Antibiotic Susceptibility of Bartonella Grown in Different Culture Conditions
Abstract
1. Introduction
2. Results
2.1. Comparison of MIC with Different Media
2.2. Comparison of MBC
2.3. Live/Dead Staining
3. Discussion
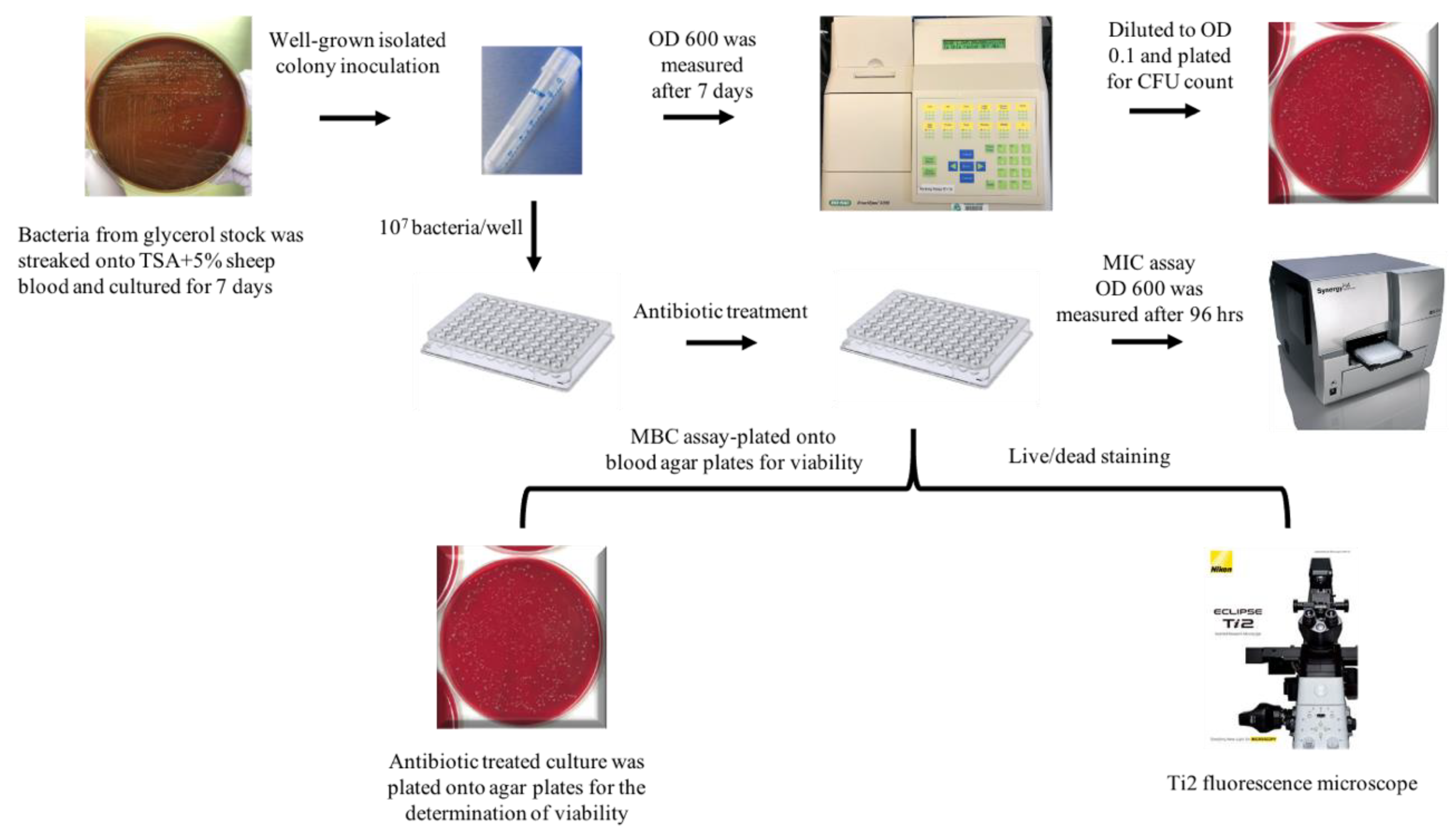
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Live/Dead Staining
4.3. DH82 Cell Culture and Seeding Density
4.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assays
4.5. Antibiotics and Their Dilution
4.6. DH82 Cell-Based, MBC Assay
4.7. Immunofluorescence Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Álvarez-Fernández, A.; Breitschwerdt, E.B.; Solano-Gallego, L. Bartonella Infections in Cats and Dogs Including Zoonotic Aspects. Parasit Vectors 2018, 11. [Google Scholar] [CrossRef]
- Lamas, C.; Curi, A.; Bóia, M.N.; Lemos, E.R.S. Human Bartonellosis: Seroepidemiological and Clinical Features with an Emphasis on Data from Brazil-A Review. Mem. Inst. Oswaldo Cruz 2008, 103, 221–235. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B. Bartonellosis: One Health Perspectives for an Emerging Infectious Disease. ILAR J. 2014, 55, 46–58. [Google Scholar] [CrossRef]
- Kaiser, P.O.; Riess, T.; O’Rourke, F.; Linke, D.; Kempf, V.A.J. Bartonella Spp.: Throwing Light on Uncommon Human Infections. Int. J. Med. Microbiol. 2011, 301, 7–15. [Google Scholar] [CrossRef]
- Ericson, M.E.; Breitschwerdt, E.B.; Reicherter, P.; Maxwell, C.; Maggi, R.G.; Melvin, R.G.; Maluki, A.H.; Bradley, J.M.; Miller, J.C.; Simmons, G.E.; et al. Bartonella Henselae Detected in Malignant Melanoma, a Preliminary Study. Pathogens 2021, 10, 326. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Bradley, J.M.; Maggi, R.G.; Lashnits, E.; Reicherter, P. Bartonella Associated Cutaneous Lesions (BACL) in People with Neuropsychiatric Symptoms. Pathogens 2020, 9, 1023. [Google Scholar] [CrossRef]
- Angelakis, E.; Raoult, D. Pathogenicity and Treatment of Bartonella Infections. Int. J. Antimicrob. Agents 2014, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G.; Bolger, A.F.; Levison, M.E.; Ferrieri, P.; Gerber, M.A.; Tani, L.Y.; Gewitz, M.H.; et al. Infective Endocarditis: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Statement for Healthcare Professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: Endorsed by the Infectious Diseases Society of America. Circulation 2005, 111, e394–e434. [Google Scholar] [CrossRef] [PubMed]
- Breitschwerdt, E.B. Bartonellosis, One Health and All Creatures Great and Small. Vet. Dermatol. 2017, 28, 96-e21. [Google Scholar] [CrossRef] [PubMed]
- Meghari, S.; Rolain, J.-M.; Grau, G.E.; Platt, E.; Barrassi, L.; Mege, J.-L.; Raoult, D. Antiangiogenic Effect of Erythromycin: An in Vitro Model of Bartonella Quintana Infection. J. Infect. Dis. 2006, 193, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.M.; Brouqui, P.; Koehler, J.E.; Maguina, C.; Dolan, M.J.; Raoult, D. Recommendations for Treatment of Human Infections Caused by Bartonella Species. Antimicrob. Agents Chemother. 2004, 48, 1921–1933. [Google Scholar] [CrossRef]
- Clemente, N.S.; Ugarte-Gil, C.A.; Solórzano, N.; Maguiña, C.; Pachas, P.; Blazes, D.; Bailey, R.; Mabey, D.; Moore, D. Bartonella Bacilliformis: A Systematic Review of the Literature to Guide the Research Agenda for Elimination. PLoS Negl. Trop. Dis. 2012, 6, e1819. [Google Scholar] [CrossRef]
- Prutsky, G.; Domecq, J.P.; Mori, L.; Bebko, S.; Matzumura, M.; Sabouni, A.; Shahrour, A.; Erwin, P.J.; Boyce, T.G.; Montori, V.M.; et al. Treatment Outcomes of Human Bartonellosis: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2013, 17, e811–e819. [Google Scholar] [CrossRef]
- Maguiña Vargas, C. Bartonellosis o enfermedad de Carrión: Nuevos aspectos de una vieja enfermedad. Rev. Inst. Med. Trop. São Paulo 2003, 45, 22. [Google Scholar] [CrossRef]
- Sharma, R.; Arshad, A.M.; Sardar, S.; Zafar, A. Hepatosplenic Bartonellosis in an Immunocompetent Teenager: An Atypical Presentation of Cat-Scratch Disease. Cureus 2021, 13. [Google Scholar] [CrossRef]
- García, J.C.; Núñez, M.J.; Castro, B.; Fernández, J.M.; Portillo, A.; Oteo, J.A. Hepatosplenic Cat Scratch Disease in Immunocompetent Adults: Report of 3 Cases and Review of the Literature. Medicine 2014, 93, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Pendle, S.; Ginn, A.; Iredell, J. Antimicrobial Susceptibility of Bartonella Henselae Using Etest Methodology. J. Antimicrob. Chemother. 2006, 57, 761–763. [Google Scholar] [CrossRef]
- Tsuneoka, H.; Yanagihara, M.; Nojima, J.; Ichihara, K. Antimicrobial Susceptibility by Etest of Bartonella Henselae Isolated from Cats and Human in Japan. J. Infect. Chemother. 2010, 16, 446–448. [Google Scholar] [CrossRef]
- Maurin, M.; Gasquet, S.; Ducco, C.; Raoult, D. MICs of 28 Antibiotic Compounds for 14 Bartonella (Formerly Rochalimaea) Isolates. Antimicrob. Agents Chemother. 1995, 39, 2387–2391. [Google Scholar] [CrossRef]
- Dörbecker, C.; Sander, A.; Oberle, K.; Schülin-Casonato, T. In Vitro Susceptibility of Bartonella Species to 17 Antimicrobial Compounds: Comparison of Etest and Agar Dilution. J. Antimicrob. Chemother. 2006, 58, 784–788. [Google Scholar] [CrossRef]
- Li, T.; Feng, J.; Xiao, S.; Shi, W.; Sullivan, D.; Zhang, Y. Identification of FDA-Approved Drugs with Activity against Stationary Phase Bartonella Henselae. Antibiot. Basel 2019, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ma, X.; Li, T.; Shi, W.; Zhang, Y. Effect of Different Drugs and Drug Combinations on Killing Stationary Phase and Biofilms Recovered Cells of Bartonella Henselae in Vitro. BMC Microbiol. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Wellman, M.L.; Krakowka, S.; Jacobs, R.M.; Kociba, G.J. A Macrophage-Monocyte Cell Line from a Dog with Malignant Histiocytosis. Vitr. Cell Dev. Biol. 1988, 24, 223–229. [Google Scholar] [CrossRef]
- Huys, G.; D’Haene, K.; Swings, J. Influence of the Culture Medium on Antibiotic Susceptibility Testing of Food-Associated Lactic Acid Bacteria with the Agar Overlay Disc Diffusion Method. Lett. Appl. Microbiol. 2002, 34, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.G.; Duncan, A.W.; Breitschwerdt, E.B. Novel Chemically Modified Liquid Medium That Will Support the Growth of Seven Bartonella Species. J. Clin. Microbiol. 2005, 43, 2651–2655. [Google Scholar] [CrossRef]
- Podsiadły, E.; Żabicka, D.; Demkow, U.; Hryniewicz, W.; Tylewska-Wierzbanowska, S. Susceptibility of Polish Bartonella Henselae Strains. Pol J. Microbiol. 2012, 61, 143–145. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and Resistance Mechanisms of Antibiotics: A Guide for Clinicians. J. Anaesthesiol. Clin Pharm. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Bartonella Species. Available online: http://www.antimicrobe.org/new/m01.asp#t7b (accessed on 24 April 2021).
- Pothineni, V.R.; Potula, H.-H.S.K.; Ambati, A.; Mallajosyula, V.V.A.; Sridharan, B.; Inayathullah, M.; Ahmed, M.S.; Rajadas, J. Azlocillin Can Be the Potential Drug Candidate against Drug-Tolerant Borrelia Burgdorferi Sensu Stricto JLB31. Sci. Rep. 2020, 10, 3798. [Google Scholar] [CrossRef]
- Chaves, B.J.; Tadi, P. Gentamicin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Battisti, J.M.; Sappington, K.N.; Smitherman, L.S.; Parrow, N.L.; Minnick, M.F. Environmental Signals Generate a Differential and Coordinated Expression of the Heme Receptor Gene Family of Bartonella Quintana. Infect. Immun. 2006, 74, 3251–3261. [Google Scholar] [CrossRef][Green Version]
- Battisti, J.M.; Smitherman, L.S.; Sappington, K.N.; Parrow, N.L.; Raghavan, R.; Minnick, M.F. Transcriptional Regulation of the Heme Binding Protein Gene Family of Bartonella Quintana Is Accomplished by a Novel Promoter Element and Iron Response Regulator. Infect. Immun. 2007, 75, 4373–4385. [Google Scholar] [CrossRef]
- Minnick, M.F.; Anderson, B.E. Chapter 105—Bartonella. In Molecular Medical Microbiology, 2nd ed.; Tang, Y.-W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Academic Press: Boston, FL, USA, 2015; pp. 1911–1939. ISBN 978-0-12-397169-2. [Google Scholar]
- Kempf, V.A.; Schaller, M.; Behrendt, S.; Volkmann, B.; Aepfelbacher, M.; Cakman, I.; Autenrieth, I.B. Interaction of Bartonella Henselae with Endothelial Cells Results in Rapid Bacterial RRNA Synthesis and Replication. Cell Microbiol. 2000, 2, 431–441. [Google Scholar] [CrossRef]
- Yoneyama, H.; Katsumata, R. Antibiotic Resistance in Bacteria and Its Future for Novel Antibiotic Development. Biosci. Biotechnol. Biochem. 2006, 70, 1060–1075. [Google Scholar] [CrossRef]
- Wise, R. A Review of the Mechanisms of Action and Resistance of Antimicrobial Agents. Can. Respir. J. 1999, 6 (Suppl. A), 2A–20A. [Google Scholar]
- Stiefel, P.; Schmidt-Emrich, S.; Maniura-Weber, K.; Ren, Q. Critical Aspects of Using Bacterial Cell Viability Assays with the Fluorophores SYTO9 and Propidium Iodide. BMC Microbiol. 2015, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Brouqui, P.; Lascola, B.; Roux, V.; Raoult, D. Chronic Bartonella Quintana Bacteremia in Homeless Patients. N. Engl. J. Med. 1999, 340, 184–189. [Google Scholar] [CrossRef]
- Droz, S.; Chi, B.; Horn, E.; Steigerwalt, A.G.; Whitney, A.M.; Brenner, D.J. Bartonella Koehlerae Sp. Nov., Isolated from Cats. J. Clin. Microbiol. 1999, 37, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.; Iverson, J.; Kosoy, M. Combining Culture Techniques for Bartonella: The Best of Both Worlds▿. J. Clin. Microbiol. 2011, 49, 1363–1368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rolain, J.-M.; Foucault, C.; Guieu, R.; Scola, B.L.; Brouqui, P.; Raoult, D. Bartonella Quintana in Human Erythrocytes. Lancet 2002, 360, 226–228. [Google Scholar] [CrossRef]
- Rolain, J.M.; La Scola, B.; Liang, Z.; Davoust, B.; Raoult, D. Immunofluorescent Detection of Intraerythrocytic Bartonella Henselae in Naturally Infected Cats. J. Clin. Microbiol. 2001, 39, 2978–2980. [Google Scholar] [CrossRef][Green Version]
- Rolain, J.-M.; Maurin, M.; Mallet, M.-N.; Parzy, D.; Raoult, D. Culture and Antibiotic Susceptibility of Bartonella Quintana in Human Erythrocytes. Antimicrob. Agents Chemother. 2003, 47, 614–619. [Google Scholar] [CrossRef][Green Version]
- Breitschwerdt, E.B.; Hegarty, B.C.; Maggi, R.; Hawkins, E.; Dyer, P. Bartonella Species as a Potential Cause of Epistaxis in Dogs. J. Clin. Microbiol. 2005, 43, 2529–2533. [Google Scholar] [CrossRef] [PubMed]
- Neupane, P.; Hegarty, B.C.; Marr, H.S.; Maggi, R.G.; Birkenheuer, A.J.; Breitschwerdt, E.B. Evaluation of Cell Culture-Grown Bartonella Antigens in Immunofluorescent Antibody Assays for the Serological Diagnosis of Bartonellosis in Dogs. J. Vet. Intern. Med. 2018, 32, 1958–1964. [Google Scholar] [CrossRef]
- Lashnits, E.; Neupane, P.; Maggi, R.G.; Linder, K.E.; Bradley, J.M.; Balakrishnan, N.; Southern, B.L.; McKeon, G.P.; Chandrashekar, R.; Breitschwerdt, E.B. Detection of Bartonella Spp. in Dogs after Infection with Rickettsia Rickettsii. J. Vet. Intern. Med. 2020, 34, 145–159. [Google Scholar] [CrossRef]
- Oteo, J.A.; Maggi, R.; Portillo, A.; Bradley, J.; García-Álvarez, L.; San-Martín, M.; Roura, X.; Breitschwerdt, E. Prevalence of Bartonella Spp. by Culture, PCR and Serology, in Veterinary Personnel from Spain. Parasites Vectors 2017, 10, 553. [Google Scholar] [CrossRef]
- Plettenberg, A.; Lorenzen, T.; Burtsche, B.T.; Rasokat, H.; Kaliebe, T.; Albrecht, H.; Mertenskötter, T.; Bogner, J.R.; Stoehr, A.; Schöfer, H. Bacillary Angiomatosis in HIV-Infected Patients--an Epidemiological and Clinical Study. Dermatology 2000, 201, 326–331. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Zhang, S.; Shi, W.; Zhang, Y. An Optimized SYBR Green I/PI Assay for Rapid Viability Assessment and Antibiotic Susceptibility Testing for Borrelia Burgdorferi. PLoS ONE 2014, 9, e111809. [Google Scholar] [CrossRef]
- Salvatore, P.; Casamassimi, A.; Sommese, L.; Fiorito, C.; Ciccodicola, A.; Rossiello, R.; Avallone, B.; Grimaldi, V.; Costa, V.; Rienzo, M.; et al. Detrimental Effects of Bartonella Henselae Are Counteracted by L-Arginine and Nitric Oxide in Human Endothelial Progenitor Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9427–9432. [Google Scholar] [CrossRef]
- Schiavano, G.F.; Dominici, S.; Rinaldi, L.; Cangiano, A.M.; Brandi, G.; Magnani, M. Modulation of Stat-1 in Human Macrophages Infected with Different Species of Intracellular Pathogenic Bacteria. J. Immunol. Res. 2016, 2016, e5086928. [Google Scholar] [CrossRef] [PubMed]
- Gadila, S.K.G.; Rosoklija, G.; Dwork, A.J.; Fallon, B.A.; Embers, M.E. Detecting Borrelia Spirochetes: A Case Study With Validation Among Autopsy Specimens. Front. Neurol. 2021, 12, 27. [Google Scholar] [CrossRef] [PubMed]




| B. henselae | B. vinsonii | ||||
|---|---|---|---|---|---|
| MIC (µg/mL) | MIC (µg/mL) | ||||
| Antibiotics | Schneider’s | BAPGM | Grace’s | Grace’s | Antibiotics |
| Ceftriaxone | >0.3 | 0.01–0.1 | 0.01–0.1 | 0–0.01 | Ceftriaxone |
| Doxycycline | >0.3 | 0.01–0.1 | 0.01–0.1 | 0.01–0.1 | Doxycycline |
| Gentamicin | >1 | 0.1–0.5 | 2.0–4.0 | 2.0–4.0 | Gentamicin |
| Azithromycin | >0.02 | 0.005–0.02 | 0.3–0.5 | 0.1–0.3 | Azithromycin |
| Azlocillin | >2 | 0.01–0.02 | 0.005–0.01 | 0.001–0.005 | Azlocillin |
| Ampicillin | 0.02–0.1 | 0.01–0.02 | Ampicillin | ||
| Cell-Free, Liquid Culture Assay | DH82 Cell-Based Assay | ||
|---|---|---|---|
| MBC (µg/mL) | MBC (µg/mL) | ||
| Antibiotics | B. henselae | B. vinsonii | B. henselae |
| Ceftriaxone | 2.0–4.0 | 0.5–1.0 | >16 |
| Doxycycline | >10 | >10 | >16 |
| Gentamycin | 2.0–4.0 | 4.0–8.0 | >16 |
| Azithromycin | 5.0–10 | 2.0-5.0 | >16 |
| Azlocillin | 1.0–2.0 | 0.25–0.5 | >16 |
| Ampicillin | >8 | >8 | >16 |
| DH82 Cell-Based Assay | Cell-Free, Liquid Culture Assay | ||
|---|---|---|---|
| Antibiotic Combination | Concentration (µg/mL) | CFU/mL after 96 h of Drug Exposure | CFU/mL after 96 h of Drug Exposure |
| Drug free control | 0 | 1.03 ± 0.76 × 104 | 1 ± 0.1 × 109 |
| Azithromycin + ampicillin | 16 | 0 | 0 |
| 8 | 0 | 0 | |
| 4 | 0 | 3.5 ± 2.12 | |
| 2 | 10 | 37.5 ± 3.54 | |
| 1 | ND | 60.5 ± 6.36 | |
| 0.5 | ND | 1.25 ± 0.35 × 103 | |
| Drug free control | 0 | 0.6 3± 0.61 × 104 | 1.15 ± 0.21 × 109 |
| Azithromycin + azlocillin | 16 | 0 | 0 |
| 8 | 0 | 0 | |
| 4 | 0 | 0 | |
| 2 | 0 | 0 | |
| 1 | ND | 11 ± 4.24 | |
| 0.5 | ND | 2.02 ± 0.33 × 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadila, S.K.G.; Embers, M.E. Antibiotic Susceptibility of Bartonella Grown in Different Culture Conditions. Pathogens 2021, 10, 718. https://doi.org/10.3390/pathogens10060718
Gadila SKG, Embers ME. Antibiotic Susceptibility of Bartonella Grown in Different Culture Conditions. Pathogens. 2021; 10(6):718. https://doi.org/10.3390/pathogens10060718
Chicago/Turabian StyleGadila, Shiva Kumar Goud, and Monica E. Embers. 2021. "Antibiotic Susceptibility of Bartonella Grown in Different Culture Conditions" Pathogens 10, no. 6: 718. https://doi.org/10.3390/pathogens10060718
APA StyleGadila, S. K. G., & Embers, M. E. (2021). Antibiotic Susceptibility of Bartonella Grown in Different Culture Conditions. Pathogens, 10(6), 718. https://doi.org/10.3390/pathogens10060718






