Observations on the Relationships between Endophytic Metarhizium robertsii, Spodoptera frugiperda (Lepidoptera: Noctuidae), and Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Inoculum
2.2. Seed Surface Sterilization and Inoculation
2.3. Plant Growth Medium
2.4. Evaluation of Endophytic Colonization of Maize
2.5. Fall Armyworm
2.6. Relative Growth Rate of Fall Armyworm
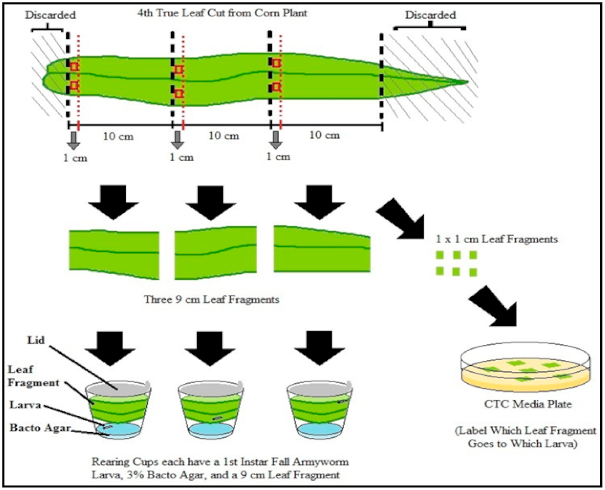
2.7. Feeding Behavior Assay and Experimental Design
2.8. Statistical Analyses
3. Results
3.1. Evaluation of Endophytic Colonization
3.2. Relative Growth Rate of Fall Armyworm
3.3. Maize Leaf Consumption by Fall Armyworm
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barlow, V.M.; Kuhar, T.P. Fall armyworm in vegetable crops. Va. Coop. Ext. 2009, 444, 1–3. [Google Scholar]
- Montezano, D.; Specht, A.; Sosa-Gómez, D.; Roque-Specht, V.; Sousa-Silva, J.; Paula-Moraes, S.; Peterson, J.; Hunt, T. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Zacarias, D.A. Global bioclimatic suitability for the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), and potential co-occurrence with major host crops under climate change scenarios. Clim. Chang. 2020, 161, 555–566. [Google Scholar] [CrossRef]
- FAO; CABI. Community-based fall armyworm (Spodoptera Frugiperda) monitoring, early warning and management; Food and Agriculture Organization: Rome, Italy, 2019; Volume 112, p. 5. [Google Scholar]
- Harrison, R.D.; Thierfelder, C.; Baudron, F.; Chinwada, P.; Midega, C.; Schaffner, U.; Berg, J.V.D. Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith) management: Providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manag. 2019, 243, 318–330. [Google Scholar] [CrossRef]
- CABI. New report reveals cost of fall armyworm to farmers in Africa, provides recommendations for control. Available online: https://www.cabi.org/news-article/new-report-reveals-cost-of-fall-armyworm-to-farmers-in-africa-provides-recommendations-for-control/ (accessed on 26 April 2021).
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Macauley, H. Cereal crops: Rice, maize, millet, sorghum, wheat: Background paper. In Proceedings of the Conference on Feeding Africa, Dakar, Senegal, 5–6 November 2015; pp. 1–31. [Google Scholar]
- Rwomushana, I.; Bateman, M.; Beale, T.; Beseh, P.; Cameron, K.; Chiluba, M.; Clottey, V.; Davis, T.; Day, R.; Early, R.; et al. Fall armyworm: Impacts and implications for Africa; CABI: Wallingford, UK, 2018. [Google Scholar]
- Kelly, P. ‘Monkey’ Business. Locke Stud. 2009, 9, 139–165. [Google Scholar] [CrossRef] [Green Version]
- De Lange, E.S.; Farnier, K.; Degen, T.; Gaudillat, B.; Aguilar-Romero, R.; Bahena-Juárez, F.; Oyama, K.; Turlings, T.C.J. Parasitic wasps can reduce mortality of teosinte plants infested with fall armyworm: Support for a defensive function of herbivore-induced plant volatiles. Front. Ecol. Evol. 2018, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Siebert, M.W.; Tindall, K.V.; Leonard, B.R.; Van Duyn, J.W.; Babcock, J.M. Evaluation of corn hybrids expressing CrylF (Herculex® I Insect Protection) against fall armyworm (Lepidoptera: Noctuidae) in the southern United States. J. Entomol. Sci. 2008, 43, 41–51. [Google Scholar] [CrossRef]
- Prasanna, B.; Huesing, J.E.; Eddy, R.; Peschke, V.M. Fall armyworm in Africa: A guide for integrated pest management; USAID CIMMYT: Texcoco, Mexico, 2018. [Google Scholar]
- Ingber, D.; Mason, C.; Flexner, L. Cry1 Bt susceptibilities of fall armyworm (Lepidoptera: Noctuidae) host strains. J. Econ. Entomol. 2018, 111, 361–368. [Google Scholar] [CrossRef]
- Behie, S.W.; Zelisko, P.M.; Bidochka, M.J. Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 2012, 336, 1576–1577. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Jiménez-Gasco, M.D.M.; Luthe, D.S.; Barbercheck, M.E. Systemic colonization by Metarhizium robertsii enhances cover crop growth. J. Fungi 2020, 6, 64. [Google Scholar] [CrossRef]
- Ahmad, I.; Zaib, S. Mighty microbes: Plant growth promoting microbes in soil health and sustainable agriculture; Springer: Cham, Switzerland, 2020; pp. 243–264. [Google Scholar]
- de Faria, M.R.; Wraight, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control. 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Lacey, L.; Grzywacz, D.; Shapiro-Ilan, D.; Frutos, R.; Brownbridge, M.; Goettel, M. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Cárdenas, O.G.; Cortez-Madrigal, H.; Malo, E.A.; Gómez-Ruíz, J.; Nord, R. Physiological and pathogenical characterization of Beauveria bassiana and Metarhizium anisopliae isolates for management of adult Spodoptera frugiperda. Southwest. Entomol. 2019, 44, 409. [Google Scholar] [CrossRef]
- Ramanujam, B.; Poornesha, B.; Shylesha, A.N. Effect of entomopathogenic fungi against invasive pest Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) in maize. Egypt. J. Biol. Pest Control. 2020, 30, 1–5. [Google Scholar] [CrossRef]
- Ramos, Y.; Taibo, A.D.; Jiménez, J.A.; Portal, O. Endophytic establishment of Beauveria bassiana and Metarhizium anisopliae in maize plants and its effect against Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) larvae. Egypt. J. Biol. Pest Control. 2020, 30, 1–6. [Google Scholar] [CrossRef]
- Hu, G.; Leger, R.J.S. Field studies using a recombinant mycoinsecticide (Metarhizium anisopliae) reveal that it is rhizosphere Competent. Appl. Environ. Microbiol. 2002, 68, 6383–6387. [Google Scholar] [CrossRef] [Green Version]
- Vega, F.E. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef] [PubMed]
- White, J.F.; Kingsley, K.L.; Zhang, Q.; Verma, R.; Obi, N.; Dvinskikh, S.; Elmore, M.T.; Verma, S.K.; Gond, S.K.; Kowalski, K.P. Review: Endophytic microbes and their potential applications in crop management. Pest Manag. Sci. 2019, 75, 2558–2565. [Google Scholar] [CrossRef]
- Ahmad, I.; Jiménez-Gasco, M.D.M.; Barbercheck, M.E. The role of endophytic insect-pathogenic fungi in biotic stress management; Springer: Singapore, 2020; pp. 379–400. [Google Scholar]
- Ahmad, I.; Jiménez-Gasco, M.D.M.; Luthe, D.S.; Shakeel, S.N.; Barbercheck, M.E. Endophytic Metarhizium robertsii promotes maize growth, suppresses insect growth, and alters plant defense gene expression. Biol. Control. 2020, 144, 104167. [Google Scholar] [CrossRef]
- Dutta, P.; Kaushik, H.; Bhawmick, P.; Puzari, K.C.; Hazarika, G.N. Metarhizium anisopliae as endophyte has the ability of plant growth enhancement. Int. J. Curr. Res. 2015, 7, 14300–14304. [Google Scholar]
- García, E.J.; Posadas, B.J.; Perticari, A.; Lecuona, R.E. Metarhizium anisopliae (Metschnikoff) Sorokin promotes growth and has endophytic activity in tomato plants. Adv. Biol. Res. 2011, 5, 22–27. [Google Scholar]
- Golo, P.S.; Gardner, D.R.; Grilley, M.M.; Takemoto, J.Y.; Krasnoff, S.B.; Pires, M.S.; Fernandes, E.; Bittencourt, V.R.E.P.; Roberts, D.W. Production of destruxins from Metarhizium spp. fungi in artificial medium and in endophytically colonized cowpea plants. PLoS ONE 2014, 9, e104946. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, H.; Dutta, P.; Himadri, K.; Pranab, D. Establishment of Metarhizium anisopliae, an entomopathogen as endophyte for biological control in tea. Res. Crop. 2016, 17, 375. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Chondrogiannis, C.; Grammatikopoulos, G. Effects of three endophytic entomopathogens on sweet sorghum and on the larvae of the stalk borer Sesamia nonagrioides. Entomol. Exp. Appl. 2015, 154, 78–87. [Google Scholar] [CrossRef]
- Behie, S.W.; Jones, S.J.; Bidochka, M.J. Plant tissue localization of the endophytic insect pathogenic fungi Metarhizium and Beauveria. Fungal Ecol. 2015, 13, 112–119. [Google Scholar] [CrossRef]
- Liao, X.; O’Brien, T.R.; Fang, W.; Leger, R.J.S. The plant beneficial effects of Metarhizium species correlate with their association with roots. Appl. Microbiol. Biotechnol. 2014, 98, 7089–7096. [Google Scholar] [CrossRef]
- Ríos-Moreno, A.; Garrido-Jurado, I.; Resquín-Romero, G.; Arroyo-Manzanares, N.; Arce, L.; Quesada-Moraga, E. Destruxin A production by Metarhizium brunneum strains during transient endophytic colonization of Solanum tuberosum. Biocontrol Sci. Technol. 2016, 26, 1574–1585. [Google Scholar] [CrossRef]
- Cruz-Avalos, A.M.; Bivián-Hernández, M.D.L.Á.; Ibarra, J.E.; Del Rincón-Castro, M.C. High virulence of Mexican entomopathogenic fungi against fall armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 2019, 112, 99–107. [Google Scholar] [CrossRef]
- Grijalba, E.P.; Espinel, C.; Cuartas, P.E.; Chaparro, M.L.; Villamizar, L.F. Metarhizium rileyi biopesticide to control Spodoptera frugiperda: Stability and insecticidal activity under glasshouse conditions. Fungal Biol. 2018, 122, 1069–1076. [Google Scholar] [CrossRef]
- Hernandez-Trejo, A.; Estrada-Drouaillet, B.; López-Santillán, J.A.; Rios-Velasco, C.; Rodríguez-Herrera, R.; Osorio-Hernández, E. Effects of native entomopathogenic fungal strains and neem extract on Spodoptera frugiperda on Maize. Southwest. Entomol. 2019, 44, 117–124. [Google Scholar] [CrossRef]
- Akutse, K.S.; Kimemia, J.W.; Ekesi, S.; Khamis, F.M.; Ombura, O.L.; Subramanian, S. Ovicidal effects of entomopathogenic fungal isolates on the invasive Fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Appl. Entomol. 2019, 143, 626–634. [Google Scholar] [CrossRef]
- de Lira, A.C.; Mascarin, G.M.; Delalibera, I., Jr. Microsclerotia production of Metarhizium spp. for dual role as plant biostimulant and control of Spodoptera frugiperda through corn seed coating. Fungal Biol. 2020, 124, 689–699. [Google Scholar] [CrossRef]
- Mwamburi, L.A. Endophytic fungi, Beauveria bassiana and Metarhizium anisopliae, confer control of the fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), in two tomato varieties. Egypt. J. Biol. Pest Control. 2021, 31, 1–6. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Mullen, C.; Barbercheck, M. Plant identity, but not diversity, and agroecosystem characteristics affect the occurrence of M. robertsii in an organic cropping system. Biol. Control. 2018, 124, 18–29. [Google Scholar] [CrossRef]
- Zimmermann, G. The ‘Galleria bait method’ for detection of entomopathogenic fungi in soil. J. Appl. Entomol. 1986, 102, 213–215. [Google Scholar] [CrossRef]
- Bischoff, J.F.; Rehner, S.A.; Humber, R.A. A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 2009, 101, 512–530. [Google Scholar] [CrossRef] [Green Version]
- Kepler, R.M.; Ugine, T.A.; Maul, J.E.; Cavigelli, M.A.; Rehner, S.A. Community composition and population genetics of insect pathogenic fungi in the genus Metarhizium from soils of a long-term agricultural research system. Environ. Microbiol. 2015, 17, 2791–2804. [Google Scholar] [CrossRef]
- Parsa, S.; Ortiz, V.; Vega, F.E. Establishing fungal entomopathogens as endophytes: Towards endophytic biological control. J. Vis. Exp. 2013, 74, e50360. [Google Scholar] [CrossRef] [Green Version]
- Jager, G.; Van Der Boon, J.; Rauw, G. The influence of soil steaming on some properties of the soil and on the growth and heading of winter glasshouse lettuce. I. Changes in chemical and physical properties. Neth. J. Agric. Sci. 1969, 17, 143–152. [Google Scholar] [CrossRef]
- Peiffer, M.; Felton, G.W. The host plant as a factor in the synthesis and secretion of salivary glucose oxidase in larval Helicoverpa zea. Arch. Insect Biochem. Physiol. 2005, 58, 106–113. [Google Scholar] [CrossRef]
- Perkins, W.D. Laboratory rearing of the fall armyworm. Fla. Entomol. 1979, 62, 87. [Google Scholar] [CrossRef]
- Chuang, W.-P.; Ray, S.; Acevedo, F.E.; Peiffer, M.; Felton, G.W.; Luthe, D.S. Herbivore cues from the fall armyworm (Spodoptera frugiperda) larvae trigger direct defenses in maize. Mol. Plant Microbe Interact. 2014, 27, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, W.A.; Poorter, H. Avoiding bias in calculations of relative growth rate. Ann. Bot. 2002, 90, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henniges-Janssen, K.; Heckel, D.G.; Groot, A.T. Preference of diamondback moth larvae for novel and original host plant after host range expansion. Insects 2014, 5, 793–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ives, A.R. For testing the significance of regression coefficients, go ahead and log-transform count data. Methods Ecol. Evol. 2015, 6, 828–835. [Google Scholar] [CrossRef]
- Sergaki, C.; Lagunas, B.; Lidbury, I.; Gifford, M.L.; Schäfer, P. Challenges and approaches in microbiome esearch: From fundamental to applied. Front. Plant Sci. 2018, 9, 1205. [Google Scholar] [CrossRef]
- Razinger, J.; Lutz, M.; Schroers, H.-J.; Urek, G.; Grunder, J. Evaluation of insect associated and plant growth promoting fungi in the control of cabbage root flies. J. Econ. Entomol. 2014, 107, 1348–1354. [Google Scholar] [CrossRef] [Green Version]
- Sasan, R.K.; Bidochka, M.J. The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am. J. Bot. 2012, 99, 101–107. [Google Scholar] [CrossRef]
- Clay, K.; Cheplick, G.P. Effect of ergot alkaloids from fungal endophyte-infected grasses on fall armyworm (Spodoptera frugiperda). J. Chem. Ecol. 1989, 15, 169–182. [Google Scholar] [CrossRef]
- Resquín-Romero, G.; Garrido-Jurado, I.; Delso, C.; Rios-Moreno, A.; Quesada-Moraga, E.J. Transient endophytic colonizations of plants improve the outcome of foliar applications of mycoinsecticides against chewing insects. J. Invertebr. Pathol. 2016, 136, 23–31. [Google Scholar] [CrossRef]
- Israni, B.; Wouters, F.C.; Luck, K.; Seibel, E.; Ahn, S.-J.; Paetz, C.; Reinert, M.; Vogel, H.; Erb, M.; Heckel, D.G.; et al. The fall armyworm Spodoptera frugiperda utilizes specific UDP-glycosyltransferases to inactivate maize defensive benzoxazinoids. Front. Physiol. 2020, 11, 604754. [Google Scholar] [CrossRef]
- Carvalho, I.; Erdmann, L.L.; Machado, L.L.; Rosa, A.P.S.A.; Zotti, M.J.; Neitzke, C.G. Metabolic resistance in the fall armyworm: An overview. J. Agric. Sci. 2018, 10, 426. [Google Scholar] [CrossRef]
- Chikate, Y.R.; Tamhane, V.; Joshi, R.S.; Gupta, V.S.; Giri, A.P. Differential protease activity augments polyphagy in Helicoverpa armigera. Insect Mol. Biol. 2013, 22, 258–272. [Google Scholar] [CrossRef]
- Giraudo, M.; Hilliou, F.; Fricaux, T.; Audant, P.; Feyereisen, R.; LE Goff, G. Cytochrome P450s from the fall armyworm (Spodoptera frugiperda): Responses to plant allelochemicals and pesticides. Insect Mol. Biol. 2014, 24, 115–128. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Xu, X.; Wang, J.; Yu, H.; Lai, R.; Gong, W. Trypsin inhibitory loop is an excellent lead structure to design serine protease inhibitors and antimicrobial peptides. FASEB J. 2007, 21, 2466–2473. [Google Scholar] [CrossRef] [Green Version]
- Després, L.; David, J.-P.; Gallet, C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef]
- Appel, H.M. The chewing herbivore gut lumen: Physicochemical conditions and their impact on plant nutrients, allelo-chemicals, and insect pathogens. In Insect-Plant Interactions, 5th ed.; CRC Press: Ann Arbor, MI, USA, 1994; Volume 5, pp. 210–223. [Google Scholar]
- Gimenez, S.; Abdelgaffar, H.; Le Goff, G.; Hilliou, F.; Blanco, C.A.; Hänniger, S.; Bretaudeau, A.; Legeai, F.; Nègre, N.; Jurat-Fuentes, J.L.; et al. Adaptation by copy number variation increases insecticide resistance in the fall armyworm. Commun. Biol. 2020, 3, 1–10. [Google Scholar] [CrossRef]
- De Bruyn, L.; Scheirs, J.; Verhagen, R. Nutrient stress, host plant quality and herbivore performance of a leaf-mining fly on grass. Oecologia 2002, 130, 594–599. [Google Scholar] [CrossRef]
- Larsson, S. Stressful times for the plant stress: Insect performance hypothesis. Oikos 1989, 56, 277. [Google Scholar] [CrossRef]
- Krell, V.; Jakobs-Schoenwandt, D.; Vidal, S.; Patel, A.V. Encapsulation of Metarhizium brunneum enhances endophytism in tomato plants. Biol. Control. 2018, 116, 62–73. [Google Scholar] [CrossRef]
- Niemeyer, H.M. Hydroxamic acids derived from 2-Hydroxy-2H-1,4-Benzoxazin-3(4H)-one: Key defense chemicals of cereals. J. Agric. Food Chem. 2009, 57, 1677–1696. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lòpez-Fernàndez, S.; Compant, S.; Vrhovsek, U.; Bianchedi, P.L.; Sessitsch, A.; Pertot, I.; Campisano, A. Grapevine colonization by endophytic bacteria shifts secondary metabolism and suggests activation of defense pathways. Plant Soil 2015, 405, 155–175. [Google Scholar] [CrossRef]
- Kunkel, B.N.; Brooks, D.M. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 2002, 5, 325–331. [Google Scholar] [CrossRef]
- Spoel, S.H.; Koornneef, A.; Claessens, S.M.C.; Korzelius, J.P.; Van Pelt, J.A.; Mueller, M.J.; Buchala, A.J.; Métraux, J.-P.; Brown, R.; Kazan, K.; et al. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 2003, 15, 760–770. [Google Scholar] [CrossRef] [Green Version]
- Clifton, E.H.; Jaronski, S.T.; Coates, B.S.; Hodgson, E.W.; Gassmann, A.J. Effects of endophytic entomopathogenic fungi on soybean aphid and identification of Metarhizium isolates from agricultural fields. PLoS ONE 2018, 13, e0194815. [Google Scholar] [CrossRef] [Green Version]
- Rasool, S.; Vidkjær, N.H.; Hooshmand, K.; Jensen, B.; Fomsgaard, I.S.; Meyling, N.V. Seed inoculations with entomopathogenic fungi affect aphid populations coinciding with modulation of plant secondary metabolite profiles across plant families. New Phytol. 2021, 229, 1715–1727. [Google Scholar] [CrossRef]
- Cachapa, J.C.; Meyling, N.V.; Burow, M.; Hauser, T.P. Induction and priming of plant defense by root-associated insect-pathogenic fungi. J. Chem. Ecol. 2021, 47, 112–122. [Google Scholar] [CrossRef]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Gichuhi, J.; Sevgan, S.; Khamis, F.; Berg, J.V.D.; Du Plessis, H.; Ekesi, S.; Herren, J. Diversity of fall armyworm, Spodoptera frugiperda and their gut bacterial community in Kenya. PeerJ 2020, 8, e8701. [Google Scholar] [CrossRef] [Green Version]
- Lezama Gutierrez, R.; Alatorre Rosas, R.; Bojalil Jaber, L.F.; Molina Ochoa, J.; Arenas Vargas, M.; Gonzalez Ramirez, M.; Rebolledo Dominguez, O. Virulence of five entomopathogenic fungi (Hyphomycetes) against Spodoptera frugiperda (Lepidop-tera: Noctuidae) eggs and neonate larvae. Vedalia Rev. Int. Control Biol. 1996, 3, 35–39. [Google Scholar]
- Schmelz, E.A.; Alborn, H.T.; Tumlinson, J.H. The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta 2001, 214, 171–179. [Google Scholar] [CrossRef]
- Acevedo, F.E.; Peiffer, M.; Tan, C.-W.; Stanley, B.A.; Stanley, A.; Wang, J.; Jones, A.G.; Hoover, K.; Rosa, C.; Luthe, D.; et al. Fall armyworm-associated gut bacteria modulate plant defense responses. Mol. Plant Microbe Interact. 2017, 30, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Santiago, R.; Cao, A.; Butrón, A.; López-Malvar, A.; Rodríguez, V.M.; Sandoya, G.V.; Malvar, R.A. Defensive changes in maize leaves induced by feeding of Mediterranean corn borer larvae. BMC Plant Biol. 2017, 17, 44. [Google Scholar] [CrossRef] [Green Version]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef]
- Cook, D.; Gardner, D.R.; Welch, K.D.; Roper, J.M.; Ralphs, M.H.; Green, B.T. Quantitative PCR method to measure the fungal endophyte in locoweeds. J. Agric. Food Chem. 2009, 57, 6050–6054. [Google Scholar] [CrossRef]
- Jaber, L.R.; Vidal, S. Fungal endophyte negative effects on herbivory are enhanced on intact plants and maintained in a subsequent generation. Ecol. Entomol. 2010, 35, 25–36. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flonc, B.; Barbercheck, M.; Ahmad, I. Observations on the Relationships between Endophytic Metarhizium robertsii, Spodoptera frugiperda (Lepidoptera: Noctuidae), and Maize. Pathogens 2021, 10, 713. https://doi.org/10.3390/pathogens10060713
Flonc B, Barbercheck M, Ahmad I. Observations on the Relationships between Endophytic Metarhizium robertsii, Spodoptera frugiperda (Lepidoptera: Noctuidae), and Maize. Pathogens. 2021; 10(6):713. https://doi.org/10.3390/pathogens10060713
Chicago/Turabian StyleFlonc, Brianna, Mary Barbercheck, and Imtiaz Ahmad. 2021. "Observations on the Relationships between Endophytic Metarhizium robertsii, Spodoptera frugiperda (Lepidoptera: Noctuidae), and Maize" Pathogens 10, no. 6: 713. https://doi.org/10.3390/pathogens10060713
APA StyleFlonc, B., Barbercheck, M., & Ahmad, I. (2021). Observations on the Relationships between Endophytic Metarhizium robertsii, Spodoptera frugiperda (Lepidoptera: Noctuidae), and Maize. Pathogens, 10(6), 713. https://doi.org/10.3390/pathogens10060713





