Over 100 Years of Rift Valley Fever: A Patchwork of Data on Pathogen Spread and Spillover
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition Strategy
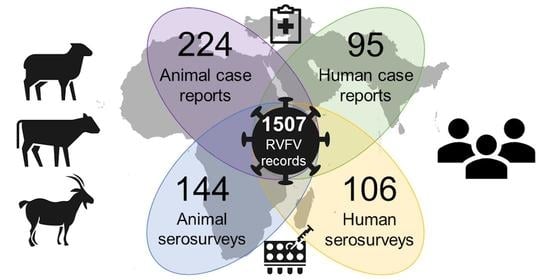
2.2. Dataset
2.2.1. RVF Case Definition
2.2.2. Geographical Information
2.2.3. Serological Records
2.3. Statistical Analyses
2.3.1. Data Preparation
2.3.2. Annual RVF Case and Serological Study Availability
2.3.3. Seroprevalence over Time, and across Regions and Outbreaks
2.3.4. Ruminant Species’ Seroprevalence and the Association with Reported Outbreaks
2.3.5. Exploring Cryptic Spread: RVFV Activity Prior to RVF Case Reporting
2.3.6. Exploring Pathogen Spillover: Concurrent Animal and Human RVFV Activity
2.3.7. Software
3. Results
3.1. Data Availability
3.1.1. Data Availability over Time
3.1.2. Spatial Distribution of Data
3.2. RVF Outbreaks and Number of Cases Affected
3.3. Variation in Seroprevalence
3.3.1. The Association of Seroprevalence with Time, Region, and Outbreaks
3.3.2. Ruminant Species’ Seroprevalence and the Association with Reported Outbreaks
3.3.3. Exploring Cryptic Spread: RVFV Activity Prior to RVF Case Reporting
3.4. Exploring Pathogen Spillover: Concurrent Animal and Human RVFV Activity
3.4.1. Association of Human RVF Outbreaks with Animal RVF Cases
3.4.2. Correlation between Animal and Human Seroprevalence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harcourt, M.P.; Bowring, C.C. Annual Report on the East Africa Protectorate for 1912–1913; Barclay and Fry: Southwark, UK, 1914; p. 27. [Google Scholar]
- Daubney, R.; Hudson, J.R.; Garnham, P.C. Enzootic Hepatitis or Rift Valley Fever. An Undescribed Virus Disease of Sheep Cattle and Man from East Africa. J. Pathol. Bacteriol. 1931, 34, 545–579. [Google Scholar] [CrossRef]
- ICTV Taxonomy History: Rift Valley Fever Phlebovirus. Available online: https://talk.ictvonline.org/taxonomy/p/taxonomy-history?taxnode_id=201900163 (accessed on 21 January 2021).
- Mehand, M.S.; Al-Shorbaji, F.; Millett, P.; Murgue, B. The WHO R&D Blueprint: 2018 Review of Emerging Infectious Diseases Requiring Urgent Research and Development Efforts. Antivir. Res. 2018, 159, 63–67. [Google Scholar] [CrossRef]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global Expansion and Redistribution of Aedes-Borne Virus Transmission Risk with Climate Change. PLoS Negl. Trop. Dis. 2019, 13. [Google Scholar] [CrossRef]
- US Department of Health and Human Services, Centers for Disease Control and Prevention. Outbreak of Rift Valley Fever--Yemen, August–October 2000. Morb. Mortal. Wkly. Rep. 2000, 49, 1065–1066. [Google Scholar]
- Ahmad, K. More Deaths from Rift Valley Fever in Saudi Arabia and Yemen. Lancet 2000, 356, 1422. [Google Scholar] [CrossRef]
- Al-Afaleq, A.I.; Hussein, M.F.; Al-Naeem, A.A.; Housawi, F.; Kabati, A.G. Seroepidemiological Study of Rift Valley Fever (RVF) in Animals in Saudi Arabia. Trop. Anim. Health Prod. 2012, 44, 1535–1539. [Google Scholar] [CrossRef]
- Madani, T.A.; Al-Mazrou, Y.Y.; Al-Jeffri, M.H.; Mishkhas, A.A.; Al-Rabeah, A.M.; Turkistani, A.M.; Al-Sayed, M.O.; Abodahish, A.A.; Khan, A.S.; Ksiazek, T.G.; et al. Rift Valley Fever Epidemic in Saudi Arabia: Epidemiological, Clinical, and Laboratory Characteristics. Clin. Infect. Dis. 2003, 37, 1084–1092. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Rojas, J.L.G.; Schmidt, C.G.; Michel, V.; et al. Rift Valley Fever—Epidemiological Update and Risk of Introduction into Europe. EFSA J. 2020, 18, e06041. [Google Scholar] [CrossRef] [PubMed]
- Hartley, D. Potential Effects of Rift Valley Fever in the United States. Emerg. Infect. Dis. 2011. [Google Scholar] [CrossRef]
- Abdo-Salem, S.; Waret-Szkuta, A.; Roger, F.; Olive, M.-M.; Saeed, K.; Chevalier, V. Risk Assessment of the Introduction of Rift Valley Fever from the Horn of Africa to Yemen via Legal Trade of Small Ruminants. Trop. Anim. Health Prod. 2011, 43, 471–480. [Google Scholar] [CrossRef] [PubMed]
- El-Harrak, M.; Martín-Folgar, R.; Llorente, F.; Fernández-Pacheco, P.; Brun, A.; Figuerola, J.; Jiménez-Clavero, M.Á. Rift Valley and West Nile Virus Antibodies in Camels, North Africa. Emerg. Infect. Dis. 2011, 17, 2372–2374. [Google Scholar] [CrossRef]
- Carroll, S.A.; Reynes, J.-M.; Khristova, M.L.; Andriamandimby, S.F.; Rollin, P.E.; Nichol, S.T. Genetic Evidence for Rift Valley Fever Outbreaks in Madagascar Resulting from Virus Introductions from the East African Mainland Rather than Enzootic Maintenance. J. Virol. 2011, 85, 6162–6167. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Kortekaas, J.; Bowden, T.A.; Warimwe, G.M. Rift Valley Fever: Biology and Epidemiology. J. Gen. Virol. 2019, 100, 1187–1199. [Google Scholar] [CrossRef]
- Turkistany, A.-H.; Mohamed, A.G.; Al-Hamdan, N. Seroprevalence of Rift Valley fever Among Slaughterhouse Personnel in Makkah During Hajj1419h (1999). J. Fam. Community Med. 2001, 8, 53–57. [Google Scholar]
- Department of Economic and Social Affairs, Population Division, United Nations. World Urbanization Prospects: The 2018 Revision; United Nations: New York, NY, USA, 2019; ISBN 978-92-1-148319-2. [Google Scholar]
- Rostal, M.K.; Liang, J.E.; Zimmermann, D.; Bengis, R.; Paweska, J.; Karesh, W.B. Rift Valley Fever: Does Wildlife Play a Role? ILAR J. 2017, 58, 359–370. [Google Scholar] [CrossRef]
- Bird, B.H.; Ksiazek, T.G.; Nichol, S.T.; MacLachlan, N.J. Rift Valley Fever Virus. J. Am. Vet. Med. Assoc. 2009, 234, 883–893. [Google Scholar] [CrossRef]
- Breiman, R.F. Decision-Support Tool for Prevention and Control of Rift Valley Fever Epizootics in the Greater Horn of Africa. Am. J. Trop. Med. Hyg. 2010, 83, 75–85. [Google Scholar] [CrossRef]
- Bird, B.H.; Nichol, S.T. Breaking the Chain: Rift Valley Fever Virus Control via Livestock Vaccination. Curr. Opin. Virol. 2012, 2, 315–323. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Use of Veterinary Vaccines for Prevention and Control of Rift Valley Fever: Memorandum from a WHO/FAO Meeting. Bull. World Health Organ. 1983, 61, 261–268. [Google Scholar]
- Smithburn, K.C. Rift Valley Fever: The Neurotropic Adaptation of the Virus and the Experimental Use of This Modified Virus as a Vaccine. Br. J. Exp. Pathol. 1949, 30, 1–16. [Google Scholar]
- Coetzer, J.A.W.; Barnard, B.J.H. Hydrops Amnii in Sheep Associated with Hydranencephaly and Arthrogryposis with Wesselsbron Disease and Rift Valley Fever Viruses as Aetiological Agents. Onderstepoort J. Vet. Res. 1977, 44, 119–126. [Google Scholar]
- Mariner, J. Rift Valley Fever Surveillance; FAO Animal Production and Health Manual No 21; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; p. 80. ISBN 978-92-5-130244-6. [Google Scholar]
- Al-Afaleq, A.I.; Hussein, M.F. The Status of Rift Valley Fever in Animals in Saudi Arabia: A Mini Review. Vector-Borne Zoonotic Dis. 2011, 11, 1513–1520. [Google Scholar] [CrossRef]
- Peyre, M.; Chevalier, V.; Abdo-Salem, S.; Velthuis, A.; Antoine-Moussiaux, N.; Thiry, E.; Roger, F. A Systematic Scoping Study of the Socio-Economic Impact of Rift Valley Fever: Research Gaps and Needs. Zoonoses Public Health 2015, 62, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Tempia, C.; Abdi, A.M. Economic Impact of Rift Valley Fever on the Somali Livestock Industry and a Novel Surveillance Approach in Nomadic Pastoral Systems. In Proceedings of the 11th International Symposium on Veterinary Epidemiology and Economics, Cairns, Australia, 2006. [Google Scholar]
- Métras, R.; Collins, L.M.; White, R.G.; Alonso, S.; Chevalier, V.; Thuranira-McKeever, C.; Pfeiffer, D.U. Rift Valley Fever Epidemiology, Surveillance, and Control: What Have Models Contributed? Vector-Borne Zoonotic Dis. 2011, 11, 761–771. [Google Scholar] [CrossRef]
- Anyamba, A.; Linthicum, K.J.; Small, J.L.; Collins, K.M.; Tucker, C.J.; Pak, E.W.; Britch, S.C.; Eastman, J.R.; Pinzon, J.E.; Russell, K.L. Climate Teleconnections and Recent Patterns of Human and Animal Disease Outbreaks. PLoS Negl. Trop. Dis. 2012, 6, e0001465. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Malherbe, J.; Weepener, H.; Majiwa, P.; Swanepoel, R. Anomalous High Rainfall and Soil Saturation as Combined Risk Indicator of Rift Valley Fever Outbreaks, South Africa, 2008–2011. Emerg. Infect. Dis. 2016, 22, 2054–2062. [Google Scholar] [CrossRef]
- Anyamba, A.; Chretien, J.-P.; Small, J.; Tucker, C.J.; Formenty, P.B.; Richardson, J.H.; Britch, S.C.; Schnabel, D.C.; Erickson, R.L.; Linthicum, K.J. Prediction of a Rift Valley Fever Outbreak. Proc. Natl. Acad. Sci. USA 2009, 106, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Davies, F.G.; Linthicum, K.J.; James, A.D. Rainfall and Epizootic Rift Valley Fever. Bull. World Health Organ. 1985, 63, 941–943. [Google Scholar] [PubMed]
- Lancelot, R.; Cêtre-Sossah, C.; Hassan, O.A.; Yahya, B.; Ould Elmamy, B.; Fall, A.G.; Lo, M.M.; Apolloni, A.; Arsevska, E.; Chevalier, V. Rift Valley Fever: One Health at Play? In Transboundary Animal Diseases in Sahelian Africa and Connected Regions; Kardjadj, M., Diallo, A., Lancelot, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 121–148. ISBN 978-3-030-25384-4. [Google Scholar]
- Davies, F.G. Risk of a Rift Valley Fever Epidemic at the Haj in Mecca, Saudi Arabia. Rev. Sci. Tech. Off. Int. Epiz. 2006, 25, 137–147. [Google Scholar] [CrossRef]
- Search ProMED Posts-ProMED-Mail. Available online: https://promedmail.org/promed-posts/ (accessed on 13 January 2021).
- OiE Handistatus II. Available online: http://web.oie.int/hs2/report.asp (accessed on 4 November 2020).
- OiE WAHIS Interface. Available online: https://www.oie.int/animal-health-in-the-world/the-world-animal-health-information-system/data-after-2004-wahis-interface/ (accessed on 13 January 2021).
- WHO. Rift Valley Fever. Available online: http://www.who.int/csr/don/archive/disease/rift_valley_fever/en/ (accessed on 5 November 2020).
- CDC. Outbreak Summaries. Rift Valley Fever. Available online: https://www.cdc.gov/vhf/rvf/outbreaks/summaries.html (accessed on 5 November 2020).
- Pienaar, N.J.; Thompson, P.N. Temporal and Spatial History of Rift Valley Fever in South Africa: 1950 to 2011. Onderstepoort J. Vet. Res. 2013, 80, 13. [Google Scholar] [CrossRef]
- Swanepoel, R.; Coetzer, J. Rift Valley Fever. Infect. Dis. Livest. 2004, 2, 1037–1070. [Google Scholar]
- Sindato, C.; Karimuribo, E.D.; Pfeiffer, D.U.; Mboera, L.E.G.; Kivaria, F.; Dautu, G.; Bernard, B.; Paweska, J.T. Spatial and Temporal Pattern of Rift Valley Fever Outbreaks in Tanzania; 1930 to 2007. PLoS ONE 2014, 9, e0088897. [Google Scholar] [CrossRef] [PubMed]
- Davies, F.G. Observations on the Epidemiology of Rift Valley Fever in Kenya. J. Hyg. 1975, 75, 219–230. [Google Scholar] [CrossRef]
- Clark, M.H.A.; Warimwe, G.M.; Di Nardo, A.; Lyons, N.A.; Gubbins, S. Systematic Literature Review of Rift Valley Fever Virus Seroprevalence in Livestock, Wildlife and Humans in Africa from 1968 to 2016. PLoS Negl. Trop. Dis. 2018, 12, e0006627. [Google Scholar] [CrossRef]
- Clements, A.C.A.; Pfeiffer, D.U.; Martin, V.; Otte, M.J. A Rift Valley Fever Atlas for Africa. Prev. Vet. Med. 2007, 82, 72–82. [Google Scholar] [CrossRef] [PubMed]
- UNSD—Methodology. Available online: https://unstats.un.org/unsd/methodology/m49/ (accessed on 5 November 2020).
- GADM. Available online: https://gadm.org/ (accessed on 5 November 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- South, A. Rnaturalearth: World Map Data from Natural Earth. 2017. Available online: https://cran.r-project.org/web/packages/rnaturalearth/vignettes/rnaturalearth.html (accessed on 4 June 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for “Ggplot2”. 2020. Available online: https://cran.r-project.org/web/packages/cowplot/index.html (accessed on 4 June 2021).
- Bażanów, B.A.; Stygar, D.; Romuk, E.; Skrzep-Poloczek, B.; Pacoń, J.; Gadzała, Ł.; Welz, M.; Pawęska, J.T. Preliminary Serological Investigation of Rift Valley Fever in Poland. J. Vector Borne Dis. 2018, 55, 324–326. [Google Scholar] [CrossRef]
- Kokernot, R.H.; Smithburn, K.C.; Weinbren, M.P. Neutralizing Antibodies to Arthropod-Borne Viruses in Human Beings and Animals in the Union of South Africa. J. Immunol. 1956, 77, 313–323. [Google Scholar]
- Shone, D.K. Rift Valley Fever in Southern Rhodesia. Cent. Afr. J. Med. 1958, 4, 284–286. [Google Scholar]
- Selmi, R. First Serological Evidence of the Rift Valley Fever Phlebovirus in Tunisian Camels. Acta Trop. 2020, 6, 105462. [Google Scholar] [CrossRef] [PubMed]
- Eisa, M. Preliminary Survey of Domestic Animals of the Sudan for Precipitating Antibodies to Rift Valley Fever Virus. J. Hyg. 1984, 93, 629–637. [Google Scholar] [CrossRef]
- Maurice, Y. Baille First Serological Record on the Incidence of Wesselsbronn’s Disease and Rift Valley Fever in Sheep and Wild Ruminants in Chad and Cameroon. Rev. Elev. Med. Vet Pays. Trop. 1967, 20, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Saleh Aghaa, O.B.; Rhaymah, M.S. Seroprevelance Study of Rift Valley Fever Antibody in Sheep and Goats in Ninevah Governorate. Iraqi J. Vet. Sci. 2013, 27, 53–61. [Google Scholar] [CrossRef]
- Fakour, S.; Naserabadi, S.; Ahmadi, E. The First Positive Serological Study on Rift Valley Fever in Ruminants of Iran. J. Vector Borne Dis. 2017, 54, 348. [Google Scholar] [CrossRef]
- Gür, S.; Kale, M.; Erol, N.; Yapici, O.; Mamak, N.; Yavru, S. The First Serological Evidence for Rift Valley Fever Infection in the Camel, Goitered Gazelle and Anatolian Water Buffaloes in Turkey. Trop. Anim. Health Prod. 2017, 49, 1531–1535. [Google Scholar] [CrossRef]
- Shoemaker, T.R.; Nyakarahuka, L.; Balinandi, S.; Ojwang, J.; Tumusiime, A.; Mulei, S.; Kyondo, J.; Lubwama, B.; Sekamatte, M.; Namutebi, A.; et al. First Laboratory-Confirmed Outbreak of Human and Animal Rift Valley Fever Virus in Uganda in 48 Years. Am. J. Trop. Med. Hyg. 2019, 100, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Métras, R.; Cavalerie, L.; Dommergues, L.; Mérot, P.; Edmunds, W.J.; Keeling, M.J.; Cêtre-Sossah, C.; Cardinale, E. The Epidemiology of Rift Valley Fever in Mayotte: Insights and Perspectives from 11 Years of Data. PLoS Negl. Trop. Dis. 2016, 10, e0004783. [Google Scholar] [CrossRef]
- Tigoi, C.; Sang, R.; Chepkorir, E.; Orindi, B.; Arum, S.O.; Mulwa, F.; Mosomtai, G.; Limbaso, S.; Hassan, O.A.; Irura, Z.; et al. High Risk for Human Exposure to Rift Valley Fever Virus in Communities Living along Livestock Movement Routes: A Cross-Sectional Survey in Kenya. PLoS Negl. Trop. Dis. 2020, 14, e0007979. [Google Scholar] [CrossRef] [PubMed]
- Sumaye, R.D.; Geubbels, E.; Mbeyela, E.; Berkvens, D. Inter-Epidemic Transmission of Rift Valley Fever in Livestock in the Kilombero River Valley, Tanzania: A Cross-Sectional Survey. PLoS Negl. Trop. Dis. 2013, 7, e0002356. [Google Scholar] [CrossRef]
- Sumaye, R.D.; Abatih, E.N.; Thiry, E.; Amuri, M.; Berkvens, D.; Geubbels, E. Inter-Epidemic Acquisition of Rift Valley Fever Virus in Humans in Tanzania. PLoS Negl. Trop. Dis. 2015, 9, e0003536. [Google Scholar] [CrossRef] [PubMed]
- Wichgers Schreur, P.J.; Oreshkova, N.; van Keulen, L.; Kant, J.; van de Water, S.; Soós, P.; Dehon, Y.; Kollár, A.; Pénzes, Z.; Kortekaas, J. Safety and Efficacy of Four-Segmented Rift Valley Fever Virus in Young Sheep, Goats and Cattle. NPJ Vaccines 2020, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. An Outbreak of Rift Valley Fever, Eastern Africa, 1997–1998. Wkly. Epidemiol. Rec. Relev. Épidémiol. Hebd. 1998, 73, 105–109. [Google Scholar]
- CDC. Signs and Symptoms. Rift Valley Fever. Available online: https://www.cdc.gov/vhf/rvf/symptoms/index.html (accessed on 21 December 2020).
- Watts, D.M.; el-Tigani, A.; Botros, B.A.; Salib, A.W.; Olson, J.G.; McCarthy, M.; Ksiazek, T.G. Arthropod-Borne Viral Infections Associated with a Fever Outbreak in the Northern Province of Sudan. J. Trop. Med. Hyg. 1994, 97, 228–230. [Google Scholar] [PubMed]
- Signs of Rift Valley Fever. Available online: http://www.fao.org/3/Y4611E/y4611e05.htm (accessed on 27 January 2021).
- Corwin, A.; Habib, M.; Watts, D.; Olson, J.; Darwis, M.; Hibbs, R.; Botros, B.; Kleinosky, M.; Shope, R.; Kilpatrick, M. Prevalence of Antibody to Rift Valley Fever Virus in the Nile River Delta of Egypt, 13 Years after a Major Outbreak. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 161. [Google Scholar] [CrossRef]
- Rostal, M.K.; Ross, N.; Machalaba, C.; Cordel, C.; Paweska, J.T.; Karesh, W.B. Benefits of a One Health Approach: An Example Using Rift Valley Fever. One Health 2018, 5, 34–36. [Google Scholar] [CrossRef] [PubMed]






| Continent | Region | Sources | Year of Publication of Used Sources | ||
|---|---|---|---|---|---|
| Earliest | Median | Most Recent | |||
| Africa | Central | 22 | 1965 | 2008 | 2019 |
| Eastern | 102 | 1931 | 2013 | 2020 | |
| Northern | 35 | 1978 | 1999 | 2020 | |
| Southern | 33 | 1951 | 2011 | 2020 | |
| Western | 47 | 1980 | 2009 | 2020 | |
| Asia | Southern | 2 | 1995 | 2000 | 2005 |
| Western | 21 | 1984 | 2013 | 2020 | |
| Multiple and other regions * | 11 | 1969 | 2011 | 2020 | |
| Total | 273 | 1931 | 2011 | 2020 | |
| Species | No Recent RVF Cases | RVF Recently Reported | ||||||
|---|---|---|---|---|---|---|---|---|
| % | (n) | aOR | 95% CI | % | (n) | aOR | 95% CI | |
| Sheep | 8.1 | (17,328) | 1 | (Reference) | 21.6 | (10,271) | 2.99 | (2.77, 3.23) |
| Buffalo | 13.8 | (1417) | 1.47 | (1.25, 1.73) | 15.7 | (1523) | 1.85 | (1.16, 2.93) |
| Camels | 10.0 | (4938) | 1.80 | (1.60, 2.03) | 20.5 | (1451) | 2.62 | (1.80, 3.82) |
| Cattle | 13.0 | (22,288) | 1.47 | (1.37, 1.58) | 18.2 | (19,519) | 2.12 | (1.67, 2.69) |
| Goats | 11.9 | (8746) | 1.24 | (1.14, 1.35) | 17.1 | (5968) | 1.83 | (1.38, 2.43) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bron, G.M.; Strimbu, K.; Cecilia, H.; Lerch, A.; Moore, S.M.; Tran, Q.; Perkins, T.A.; ten Bosch, Q.A. Over 100 Years of Rift Valley Fever: A Patchwork of Data on Pathogen Spread and Spillover. Pathogens 2021, 10, 708. https://doi.org/10.3390/pathogens10060708
Bron GM, Strimbu K, Cecilia H, Lerch A, Moore SM, Tran Q, Perkins TA, ten Bosch QA. Over 100 Years of Rift Valley Fever: A Patchwork of Data on Pathogen Spread and Spillover. Pathogens. 2021; 10(6):708. https://doi.org/10.3390/pathogens10060708
Chicago/Turabian StyleBron, Gebbiena M., Kathryn Strimbu, Hélène Cecilia, Anita Lerch, Sean M. Moore, Quan Tran, T. Alex Perkins, and Quirine A. ten Bosch. 2021. "Over 100 Years of Rift Valley Fever: A Patchwork of Data on Pathogen Spread and Spillover" Pathogens 10, no. 6: 708. https://doi.org/10.3390/pathogens10060708
APA StyleBron, G. M., Strimbu, K., Cecilia, H., Lerch, A., Moore, S. M., Tran, Q., Perkins, T. A., & ten Bosch, Q. A. (2021). Over 100 Years of Rift Valley Fever: A Patchwork of Data on Pathogen Spread and Spillover. Pathogens, 10(6), 708. https://doi.org/10.3390/pathogens10060708