- Solli, G.S.; Tønnessen, E.; Sandbakk, Ø. The Training Characteristics of the World’s Most Successful Female Cross-Country Skier. Front. Physiol. 2017, 8, 1069. [Google Scholar] [CrossRef]
- Komatsu, H.; Inui, A.; Sogo, T.; Kuroda, K.; Tanaka, T.; Fujisawa, T. TTV Infection in Children Born to Mothers Infected with TTV but Not with HBV, HCV, or HIV. J. Med. Virol. 2004, 74, 499–506. [Google Scholar] [CrossRef]
- Görzer, I.; Jaksch, P.; Kundi, M.; Seitz, T.; Klepetko, W.; Puchhammer-Stöckl, E. Pre-Transplant Plasma Torque Teno Virus Load and Increase Dynamics after Lung Transplantation. PLoS ONE 2015, 10, e0122975. [Google Scholar]
- Haloschan, M.; Bettesch, R.; Görzer, I.; Weseslindtner, L.; Kundi, M.; Puchhammer-Stöckl, E. TTV DNA Plasma Load and Its Association with Age, Gender, and HCMV IgG Serostatus in Healthy Adults. Age Omaha 2014, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.D.; Eugen-Olsen, J.; Kirk, O.; Parner, J.; Christensen, J.K.; Brasholt, M.S.; Nielsen, J.O.; Krogsgaard, K. TTV Viral Load as a Marker for Immune Reconstitution after Initiation of HAART in HIV-Infected Patients. HIV Clin. Trials 2002, 3, 287–295. [Google Scholar] [PubMed]
- Masouridi-Levrat, S.; Pradier, A.; Simonetta, F.; Kaiser, L.; Chalandon, Y.; Roosnek, E. Torque Teno Virus in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation for Hematological Malignancies. Bone Marrow Transpl. 2016, 51, 440–442. [Google Scholar] [CrossRef]
- Strassl, R.; Schiemann, M.; Doberer, K.; Görzer, I.; Puchhammer-Stöckl, E.; Eskandary, F.; Kikić, Ž.; Gualdoni, G.A.; Vossen, M.G.; Rasoul-Rockenschaub, S.; et al. Quantification of Torque Teno Virus Viremia as a Prospective Biomarker for Infectious Disease in Kidney Allograft Recipients. J. Infect. Dis. 2018, 218, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Martín-López, M.; Albert, E.; Fernández-Ruiz, M.; González-Álvaro, I.; Rodríguez, E.; Aguado, J.M.; Navarro, D.; Pablos, J.L. Torque Teno Virus Viremia in Patients with Chronic Arthritis: Influence of Biologic Therapies. Semin. Arthritis Rheum. 2020, 50, 166–171. [Google Scholar] [CrossRef]
- Schmidt, L.; Jensen, B.E.O.; Walker, A.; Keitel-Anselmino, V.; di Cristanziano, V.; Böhm, M.; Knops, E.; Heger, E.; Kaiser, R.; de Luca, A.; et al. Torque Teno Virus Plasma Level as Novel Biomarker of Retained Immunocompetence in HIV-Infected Patients. Infection 2021, 1, 3. [Google Scholar]
- Rueschenbaum, S.; Ciesek, S.; Queck, A.; Widera, M.; Schwarzkopf, K.; Brüne, B.; Welsch, C.; Wedemeyer, H.; Zeuzem, S.; Weigert, A.; et al. Dysregulated Adaptive Immunity Is an Early Event in Liver Cirrhosis Preceding Acute-on-Chronic Liver Failure. Front. Immunol. 2021, 11, 534731. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Macera, L.; Pistello, M.; Maggi, F. Torque Teno Virus Viremia Correlates With Intensity of Maintenance Immunosuppression in Adult Orthotopic Liver Transplant. J. Infect. Dis. 2014, 210, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Wolfarth, B.; Hörterer, H.G.; Halle, M.; Reichhuber, C.; Nadas, K.; Tora, C.; Erfle, V.; Protzer, U.; Schätzl, H.M. Elevated Epstein-Barr Virus Loads and Lower Antibody Titers in Competitive Athletes. J. Med. Virol. 2010, 82, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Pyne, D.B.; Austin, J.P.; Francis, J.L.; Clancy, R.L.; McDonald, W.A.; Fricker, P.A. Epstein-Barr Virus Reactivation and Upper-Respiratory Illness in Elite Swimmers. Med. Sci. Sports Exerc. 2002, 34, 411–417. [Google Scholar] [CrossRef]
- Reid, V.L.; Gleeson, M.; Williams, N.; Clancy, R.L. Clinical Investigation of Athletes with Persistent Fatigue and/or Recurrent Infections. Br. J. Sports Med. 2004, 38, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.L.; Gleeson, M.; Cox, A.; Callister, R.; Dorrington, M.; D’Este, C.; Pang, G.; Pyne, D.; Fricker, P.; Henriksson, A. Reversal in Fatigued Athletes of a Defect in Interferon γ Secretion after Administration of Lactobacillus Acidophilus. Br. J. Sports Med. 2006, 40, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Venables, P.J.W.; Teo, C.G.; Baboonian, C.; Griffin, B.E.; Hughes, R.A.; Maini, R.N. Persistence of Epstein-Barr Virus in Salivary Gland Biopsies from Healthy Individuals and Patients with Sjogren’s Syndrome. Clin. Exp. Immunol. 1989, 75, 359–364. [Google Scholar]
- Maurmann, S.; Fricke, L.; Wagner, H.J.; Schlenke, P.; Hennig, H.; Steinhoff, J.; Jabs, W.J. Molecular Parameters for Precise Diagnosis of Asymptomatic Epstein-Barr Virus Reactivation in Healthy Carriers. J. Clin. Microbiol. 2003, 41, 5419–5428. [Google Scholar] [CrossRef]
- Hadinoto, V.; Shapiro, M.; Sun, C.C.; Thorley-Lawson, D.A. The Dynamics of EBV Shedding Implicate a Central Role for Epithelial Cells in Amplifying Viral Output. PLoS Pathog. 2009, 5, e1000496. [Google Scholar] [CrossRef]
- Pyöriä, L.; Toppinen, M.; Mäntylä, E.; Hedman, L.; Aaltonen, L.M.; Vihinen-Ranta, M.; Ilmarinen, T.; Söderlund-Venermo, M.; Hedman, K.; Perdomo, M.F. Extinct Type of Human Parvovirus B19 Persists in Tonsillar B Cells. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Hara, S.; Kimura, H.; Hoshino, Y.; Tanaka, N.; Nishikawa, K.; Ihira, M.; Yoshikawa, T.; Morishima, T. Detection of Herpesvirus DNA in the Serum of Immunocompetent Children. Microbiol. Immunol. 2002, 46, 177–180. [Google Scholar] [CrossRef]
- Aalto, S.M.; Juvonen, E.; Tarkkanen, J.; Volin, L.; Ruutu, T.; Mattila, P.S.; Piiparinen, H.; Knuutila, S.; Hedman, K. Lymphoproliferative Disease after Allogeneic Stem Cell Transplantation—Pre-Emptive Diagnosis by Quantification of Epstein-Barr Virus DNA in Serum. J. Clin. Virol. 2003, 28, 275–283. [Google Scholar] [CrossRef]
- Kullberg-Lindh, C.; Olofsson, S.; Brune, M.; Lindh, M. Comparison of Serum and Whole Blood Levels of Cytomegalovirus and Epstein-Barr Virus DNA. Transpl. Infect. Dis. 2008, 10, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Puhakka, L.; Sarvikivi, E.; Lappalainen, M.; Surcel, H.M.; Saxen, H. Decrease in Seroprevalence for Herpesviruses among Pregnant Women in Finland: Cross-Sectional Study of Three Time Points 1992, 2002 and 2012. Infect. Dis. Auckl. 2016, 48, 406–410. [Google Scholar] [CrossRef]
- Focosi, D.; Antonelli, G.; Pistello, M.; Maggi, F. Torquetenovirus: The Human Virome from Bench to Bedside. Clin. Microbiol. Infect. 2016, 22, 589–593. [Google Scholar] [CrossRef]
- Balfour, H.H.; Sifakis, F.; Sliman, J.A.; Knight, J.A.; Schmeling, D.O.; Thomas, W. Age-Specific Prevalence of Epstein-Barr Virus Infection among Individuals Aged 6-19 Years in the United States and Factors Affecting Its Acquisition. J. Infect. Dis. 2013, 208, 1286–1293. [Google Scholar] [CrossRef]
- Rostgaard, K.; Balfour, H.H.; Jarrett, R.; Erikstrup, C.; Pedersen, O.; Ullum, H.; Nielsen, L.P.; Voldstedlund, M.; Hjalgrim, H. Primary Epstein-Barr Virus Infection with and without Infectious Mononucleosis. PLoS ONE 2019, 14, e0226436. [Google Scholar] [CrossRef]
- Theall, B.; Wang, H.; Kuremsky, C.A.; Cho, E.; Hardin, K.; Robelot, L.; Marucci, J.; Mullenix, S.; Lemoine, N.; Johannsen, N.M.; et al. Allostatic Stress Load and CMV Serostatus Impact Immune Response to Maximal Exercise in Collegiate Swimmers. J. Appl. Physiol. 2020, 128, 178–188. [Google Scholar] [CrossRef]
- Mabilangan, C.; Burton, C.; O’brien, S.; Plitt, S.; Eurich, D.; Preiksaitis, J. Using Blood Donors and Solid Organ Transplant Donors and Recipients to Estimate the Seroprevalence of Cytomegalovirus and Epstein–Barr Virus in Canada: A Cross-Sectional Study. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2020, 5, 158–176. [Google Scholar] [CrossRef]
- Pyöriä, L.; Jokinen, M.; Toppinen, M.; Salminen, H.; Vuorinen, T.; Hukkanen, V.; Schmotz, C.; Elbasani, E.; Ojala, P.M.; Hedman, K.; et al. HERQ-9 Is a New Multiplex PCR for Differentiation and Quantification of All Nine Human Herpesviruses. mSphere 2020, 5, e00265-20. [Google Scholar] [CrossRef] [PubMed]
- Toppinen, M.; Pratas, D.; Väisänen, E.; Söderlund-Venermo, M.; Hedman, K.; Perdomo, M.F.; Sajantila, A. The Landscape of Persistent Human DNA Viruses in Femoral Bone. Forensic Sci. Int. Genet. 2020, 48, 102353. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).