Periodontal Pathogens and Preterm Birth: Current Knowledge and Further Interventions
Abstract
:1. Introduction. Preterm Labor
1.1. Definition and Epidemiology
1.2. Etiology and Risk Factors
2. Materials and Methods
3. The Link between Infectious Pathogens and Preterm Labor
3.1. Infectious Pathogens as a Cause of Preterm Labor
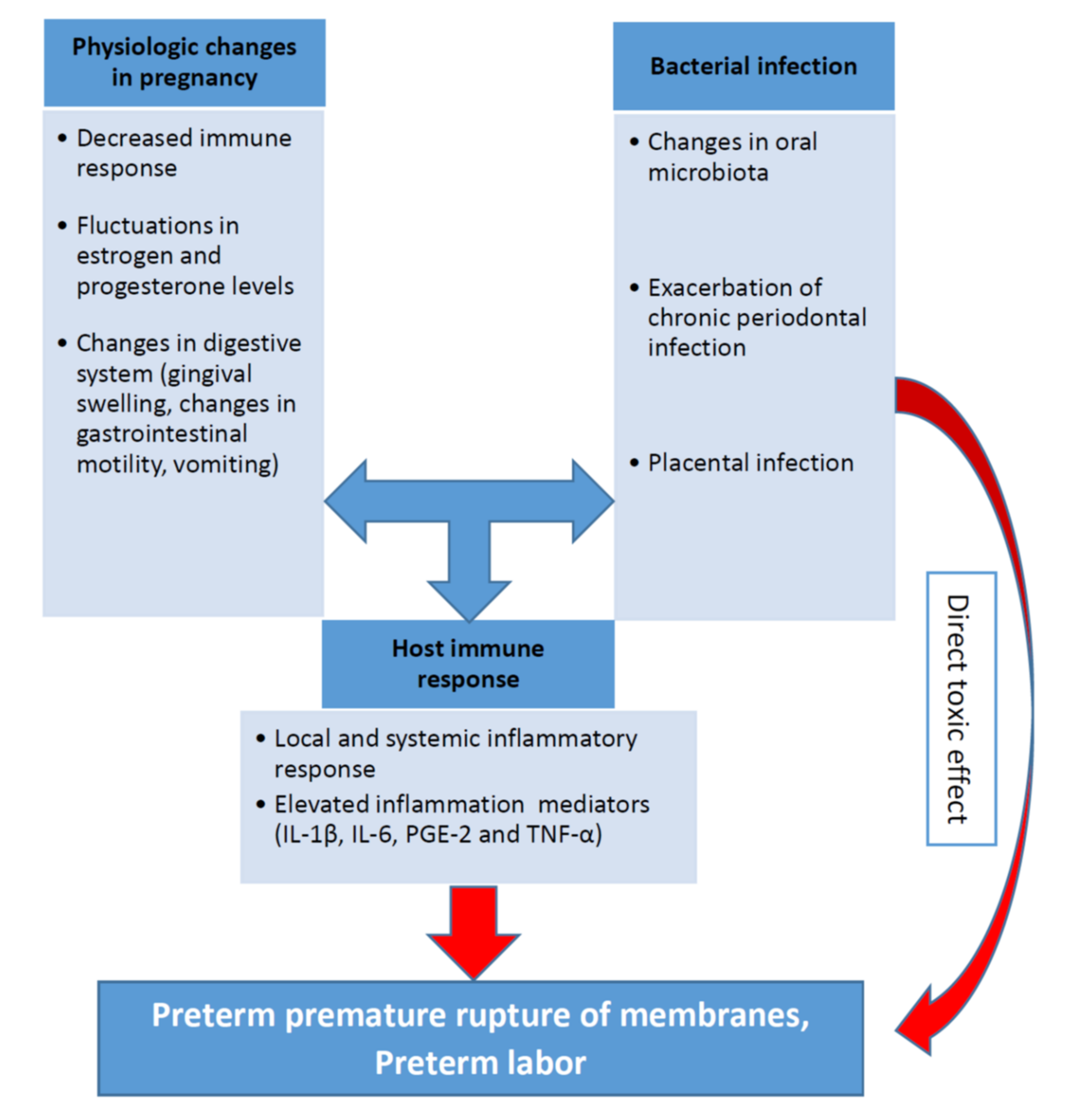
3.2. Pathophysiological Mechanisms
4. Periodontal Pathogens and Pregnancy
4.1. Anatomical and Physiological Background
4.1.1. Gingival Changes in Pregnancy
4.1.2. Factors Influencing Gingival Changes
4.2. Relationship between the Presence of Periodontal Pathogens in the Oral Cavity and the Preterm Labor
4.2.1. Definition, Epidemiology and Classification of Periodontal Disease
4.2.2. Pathophysiologic Mechanisms of Periodontal Disease
4.2.3. The Link of Periodontal Disease in Pregnancy and Preterm Birth: Studies Contradictions
4.3. Development of the Periodontal Disease in Pregnancy
5. Possible Management
5.1. Dental Management during Pregnancy
5.2. Treatment Modalities Based on the Gestational Age
5.3. The Importance of Oral Care before Conception
5.4. Future Tasks for Gynecologists and Dentists
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [Green Version]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 171: Management of Preterm Labor. Obs. Gynecol. 2016, 128, e155–e164. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, G.; Arabin, B.; Antsaklis, A.; Cabero Roura, L. Preterm Labor: Up to Date. Biomed. Res. Int. 2019, 2019, 4870938. [Google Scholar] [CrossRef]
- Slattery, M.M.; Morrison, J.J. Preterm delivery. Lancet 2002, 360, 1489–1497. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Berger, R.; Abele, H.; Bahlmann, F.; Bedei, I.; Doubek, K.; Felderhoff-Müser, U.; Fluhr, H.; Garnier, Y.; Grylka-Baeschlin, S.; Helmer, H.; et al. Prevention and Therapy of Preterm Birth. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry Number 015/025, February 2019)—Part 1 with Recommendations on the Epidemiology, Etiology, Prediction, Primary and Secondary Prevention of Preterm Birth. Geburtshilfe und Frauenheilkunde 2019, 79, 800–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.; Du, M. Role of Maternal Periodontitis in Preterm Birth. Front. Immunol. 2017, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Glover, A.V.; Manuck, T.A. Screening for spontaneous preterm birth and resultant therapies to reduce neonatal morbidity and mortality: A review. Semin. Fetal Neonatal. Med. 2018, 23, 126–132. [Google Scholar] [CrossRef]
- Figuero, E.; Han, Y.W.; Furuichi, Y. Periodontal diseases and adverse pregnancy outcomes: Mechanisms. Periodontol 2000 2020, 83, 175–188. [Google Scholar] [CrossRef]
- Jajoo, N.S.; Shelke, A.U.; Bajaj, R.S.; Patil, P.P.; Patil, M.A. Association of periodontitis with pre term low birth weight—A review. Placenta 2020, 95, 62–68. [Google Scholar] [CrossRef]
- Gonçalves, L.F.; Chaiworapongsa, T.; Romero, R. Intrauterine infection and prematurity. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 3–13. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 17–42. [Google Scholar] [CrossRef]
- Leitich, H. Secondary predictors of preterm labour. Int. J. Obstet. Gynaecol. 2005, 112, 48–50. [Google Scholar] [CrossRef]
- Fidel, P.L.; Romero, R.; Wolf, N.; Cutright, J.; Ramirez, M.; Araneda, H.; Cotton, D.B. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am. J. Obstet. Gynecol. 1994, 170, 1467–1475. [Google Scholar] [CrossRef]
- Wang, H.; Hirsch, E. Bacterially-Induced Preterm Labor and Regulation of Prostaglandin-Metabolizing Enzyme Expression in Mice: The Role of Toll-Like Receptor 41. Biol. Reprod. 2003, 69, 1957–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaul, A.K.; Khan, S.; Martens, M.G.; Crosson, J.T.; Lupo, V.R.; Kaul, R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect. Immun. 1999, 67, 5958–5966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Offenbacher, S. Maternal periodontal infections, prematurity, and growth restriction. Clin. Obstet. Gynecol. 2004, 47, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Offenbacher, S.; Boggess, K.A.; Murtha, A.P.; Jared, H.L.; Lieff, S.; McKaig, R.G.; Mauriello, S.M.; Moss, K.L.; Beck, J.D. Progressive Periodontal Disease and Risk of Very Preterm Delivery. Obstet. Gynecol. 2006, 107, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smaill, F.M.; Vazquez, J.C. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst. Rev. 2019, 11, CD000490. [Google Scholar] [CrossRef]
- Dautt-Leyva, J.G.; Canizalez-Román, A.; Acosta Alfaro, L.F.; Gonzalez-Ibarra, F.; Murillo-Llanes, J. Maternal and perinatal complications in pregnant women with urinary tract infection caused by Escherichia coli. J. Obstet. Gynaecol. Res. 2018, 44, 1384–1390. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine Infection and Preterm Delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef]
- Fischer, L.A.; Demerath, E.; Bittner-Eddy, P.; Costalonga, M. Placental colonization with periodontal pathogens: The potential missing link. Am. J. Obstet. Gynecol. 2019, 221, 383–392.e3. [Google Scholar] [CrossRef]
- Brabant, G. Vaginose bactérienne et prématurité spontanée [Bacterial vaginosis and spontaneous preterm birth]. J. Gynecol. Obstet. Biol. Reprod. 2016, 45, 1247–1260. [Google Scholar] [CrossRef]
- Klebanoff, M.A.; Brotman, R.M. Treatment of bacterial vaginosis to prevent preterm birth. Lancet 2018, 392, 2141–2142. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Johnson, D.C. Maternal Infection and Adverse Fetal and Neonatal Outcomes. Clin. Perinatol. 2005, 32, 523–559. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Andrews, W.W.; Yuan, A.C.; Mackay, H.T.; Louis, M.E.S. Sexually Transmitted Diseases and Adverse Outcomes of Pregnancy. Clin. Perinatol. 1997, 24, 23–41. [Google Scholar] [CrossRef]
- Sanz, M.; Kornman, K.; Working group 3 of the joint EFP/AAP workshop. Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S164–S169. [Google Scholar] [CrossRef]
- Gürsoy, M.; Könönen, E.; Gursoy, U.K.; Tervahartiala, T.; Pajukanta, R.; Sorsa, T. Periodontal Status and Neutrophilic Enzyme Levels in Gingival Crevicular Fluid During Pregnancy and Postpartum. J. Periodontol. 2010, 81, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Moosa, Y.; Kwon, D.; De Oliveira, T.; Wong, E.B. Determinants of Vaginal Microbiota Composition. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Yenen, Z.; Ataçağ, T. Oral care in pregnancy. J. Turk. Gynecol. Assoc. 2019, 20, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, E.; Robinson, P.J. Influence of Pregnancy on the Oral Cavity. Glob. Libr. Women’s Med 2009. [Google Scholar] [CrossRef]
- Silk, H.; Douglass, A.B.; Douglass, J.M.; Silk, L. Oral health during pregnancy. Am. Fam. Physician. 2008, 77, 1139–1144. [Google Scholar]
- Offenbacher, S.; Katz, V.; Fertik, G.; Collins, J.; Boyd, D.; Maynor, G.; McKaig, R.; Beck, J. Periodontal infection as a possible risk factor for preterm low birth weight. J. Periodontol. 1996, 67, 1103–1113. [Google Scholar] [CrossRef]
- Musskopf, M.L.; Milanesi, F.C.; da Rocha, J.M.; Fiorini, T.; Moreira, C.H.C.; Susin, C.; Rosing, C.K.; Weidlich, P.; Oppermann, R.V. Oral health related quality of life among pregnant women: A randomized controlled trial. Braz. Oral Res. 2018, 32, e002. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.K.; Parry, S. Periodontal Disease and Pregnancy Outcomes: Time to Move On? J. Women’s Health 2012, 21, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Saadaoui, M.; Singh, P.; Al Khodor, S. Oral microbiome and pregnancy: A bidirectional relationship. J. Reprod. Immunol. 2021, 145, 103293. [Google Scholar] [CrossRef] [PubMed]
- Daalderop, L.A.; Wieland, B.V.; Tomsin, K.; Reyes, L.; Kramer, B.W.; Vanterpool, S.F.; Been, J.V. Periodontal Disease and Pregnancy Outcomes: Overview of Systematic Reviews. JDR Clin. Trans. Res. 2018, 3, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Calixto, N.; Alves, C.; Abreu, L.; Thomaz, E.; Vidal, F.; Gomes-Filho, I.; Lopes, F. Detection of periodontal pathogens in mothers of preterm birth and/or low weight. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e776–e781. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinane, D.; Bouchard, P.; Group E of the European Workshop on Periodontology. Periodontal diseases and health: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 333–337. [Google Scholar] [CrossRef]
- Mealey, B.L.; Rose, L.F. Diabetes mellitus and inflammatory periodontal diseases. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kannosh, I.; Staletovic, D.; Toljic, B.; Radunovic, M.; Pucar, A.; Petrovic, S.M.; Grubisa, I.; Lazarevic, M.; Brkic, Z.; Vukcevic, J.K.; et al. The presence of periopathogenic bacteria in subgingival and atherosclerotic plaques–An age related comparative analysis. J. Infect. Dev. Count. 2018, 12, 1088–1095. [Google Scholar] [CrossRef] [Green Version]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Kaur, S.; White, S.; Bartold, P.M. Periodontal disease and rheumatoid arthritis: A systematic review. J. Dental Res. 2013, 92, 399–408. [Google Scholar] [CrossRef]
- Whitmore, S.E.; Lamont, R.J. Oral Bacteria and Cancer. PLoS Pathog. 2014, 10, e1003933. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S.; Page, R.C.; Tonetti, M.S. The host response to the microbial challenge in periodontitis: Assembling the players. Periodontol. 2000 1997, 14, 33–53. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feres, M.; Teles, F.; Teles, R.; Figueiredo, L.C.; Faveri, M. The subgingival periodontal microbiota of the aging mouth. Periodontol. 2000 2016, 72, 30–53. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol. 2000 2016, 70, 142–163. [Google Scholar] [CrossRef] [PubMed]
- Caneiro-Queija, L.; López-Carral, J.; Martin-Lancharro, P.; Limeres-Posse, J.; Diz-Dios, P.; Blanco-Carrion, J. Non-Surgical Treatment of Periodontal Disease in a Pregnant Caucasian Women Population: Adverse Pregnancy Outcomes of a Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2019, 16, 3638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carta, G.; Persia, G.; Falciglia, K.; Iovenitti, P. Periodontal disease and poor obstetrical outcome. Clin. Exp. Obstet. Gynecol. 2004, 31, 47–49. [Google Scholar]
- López, N.J.; Da Silva, I.; Ipinza, J.N.; Gutiérrez, J. Periodontal Therapy Reduces the Rate of Preterm Low Birth Weight in Women with Pregnancy-Associated Gingivitis. J. Periodontol. 2005, 76, 2144–2153. [Google Scholar] [CrossRef]
- Cruz, I.S.; Herrera, D.; Martin, C.; Herrero, A.; Sanz, M. Association between periodontal status and pre-term and/or low-birth weight in Spain: Clinical and microbiological parameters. J. Periodontal Res. 2013, 48, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Puertas, A.; Magan-Fernandez, A.; Blanc, V.; Revelles, L.; O’Valle, F.; Pozo, E.; León, R.; Mesa, F. Association of periodontitis with preterm birth and low birth weight: A comprehensive review. J. Materm. Fetal Neonatal. Med. 2018, 31, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Offenbacher, S.; Beck, J.D.; Jared, H.L.; Mauriello, S.M.; Mendoza, L.C.; Couper, D.; Stewart, D.D.; Murtha, A.P.; Cochran, D.L.; Dudley, D.J.; et al. Effects of Periodontal Therapy on Rate of Preterm Delivery. Obstet. Gynecol. 2009, 114, 551–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambrone, L.; Pannuti, C.M.; Guglielmetti, M.R.; Chambrone, L.A. Evidence grade associating periodontitis with preterm birth and/or low birth weight: II. A systematic review of randomized trials evaluating the effects of periodontal treatment. J. Clin. Periodontol. 2011, 38, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Song, I.-S.; Kim, E.-S.; Ahn, K.H. Determinants of Spontaneous Preterm Labor and Birth Including Gastroesophageal Reflux Disease and Periodontitis. J. Korean Med. Sci. 2020, 35, e105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Ren, H.; Guo, H.; Xing, W.; Liu, C.; Ji, Y.; Jiang, H.; Zhang, P.; Du, M. Periodontal infection with Porphyromonas gingivalis induces preterm birth and lower birth weight in rats. Mol. Oral Microbiol. 2018, 33, 312–321. [Google Scholar] [CrossRef]
- Rangel-Rincón, L.J.; Vivares-Builes, A.M.; Botero, J.E.; Agudelo-Suárez, A.A. An Umbrella Review Exploring the Effect of Periodontal Treatment in Pregnant Women on the Frequency of Adverse Obstetric Outcomes. J. Évid. Based Dent. Pract. 2018, 18, 218–239. [Google Scholar] [CrossRef]
- Krüger, M.S.D.M.; Casarin, R.P.; Pinto, G.D.S.; Pappen, F.G.; Camargo, M.B.J.; Correa, F.O.B.; Romano, A.R. Maternal periodontal disease and adverse perinatal outcomes: Is there an association? A hospital-based case-control study. J. Matern. Neonatal Med. 2019, 32, 3401–3407. [Google Scholar] [CrossRef]
- Morelli, E.L.; Broadbent, J.M.; Leichter, J.W.; Thomson, W.M. Pregnancy, parity and periodontal disease. Aust. Dent. J. 2018, 63, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Uriza, C.L.; Velosa-Porras, J.; Roa, N.S.; Lara, S.M.Q.; Silva, J.; Ruiz, A.J.; Arregoces, F.M.E. Periodontal Disease, Inflammatory Cytokines, and PGE2 in Pregnant Patients at Risk of Preterm Delivery: A Pilot Study. Infect. Dis. Obstet. Gynecol. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ao, M.; Miyauchi, M.; Furusho, H.; Inubushi, T.; Kitagawa, M.; Nagasaki, A.; Sakamoto, S.; Kozai, K.; Takata, T. Dental Infection of Porphyromonas gingivalis Induces Preterm Birth in Mice. PLoS ONE 2015, 10, e0137249. [Google Scholar] [CrossRef]
- Komine-Aizawa, S.; Aizawa, S.; Hayakawa, S. Periodontal diseases and adverse pregnancy outcomes. J. Obstet. Gynaecol. Res. 2019, 45, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Stadelmann, P.; Alessandri, R.; Eick, S.; Salvi, G.E.; Surbek, D.; Sculean, A. The potential association between gingival crevicular fluid inflammatory mediators and adverse pregnancy outcomes: A systematic review. Clin. Oral Investig. 2013, 17, 1453–1463. [Google Scholar] [CrossRef] [Green Version]
- Brikos, C.; O’Neill, L.A. Signalling of toll-like receptors. Handb. Exp. Pharmacol. 2008, 183, 21–50. [Google Scholar]
- Arce, R.M.; Diaz, P.; Barros, S.; Galloway, P.; Bobetsis, Y.; Threadgill, D.; Offenbacher, S. Characterization of the invasive and inflammatory traits of oral Campylobacter rectus in a murine model of fetoplacental growth restriction and in trophoblast cultures. J. Reprod. Immunol. 2010, 84, 145–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manrique-Corredor, E.J.; Orozco-Beltran, D.; Lopez-Pineda, A.; Quesada, J.A.; Gil-Guillen, V.F.; Carratala-Munuera, C. Maternal periodontitis and preterm birth: Systematic review and meta-analysis. Community Dent. Oral Epidemiol. 2019, 47, 243–251. [Google Scholar] [CrossRef]
- Makeeva, I.M.; Ignatko, A.A.; Churganova, A.A.; Lebedev, V.A.; Makeeva, M.K. Periodontal diseases and complicated pregnancy. Stomatology 2019, 98, 70–73. [Google Scholar] [CrossRef]
- Vamos, C.A.; Thompson, E.L.; Avendano, M.; Daley, E.M.; Quinonez, R.B.; Boggess, K. Oral health promotion interventions during pregnancy: A systematic review. Community Dent. Oral Epidemiol. 2015, 43, 385–396. [Google Scholar] [CrossRef]
- Iheozor-Ejiofor, Z.; Middleton, P.; Esposito, M.; Glenny, A.-M. Treating periodontal disease for preventing adverse birth outcomes in pregnant women. Cochrane Database Syst. Rev. 2017, 6, CD005297. [Google Scholar] [CrossRef] [PubMed]
- Michalowicz, B.S.; Hodges, J.S.; DiAngelis, A.J.; Lupo, V.R.; Novak, M.J.; Ferguson, J.E.; Buchanan, W.; Bofill, J.; Papapanou, P.N.; Mitchell, D.A.; et al. Treatment of Periodontal Disease and the Risk of Preterm Birth. N. Engl. J. Med. 2006, 355, 1885–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merchant, A.T.; Sutherland, M.W.; Liu, J.; Pitiphat, W.; Dasanayake, A. Periodontal treatment among mothers with mild to moderate periodontal disease and preterm birth: Reanalysis of OPT trial data accounting for selective survival. Int. J. Epidemiol. 2018, 47, 1670–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huck, O.; Tenenbaum, H.; Davideau, J.-L. Relationship between Periodontal Diseases and Preterm Birth: Recent Epidemiological and Biological Data. J. Pregnancy 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albandar, J.M.; Rams, T.E. Global epidemiology of periodontal diseases: An overview. Periodontol. 2000 2002, 29, 7–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otomo-Corgel, J. Dental management of the female patient. Periodontol. 2000 2013, 61, 219–231. [Google Scholar] [CrossRef]
- Doucède, G.; Dehaynin-Toulet, E.; Kacet, L.; Jollant, B.; Tholliez, S.; Deruelle, P.; Subtil, D. Dents et grossesse, un enjeu de santé publique. Presse Méd. 2019, 48, 1043–1050. [Google Scholar] [CrossRef]
- Brito, I.L.; Gurry, T.; Zhao, S.; Huang, K.; Young, S.K.; Shea, T.P.; Naisilisili, W.; Jenkins, A.; Jupiter, S.D.; Gevers, D.; et al. Transmission of human-associated microbiota along family and social networks. Nat. Microbiol. 2019, 4, 964–971. [Google Scholar] [CrossRef]
- Mei, F.; Xie, M.; Huang, X.; Long, Y.; Lu, X.; Wang, X.; Chen, L. Porphyromonas gingivalis and Its Systemic Impact: Current Status. Pathogens 2020, 9, 944. [Google Scholar] [CrossRef]
| Risk Factors | OR | 95% CI | Reference |
|---|---|---|---|
| Second trimester cervical length ≤2.50 cm | 6.9 | 4.3–11.1 | [8] |
| Vaginal bleeding in third trimester | 5.9 | 5.1–6.9 | [6] |
| Short interval between pregnancies (<12 months) | 4.2 | 3.0–6.0 | [6] |
| Previous preterm birth with a single newborn | 2.62 | 1.99–3.44 | [8] |
| Vaginal bleeding in first trimester | 2.0 | 1.7–2.3 | [6] |
| Periodontal disease | 2.0 | 1.2–3.2 | [6] |
| Prior cervical conization | 1.7 | 1.24–2.35 | [6] |
| Age younger than 18 | 1.7 | 1.02–3.08 | [6] |
| Low socioeconomic condition | 1.66 | 1.06–2.61 | [8] |
| 1.75 | 1.65–1.86 | [6] | |
| Pregnancy with male fetus | 1.51 | 1.02–2.24 | [8] |
| Asymptomatic bacteriuria | 1.5 | 1.2–1.9 | [6] |
| Bacterial vaginosis | 1.4 | 1.1–1.8 | [6] |
| Family history of preterm birth | 1.35 | 1.12–1.63 | [8] |
| Maternal smoking | 1.27 | 1.21–1.33 | [8] |
| 1.7 | 1.3–2.2 | [6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terzic, M.; Aimagambetova, G.; Terzic, S.; Radunovic, M.; Bapayeva, G.; Laganà, A.S. Periodontal Pathogens and Preterm Birth: Current Knowledge and Further Interventions. Pathogens 2021, 10, 730. https://doi.org/10.3390/pathogens10060730
Terzic M, Aimagambetova G, Terzic S, Radunovic M, Bapayeva G, Laganà AS. Periodontal Pathogens and Preterm Birth: Current Knowledge and Further Interventions. Pathogens. 2021; 10(6):730. https://doi.org/10.3390/pathogens10060730
Chicago/Turabian StyleTerzic, Milan, Gulzhanat Aimagambetova, Sanja Terzic, Milena Radunovic, Gauri Bapayeva, and Antonio Simone Laganà. 2021. "Periodontal Pathogens and Preterm Birth: Current Knowledge and Further Interventions" Pathogens 10, no. 6: 730. https://doi.org/10.3390/pathogens10060730








