Evaluation of Lesions and Viral Antigen Distribution in Domestic Pigs Inoculated Intranasally with African Swine Fever Virus Ken05/Tk1 (Genotype X)
Abstract
:1. Introduction
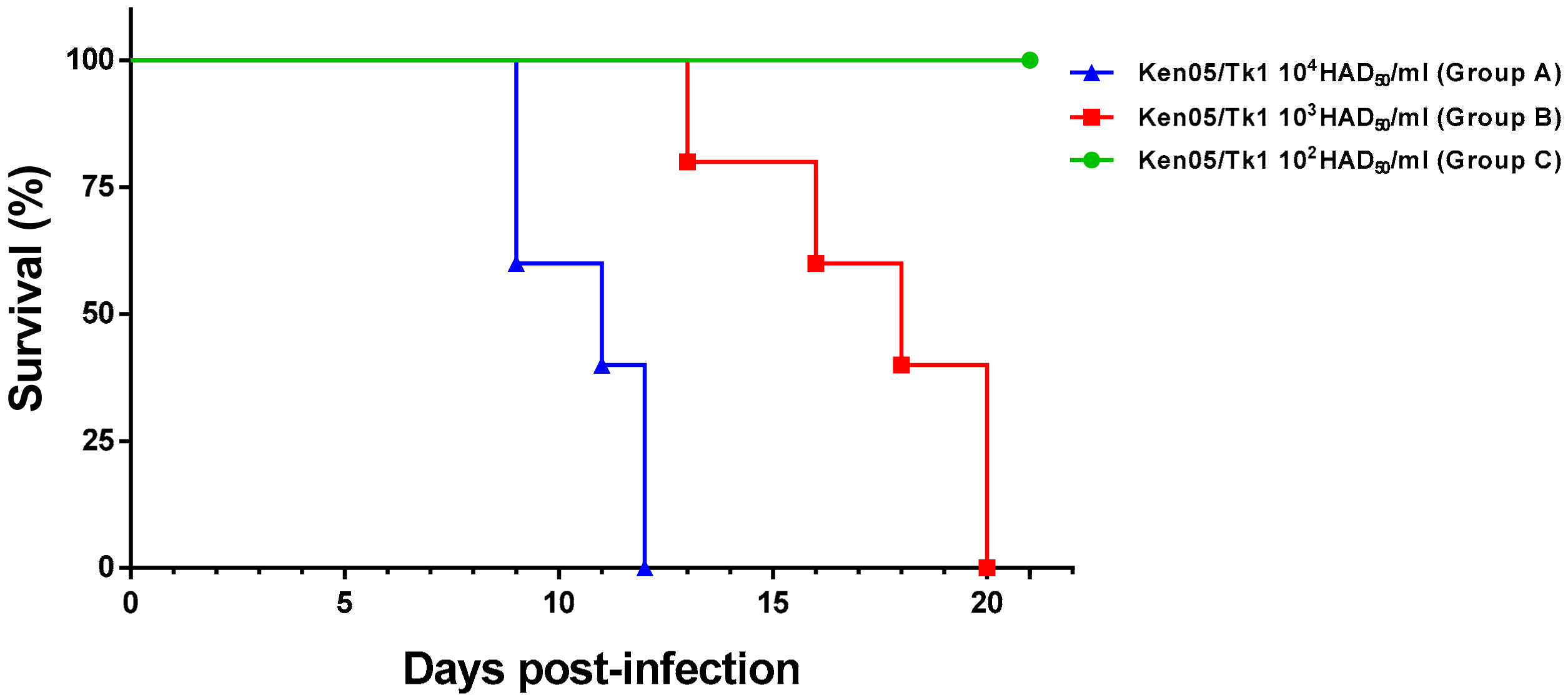
2. Results
2.1. Macroscopic Evaluation of Lesions
2.1.1. Group A—High Dose (104 HAD)
2.1.2. Group B—Medium Dose (103 HAD)
2.1.3. Group C—Low Dose (102 HAD)
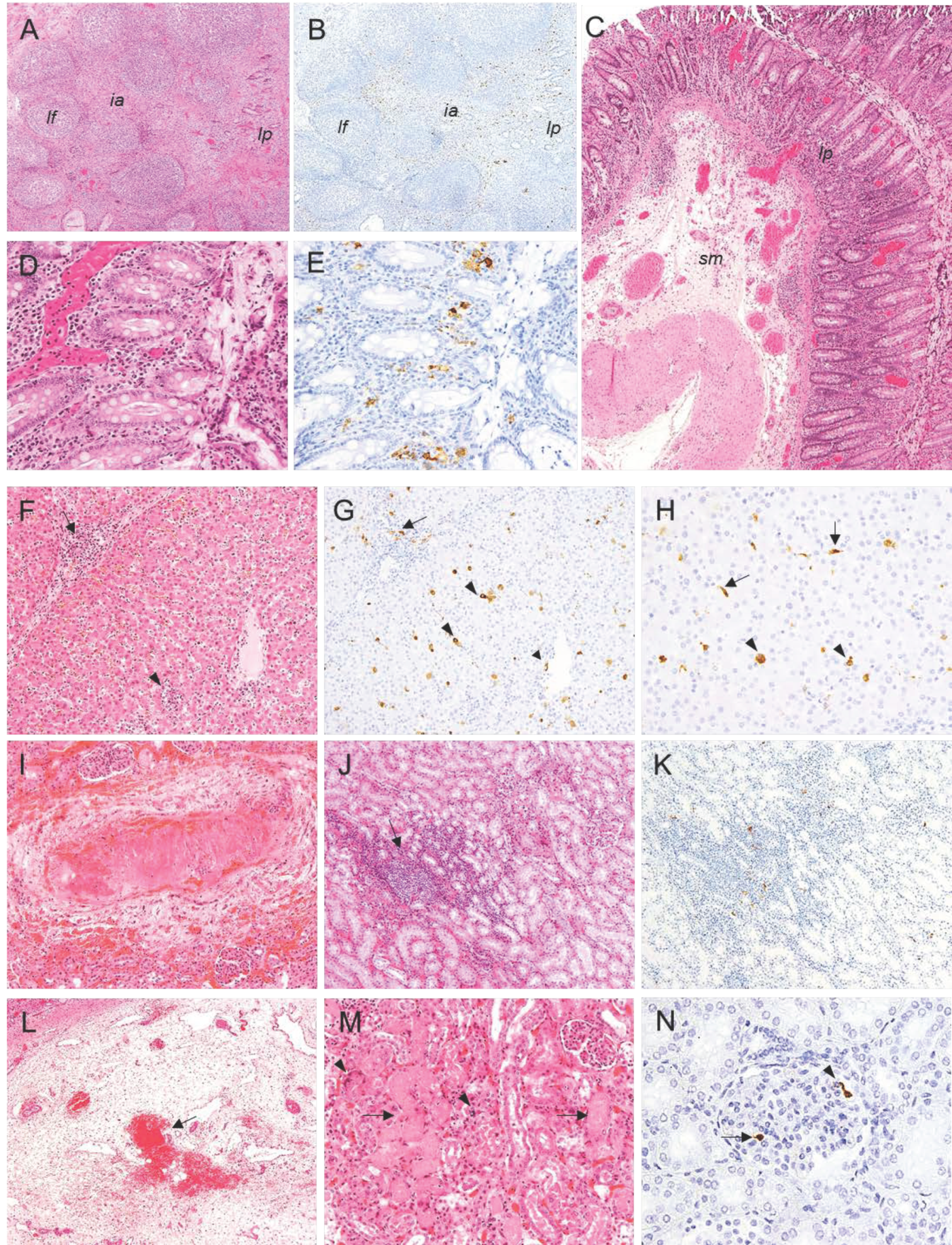
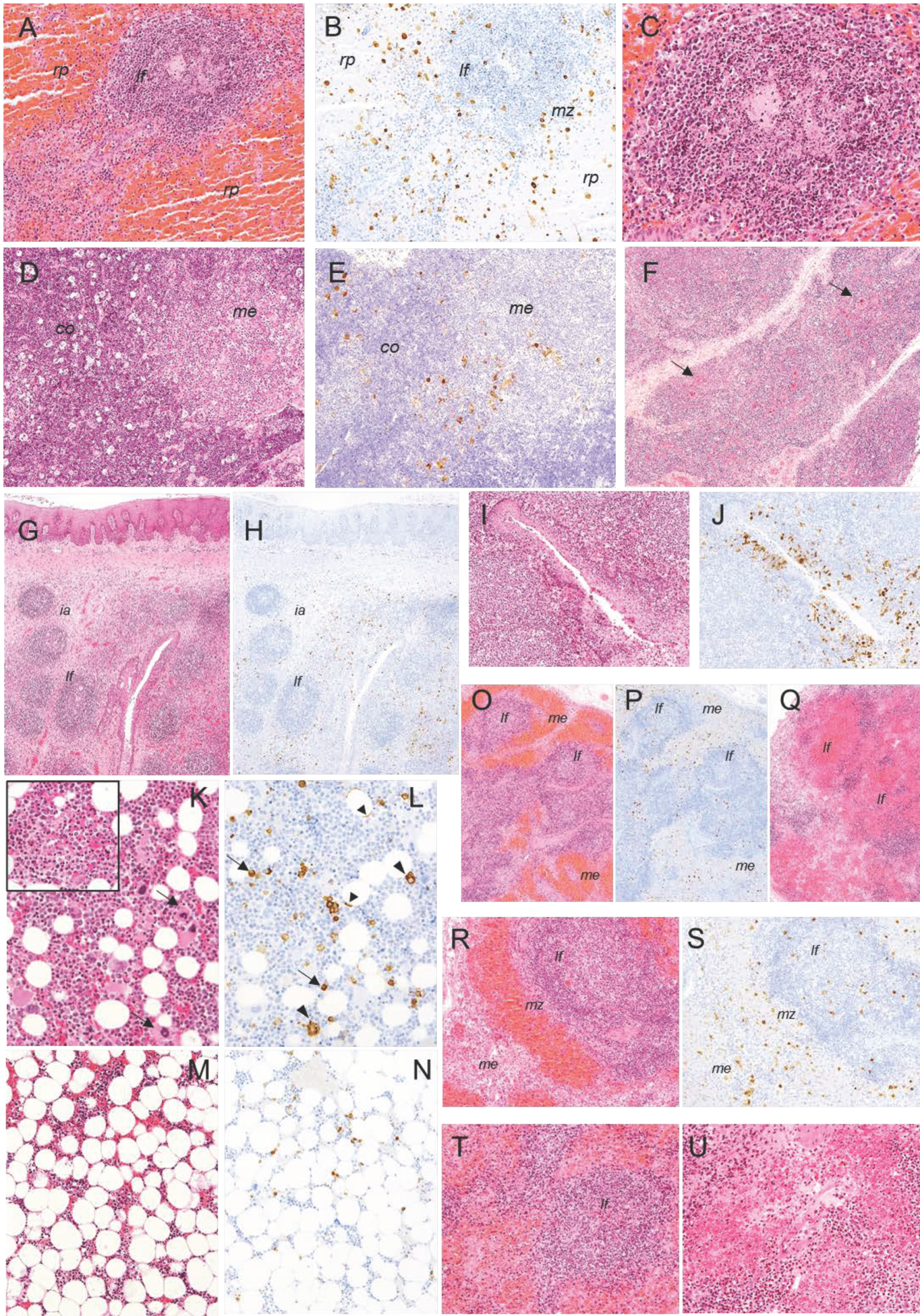
2.2. Histopathological Evaluation of Lesions
2.2.1. Group A—High Dose (104 HAD) and Group B—Medium Dose (103 HAD)
2.2.2. Group C—Low Dose (102 HAD)
2.3. Evaluation of Viral Antigen Distribution
3. Discussion
4. Materials and Methods
4.1. Experimental Design, Clinical Evaluations, Sampling and Quantification of Viral DNA
4.2. Evaluation of Lesions and Detection of African Swine Fever Virus in Tissue Samples
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M. Ictv Report Consortium. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef]
- Wilkinson, P.J. African swine fever virus. In Virus Infections of Porcines; Pensaert, M.B., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1989; pp. 17–35. [Google Scholar]
- Montgomery, R.E. On a form of swine fever occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef] [Green Version]
- Linden, A.; Licoppe, A.; Volpe, R.; Paternostre, J.; Lesenfants, C.; Cassart, D.; Garigliany, M.; Tignon, M.; van den Berg, T.; Desmecht, D.; et al. Summer 2018: African swine fever virus hits north-western Europe. Transbound. Emerg. Dis. 2019, 66, 54–55. [Google Scholar] [CrossRef]
- Sauter-Louis, C.; Forth, J.H.; Probst, C.; Staubach, C.; Hlinak, A.; Rudovsky, A.; Holland, D.; Schlieben, P.; Göldner, M.; Schatz, J.; et al. Joining the club: First detection of African swine fever in wild boar in Germany. Transbound. Emerg. Dis. 2020. [Google Scholar] [CrossRef]
- Ge, S.; Li, J.; Fan, X.; Liu, F.; Li, L.; Wang, Q.; Ren, W.; Bao, J.; Liu, C.; Wang, H.; et al. Molecular characterization of African swine fever Virus, China. Transbound. Emerg. Dis. 2018, 24, 2131–2133. [Google Scholar] [CrossRef] [Green Version]
- OIE, African swine fever (ASF) Report N° 64: 5–18 February 2021. Available online: https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/ASF/Report_64_Current_situation_of_ASF.pdf. (accessed on 18 February 2021).
- Bastos, A.D.; Penrith, M.L.; Cruciere, C.; Edrich, J.L.; Hutchings, G.; Roger, F.; Couacy- Hymann, E.; Thomson, G.R. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 2003, 148, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, J.E.; Gallardo, C.; Nieto-Pelegrin, E.; Rivera-Arroyo, B.; Degefa-Negi, T.; Arias, M.; Jenberie, S.; Mulisa, D.D.; Gizaw, D.; Gelaye, E.; et al. Identification of a new genotype of African swine fever virus in domestic pigs from Ethiopia. Transbound. Emerg. Dis. 2017, 5, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Forth, J.H.; Forth, L.F.; King, J.; Groza, O.; Hübner, A.; Olesen, A.S.; Höper, D.; Dixon, L.K.; Netherton, C.L.; Rasmussen, T.B.; et al. A Deep-Sequencing Workflow for the Fast and Efficient Generation of High-Quality African Swine Fever Virus Whole-Genome Sequences. Viruses 2019, 11, 846. [Google Scholar] [CrossRef] [Green Version]
- Sierra, M.A.; Bernabé, A.; Mozos, E.; Méndez, A.; Jover, A. Ultrastructure of the liver in pigs with experimental African swine fever. Vet. Pathol. 1987, 24, 460–462. [Google Scholar] [CrossRef] [Green Version]
- Sierra, M.A.; Carrasco, L.; Gómez-Villamandos, J.C.; Martín de las Muías, J.; Méndez, A.; Jover, A. Pulmonary intravascular macrophages in pigs inoculated with African swine fever virus of differing virulence. J. Comp. Pathol. 1990, 102, 323–334. [Google Scholar] [CrossRef]
- Gómez-Villamandos, J.C.; Hervás, J.; Méndez, A.; Carrasco, L.; Villeda, C.J.; Wilkinson, P.J.; Sierra, M.A. Pathological changes of renal interstitial capillaries in pigs inoculated with two different strains of African swine fever virus. J. Comp. Pathol. 1995, 112, 283–298. [Google Scholar] [CrossRef]
- Rodríguez, F.; Fernández, A.; Martín de las Mulas, J.P.; Sierra, M.A.; Jover, A. African swine fever: Morphopathology of a viral haemorrhagic disease. Vet. Rec. 1996, 139, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Salguero, F.J.; Ruiz-Villamor, E.; Bautista, M.J.; Sánchez-Cordón, P.J.; Carrasco, L.; Gómez-Villamandos, J.C. Changes in macrophages in spleen and lymph nodes during acute African swine fever: Expression of cytokines. Vet. Immunol. Immunopathol. 2002, 90, 11–22. [Google Scholar] [CrossRef]
- Boinas, F.S.; Hutchings, G.H.; Dixon, L.K.; Wilkinson, P.J. Characterization of pathogenic and non-pathogenic African swine fever virus isolates from Ornithodoros erraticus inhabiting pig premises in Portugal. J. Gen. Virol. 2004, 85, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cordón, P.J.; Romero-Trevejo, J.L.; Pedrera, M.; Sánchez-Vizcaíno, J.M.; Bautista, M.J.; Gómez-Villamandos, J.C. Role of hepatic macrophages during the viral haemorrhagic fever induced by African swine fever virus. Histol. Histopathol. 2008, 23, 683–691. [Google Scholar] [CrossRef]
- De Carvalho Ferreira, H.C.; Weesendorp, E.; Elbers, A.R.W.; Bouma, A.; Quak, S.; Stegeman, J.A.; Loeffen, W.L.A. African swine fever virus excretion patterns in persistently infected animals: A quantitative approach. Vet. Microbiol. 2012, 160, 327–340. [Google Scholar] [CrossRef]
- Howey, E.B.; O’Donnell, V.; de Carvalho Ferreira, H.C.; Borca, M.V.; Arzt, J. Pathogenesis of highly virulent African swine fever virus in domestic pigs exposed via intraoropharyngeal, intranasopharyngeal, and intramuscular inoculation, and by direct contact with infected pigs. Virus Res. 2013, 178, 328–339. [Google Scholar] [CrossRef]
- Post, J.; Weesendorp, E.; Montoya, M.; Loeffen, W.L. Influence of age and dose of African swine fever virus infections on clinical outcome and blood parameters in pigs. Viral Immunol. 2017, 30, 58–69. [Google Scholar] [CrossRef]
- Gabriel, C.; Blome, S.; Malogolovkin, A.; Parilov, S.; Kolbasov, D.; Teifke, J.P.; Beer, M. Characterization of African swine fever virus Caucasus isolate in European wild boars. Emerg. Infect. Dis. 2011, 17, 2342–2345. [Google Scholar] [CrossRef]
- Blome, S.; Gabriel, C.; Dietze, K.; Breithaupt, A.; Beer, M. High virulence of African swine fever virus Caucasus isolate in European wild boars of all ages. Emerg. Infect. Dis. 2012, 18, 708. [Google Scholar] [CrossRef]
- Guinat, C.; Reis, A.L.; Netherton, C.L.; Goatley, L.; Pfeier, D.U.; Dixon, L. Dynamics of African swine fever virus shedding and excretion in domestic pigs infected by intramuscular inoculation and contact transmission. Vet. Res. 2014, 45, 93. [Google Scholar] [CrossRef]
- Pietschmann, J.; Guinat, C.; Beer, M.; Pronin, V.; Tauscher, K.; Petrov, A.; Keil, G.; Blome, S. Course and transmission characteristics of oral low-dose infection of domestic pigs and European wild boar with a Caucasian African swine fever virus isolate. Arch. Virol. 2015, 160, 1657–1667. [Google Scholar] [CrossRef]
- Gallardo, C.; Nurmoja, I.; Soler, A.; Delicado, V.; Simón, A.; Martin, E.; Perez, C.; Nieto, R.; Arias, M. Evolution in Europe of African Swine Fever Genotype II Viruses from Highly to Moderately Virulent. Vet. Microbiol. 2018, 219, 70–79. [Google Scholar] [CrossRef]
- Gallardo, C.; Soler, A.; Rodze, I.; Nieto, R.; Cano-Gómez, C.; Fernandez-Pinero, J.; Arias, M. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound. Emerg. Dis. 2019, 66, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Global African Swine Fever Research Alliance (GARA) Gap Analysis Report. 2018; pp. 25–28. Available online: https://www.ars.usda.gov/ARSUserFiles/np103/SymposiumWorkshopsMeetings/GARA%20Gap%20Analysis%20Report%202018%2011-11-18.pdf (accessed on 15 November 2018).
- Gallardo, C.; Fernández-Pinero, J.; Pelayo, V.; Gazaev, I.; Markowska-Daniel, I.; Pridotkas, G.; Nieto, R.; Fernández-Pacheco, P.; Bokhan, S.; Nevolko, O.; et al. Genetic variation among African swine fever genotype II viruses, eastern and central Europe. Emerg. Infect. Dis. 2014, 20, 1544–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luka, P.D.; Achenbach, J.E.; Mwiine, F.N.; Lamien, C.E.; Shamaki, D.; Unger, H.; Erume, J. Genetic characterization of circulating African swine fever viruses in Nigeria (2007–2015). Transbound. Emerg. Dis. 2017, 64, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Quembo, C.J.; Jori, F.; Vosloo, W.; Heath, L. Genetic characterization of African swine fever virus isolates from soft ticks at the wildlife/domestic interface in Mozambique and identification of a novel genotype. Transbound. Emerg. Dis. 2018, 65, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Tignon, M.; Gallardo, C.; Iscaro, C.; Hutet, E.; Van der Stede, Y.; Kolbasov, D.; De Mia, G.M.; Le Potier, M.F.; Bishop, R.P.; Arias, M.; et al. Development and inter-laboratory validation study of an improved new real-time PCR assay with internal control for detection and laboratory diagnosis of African swine fever virus. J. Virol. Methods. 2011, 178, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Gomez-Villamandos, J.C.; Carrasco, L. An update on the epidemiology and pathology of African swine fever. J. Comp. Pathol. 2015, 152, 9–21. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Chapman, D.; Jabbar, T.; Reis, A.L.; Goatley, L.; Netherton, C.L.; Taylor, G.; Montoya, M.; Dixon, L. Different routes and doses influence protection in pigs immunised with the naturally attenuated African swine fever virus isolate OURT88/3. Antivir. Res. 2017, 138, 1–8. [Google Scholar] [CrossRef]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Martinez-Lopez, B. African swine fever: An epidemiological update. Transbound. Emerg. Dis. 2012, 59, 27–35. [Google Scholar] [CrossRef] [PubMed]
- McCleary, S.; Strong, R.; McCarthy, R.R.; Edwards, J.C.; Howes, E.L.; Stevens, L.M.; Sánchez-Cordón, P.J.; Núñez, A.; Watson, S.; Mileham, A.J.; et al. Substitution of warthog NF-κB motifs into RELA of domestic pigs is not sufficient to confer resilience to African swine fever virus. Sci. Rep. 2020. accepted. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Okoth, E.; Pelayo, V.; Anchuelo, R.; Martín, E.; Simón, A.; Llorente, A.; Nieto, R.; Soler, A.; Martín, R.; et al. African swine fever viruses with two different genotypes, both of which occur in domestic pigs, are associated with ticks and adult warthogs, respectively, at a single geographical site. J. Gen. Virol. 2011, 92, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Cardiel, I.; Ballester, M.; Solanes, D.; Nofrarías, M.; López-Soria, S.; Argilaguet, J.M.; Lacasta, A.; Accensi, F.; Rodríguez, F.; Segalés, J. Standardization of pathological investigations in the framework of experimental ASFV infections. Virus Res. 2013, 173, 180–190. [Google Scholar] [CrossRef]
- Gomez-Villamandos, J.C.; Bautista, M.J.; Sanchez-Cordon, P.J.; Carrasco, L. Pathology of African swine fever: The role of monocyte–macrophage. Virus Res. 2013, 173, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Villamandos, J.C.; Hervás, J.; Méndez, A.; Carrasco, L.; Martín de las Mulas, J.; Villeda, C.J.; Wilkinson, P.J.; Sierra, M.A. Experimental African swine fever: Apoptosis of lymphocytes and virus replication in other cells. J. Gen. Virol. 1995, 76, 2399–2405. [Google Scholar] [CrossRef]
- Gómez-Villamandos, J.C.; Bautista, M.J.; Carrasco, L.; Chacón-Manrique de Lara, F.; Hervás, J.; Wilkinson, P.J.; Sierra, M.A. Thrombocytopenia associated with apoptotic megakaryocytes in a viral haemorrhagic syndrome induced by a moderately virulent strain of African swine fever virus. J. Comp. Pathol. 1998, 118, 1–13. [Google Scholar] [CrossRef]
- Romero-Palomo, F.; Sánchez-Cordón, P.J.; Pedrera, M.; Risalde, M.A.; Molina, V.; Sánchez-Vizcaíno, J.M.; Gómez-Villamandos, J.C. Histopathologic study and viral antigen distribution in tissues of pigs inoculated with isolate Ken05/Tk1 of African swine fever virus. Proc. XI Natl. Congr. Virol. Granada Spain 2011, 14, 240–241. [Google Scholar]
- Zaman, M.; Chandrudu, S.; Toth, I. Strategies for intranasal delivery of vaccines. Drug Deliv. Transl. Res. 2013, 3, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Heuschele, W.P. Studies on the pathogenesis of African swine fever. I. Quantitative studies on the sequential development of virus in pig tissues. Arch. Gesamte. Virusforsch. 1967, 21, 349–356. [Google Scholar] [CrossRef]
- Plowright, W.; Parker, J.; Staple, R.F. The growth of a virulent strain of African swine fever virus in domestic pigs. J. Hyg. 1968, 66, 117–134. [Google Scholar] [CrossRef] [Green Version]
- Misinzo, G.; Kwavi, D.E.; Sikombe, C.D.; Makange, M.; Peter, E.; Muhairwa, A.P.; Madege, M.J. Molecular characterization of African swine fever virus from domestic pigs in northern Tanzania during an outbreak in 2013. Trop. Anim. Health Prod. 2014, 46, 1199–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, R.P.; Fleischauer, C.; de Villiers, E.P.; Okoth, E.A.; Arias, M.; Gallardo, C.; Upton, C. Comparative analysis of the complete genome sequences of Kenyan African swine fever virus isolates within p72 genotypes IX and X. Virus Genes 2015, 50, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Nieto, R.; Mur, L.; Soler, A.; Pelayo, V.; Bishop, R.; Sánchez-Cordón, P.J.; Martins, C.; Sánchez-Vizcaíno, J.M.; Arias, M. African swine fever (ASF) in Africa. The role of the African indigenous pigs in the transmission of the disease. In Proceedings of the 6th Annual Meeting Epizone, Brighton, UK, 12–14 June 2012; p. 15. [Google Scholar]
- Gallardo, C.; Soler, A.; Nieto, R.; Carrascosa, A.L.; De Mia, G.M.; Bishop, R.P.; Martins, C.; Fasina, F.O.; Couacy-Hymman, E.; Heath, L.; et al. Comparative evaluation of novel African swine fever virus (ASF) antibody detection techniques derived from specific ASF viral genotypes with the OIE internationally prescribed serological tests. Vet. Microbiol. 2013, 162, 32–43. [Google Scholar] [CrossRef]
- Okoth, E.; Gallardo, C.; Macharia, J.M.; Omore, A.; Pelayo, V.; Bulimo, D.W.; Arias, M.; Kitala, P.; Baboon, K.; Lekolol, I.; et al. Comparison of African swine fever virus prevalence and risk in two contrasting pig-farming systems in South-west and Central Kenya. Prev. Vet. Med. 2013, 110, 198–205. [Google Scholar] [CrossRef]
- Abworo, E.O.; Onzere, C.; Oluoch Amimo, J.; Riitho, V.; Mwangi, W.; Davies, J.; Blome, S.; Bishop, R.P. Detection of African swine fever virus in the tissues of asymptomatic pigs in smallholder farming systems along the Kenya-Uganda border: Implications for transmission in endemic areas and ASF surveillance in East Africa. J. Gen. Virol. 2017, 98, 1806–1814. [Google Scholar] [CrossRef]
- Costard, S.; Mur, L.; Lubroth, J.; Sanchez-Vizcaino, J.M.; Pfeiffer, D.U. Epidemiology of African swine fever virus. Virus Res. 2013, 173, 191–197. [Google Scholar] [CrossRef]
- Gallardo, C.; Soler, A.; Nieto, R.; Sánchez, M.A.; Martins, C.; Pelayo, V.; Carrascosa, A.; Revilla, Y.; Simón, A.; Briones, V.; et al. Experimental Transmission of African swine fever low virulent isolate NH/P68 by surviving pigs. Transbound. Emerg. Dis. 2015, 62, 612–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ståhl, K.; Sternberg-Lewerin, S.; Blome, S.; Viltrop, A.; Penrith, M.L.; Chenais, E. Lack of evidence for long term carriers of African swine fever virus—A systematic review. Virus Res. 2019, 272, 197725. [Google Scholar] [CrossRef]
- Oura, C.A.; Powell, P.P.; Parkhouse, R.M. African swine fever: A disease characterized by apoptosis. J. Gen. Virol. 1998, 79, 1427–1438. [Google Scholar] [CrossRef]
- Ramiro-Ibáñez, F.; Escribano, J.M.; Alonso, C. Application of a monoclonal antibody recognizing protein p30 to detect African swine fever virus-infected cells in peripheral blood. J. Virol. Methods. 1995, 55, 339–345. [Google Scholar] [CrossRef]
- Sánchez-Torres, C.; Gómez-Puertas, P.; Gómez del Moral, M.; Alonso, F.; Escribano, J.M.; Ezquerra, A.; Domínguez, J. Expression of porcine CD163 on monocytes/macrophages correlates with permissiveness to African swine fever infection. Arch Virol. 2003, 148, 2307–2323. [Google Scholar] [CrossRef] [PubMed]
- Lithgow, P.; Takamatsu, H.; Werling, D.; Dixon, L.; Chapman, D. Correlation of cell surface marker expression with African swine fever virus infection. Vet. Microbiol. 2014, 31, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcami, A.; Carrascosa, A.L.; Viñuela, E. Interaction of African swine fever virus with macrophages. Virus Res. 1990, 17, 93–104. [Google Scholar] [CrossRef]


| Pig No. | Viraemia * | Incubation Period | Clinical Course | Euthanasia |
|---|---|---|---|---|
| Group A—High dose (104 HAD50) | ||||
| 86 | 5 pi (low levels) | 7 | 5 | 11 pi |
| 87 | 5 pi (high levels) | 5 | 5 | 9 pi |
| 88 | 10 pi (low levels) | NCS | NCS | 12 pi ** |
| 89 | 5 pi (high levels) | 6 | 7 | 12 pi |
| 90 | 5 pi (high levels) | 5 | 5 | 9 pi |
| Group B—Medium dose (103 HAD50) | ||||
| 81 | 10 pi (moderate levels) | 7 | 14 | 20 pi ** |
| 82 | 13 pi (high levels) | 7 | 7 | 13 pi |
| 83 | 12 pi (low levels) | 17 | 4 | 20 pi |
| 84 | 12 pi (low levels) | 15 | 4 | 18 pi |
| 85 | 12 pi (low levels) | 13 | 4 | 16 pi |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the Crown. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Cordón, P.J.; Floyd, T.; Hicks, D.; Crooke, H.R.; McCleary, S.; McCarthy, R.R.; Strong, R.; Dixon, L.K.; Neimanis, A.; Wikström-Lassa, E.; et al. Evaluation of Lesions and Viral Antigen Distribution in Domestic Pigs Inoculated Intranasally with African Swine Fever Virus Ken05/Tk1 (Genotype X). Pathogens 2021, 10, 768. https://doi.org/10.3390/pathogens10060768
Sánchez-Cordón PJ, Floyd T, Hicks D, Crooke HR, McCleary S, McCarthy RR, Strong R, Dixon LK, Neimanis A, Wikström-Lassa E, et al. Evaluation of Lesions and Viral Antigen Distribution in Domestic Pigs Inoculated Intranasally with African Swine Fever Virus Ken05/Tk1 (Genotype X). Pathogens. 2021; 10(6):768. https://doi.org/10.3390/pathogens10060768
Chicago/Turabian StyleSánchez-Cordón, Pedro J., Tobias Floyd, Daniel Hicks, Helen R. Crooke, Stephen McCleary, Ronan R. McCarthy, Rebecca Strong, Linda K. Dixon, Aleksija Neimanis, Emil Wikström-Lassa, and et al. 2021. "Evaluation of Lesions and Viral Antigen Distribution in Domestic Pigs Inoculated Intranasally with African Swine Fever Virus Ken05/Tk1 (Genotype X)" Pathogens 10, no. 6: 768. https://doi.org/10.3390/pathogens10060768
APA StyleSánchez-Cordón, P. J., Floyd, T., Hicks, D., Crooke, H. R., McCleary, S., McCarthy, R. R., Strong, R., Dixon, L. K., Neimanis, A., Wikström-Lassa, E., Gavier-Widén, D., & Núñez, A. (2021). Evaluation of Lesions and Viral Antigen Distribution in Domestic Pigs Inoculated Intranasally with African Swine Fever Virus Ken05/Tk1 (Genotype X). Pathogens, 10(6), 768. https://doi.org/10.3390/pathogens10060768







