Whole Genome Sequence Analysis of Mycobacterium bovis Cattle Isolates, Algeria
Abstract
:1. Introduction
2. Results
2.1. Data Analysis
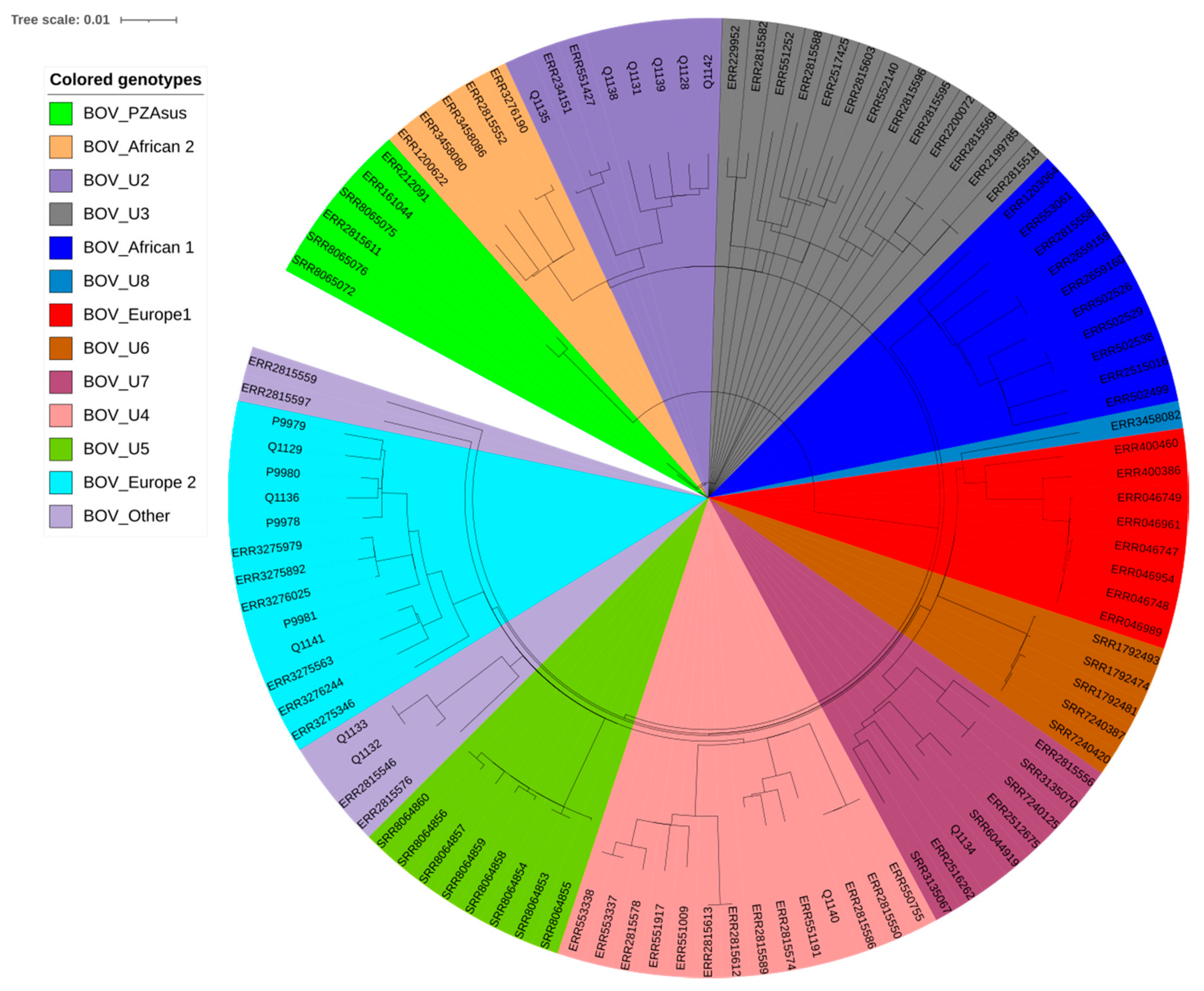
2.2. Genomic Typing Analysis
3. Discussion
4. Materials and Methods
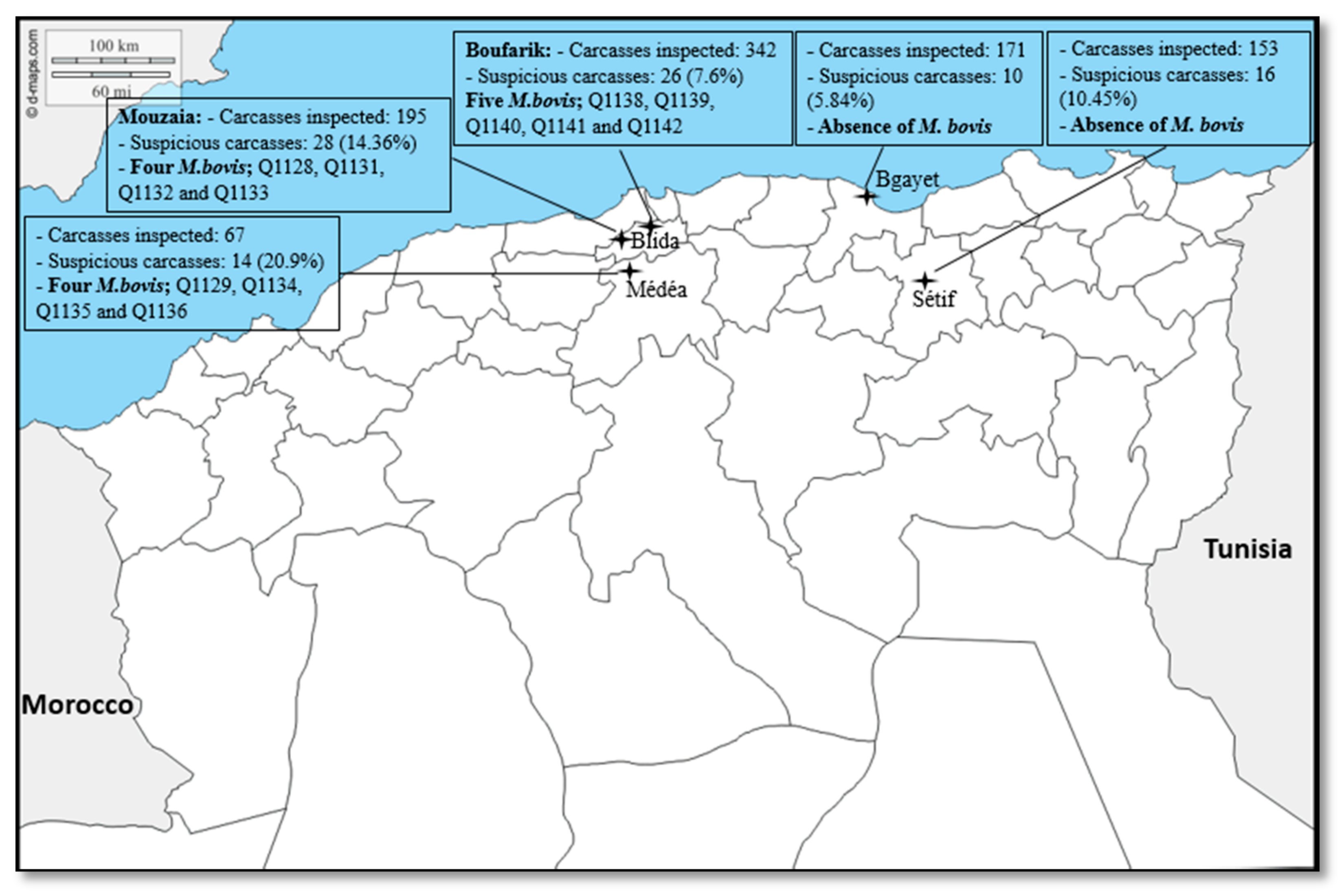
4.1. Study Area and Sample Collection
4.2. Tissue Preparation and Culture
4.3. Isolate Identification
4.4. Genome Sequence Analyses
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boukary, A.R.; Thys, E.; Rigouts, L.; Matthys, F.; Berkvens, D.; Mahamadou, I.; Yenikoye, A.; Saegerman, C. Ris factors associated with bovine tuberculosis and molecular characterization of Mycobacterium bovis strains in urban settings in Niger. Transbound. Emerg. Dis. 2012, 59, 490–502. [Google Scholar] [CrossRef]
- Lorente-Leal, V.; Liandris, E.; Castellanos, E.; Bezos, J.; Domínguez, L.; de Juan, L.; Romero, B. Validation of a Real-Time PCR for the Detection of Mycobacterium tuberculosis Complex Members in Bovine Tissue Samples. Front. Vet. Sci. 2019, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Eroksuz, Y.; Baydar, E.; Otlu, B.; Dabak, M.; Eroksuz, H.; Karabulut, B.; Incili, C.A.; Timurkan, M.O. Case report: Systemic tuberculosis caused by Mycobacterium bovis in a cat. BMC Vet. Res. 2019, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Nebreda-Mayoral, T.; Brezmes-Valdivieso, M.F.; Gutiérrez-Zufiaurre, N.; García-de Cruz, S.; Labayru-Echeverría, C.; López-Medrano, R.; López-Urrutia-Lorente, L.; Tinajas-Puertas, A.; Rivero-Lezcano, O. Human Mycobacterium bovis infection in Castile and León (Spain), 2006–2015. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2019, 37, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Belkhiri, M.; Tlidjane, M.; Benhathat, Y.; Meziane, T. Histopathological study and pulmonary classification of bovine lesions. Afr. J. Agric. Res. 2009, 4, 584–591. [Google Scholar]
- Boland, F.; Kelly, G.E.; Good, M.; More, S.J. Bovine tuberculosis and milk production in infected dairy herds in Ireland. Prev. Vet. Med. 2010, 93, 153–161. [Google Scholar] [CrossRef]
- Cousins, D.V. Mycobacterium bovis infection and control in domestic livestock. Rev. Sci. Tech. Off. Int. Epiz. 2001, 20, 71–85. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health. Bovine Tuberculosis. 2020. Available online: https://www.oie.int/en/animal-health-in-the-world/animal-diseases/bovine-tuberculosis/#A (accessed on 19 February 2020).
- Ministry of Agriculture and Rural Development and Fishing of Algeria. Department of Veterinary Services. Establishment of an Animal Identification, Registration and Monitoring System in Algeria. K. Boughalem CVO Algeria Algiers, Algeria—November 24 and 25, 2015. CPC/REMESA. Available online: https://docplayer.fr/43882308-Dr-k-boughalem-cvo-algerie-alger-algerie-24-et-25-novembre-2015-cpc-remesa.html?fbclid=IwAR37DQnECLe-WWs85AseZi5iXI31I8cFkFxFUNkMbA5o19_FJdprpa-uPog (accessed on 19 February 2020).
- Sahraoui, N.; Müller, B.; Guetarni, D.; Boulahbal, F.; Yala, D.; Ouzrout, R.; Berg, S.; Smith, N.H.; Zinsstag, J. Molecular characterization of Mycobacterium bovis strains isolated from cattle slaughtered at two abattoirs in Algeria. BMC Vet. Res. 2009, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Michel, A.L.; Müller, B.; van Helden, P.D. Mycobacterium bovis at the animal–human interface: A problem, or not? Vet. Microbiol. 2010, 140, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Sahraoui, N.; Muller, B.; Mamache, B.; Yala, D.; Boulahbal, F.; Zinsstag, J.; Guetarni, D. Tuberculosis in cattle and Goat of North Algeria. Vet. Res. 2011, 4, 100–103. [Google Scholar]
- Loiseau, C.; Menardo, F.; Aseffa, A.; Hailu, E.; Gumi, B.; Ameni, G.; Berg, S.; Rigouts, L.; Robbe-Austerman, S.; Zinsstag, J.; et al. An African origin for Mycobacterium bovis. Evol. Med. Public Health 2020, 2020, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Luo, T.; Shen, X.; Wu, J.; Gan, M.; Xu, P.; Wu, Z.; Lin, S.; Tian, J.; Liu, Q.; et al. Transmission of multidrug-resistant Mycobacterium tuberculosis in Shanghai, China: A retrospective observational study using whole-genome sequencing and epidemiological investigation. Lancet Infect. Dis. 2017, 17, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Tazerart, F.; Saad, J.; Niar, A.; Sahraoui, N.; Drancourt, M. Mycobacterium bovis Pulmonary Tuberculosis, Algeria. Emerg. Infect. Dis. 2021, 27, 972. [Google Scholar] [CrossRef]
- Teklu, A.; Asseged, B.; Yimer, E.; Gebeyehu, M.; Woldesenbet, Z. Tuberculous lesions not detected by routine abattoir inspection: The experience of the Hossana municipal abattoir, southern Ethiopia. Rev. Sci. Tech. 2004, 23, 957–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebrezgabiher, G.; Romha, G.; Ameni, G. Prevalence study of bovine tuberculosis and genus typing of its causative agents in cattle slaughtered at Dilla Municipal abattoir, Southern Ethiopia. Afr. J. Basic Appl. Sci. 2014, 6, 103–109. [Google Scholar]
- Terefe, D. Gross pathological lesions of bovine tuberculosis and efficiency of meat inspection procedure to detect-infected cattle in Adama municipal abattoir. J. Vet. Med. Anim. Health 2014, 6, 48–53. [Google Scholar]
- Yahyaoui-Azami, H.; Aboukhassib, H.; Bouslikhane, M.; Berrada, J.; Rami, S.; Reinhard, M.; Gagneux, S.; Feldmann, J.; Borrell, S.; Zinsstag, J. Molecular characterization of bovine tuberculosis strains in two slaughterhouses in Morocco. BMC Vet. Res. 2017, 13, 272. [Google Scholar] [CrossRef] [Green Version]
- Chougar, L.; Amor, N.; Farjallah, S.; Harhoura, K.; Aissi, M.; Alagaili, A.N.; Merella, P. New insight into genetic variation and haplotype diversity of Fasciola hepatica from Algeria. Parasitol. Res. 2019, 118, 1179–1192. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Food and Environment of Spain. Livestock Foreign Trade: MAPAMA’s Role. Conference on the Export of Live Cattle to Third Countries. 2017. Available online: https://www.mapa.gob.es/es/ganaderia/temas/comercio-exterior-ganadero/01comercioexteriorganaderopapeldelmapama_tcm30-426534.pdf (accessed on 25 March 2020).
- Pavlik, I.; Machackova, M.; Yayo Ayele, W.; Lamka, J.; Parmova, I.; Melicharek, I.; Hanzlikova, M.; Körmendy, B.; Nagy, G.; Cvetnic, Z.; et al. Incidence of bovine tuberculosis in wild and domestic animals other than cattle in six Central European countries during 1990–1999. Vet. Med. Praha 2002, 47, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Krajewska-Wędzina, M.; Didkowska, A.; Sridhara, A.A.; Elahi, R.; Johnathan-Lee, A.; Radulski, Ł.; Lipiec, M.; Anusz, K.; Lyashchenko, K.P.; Miller, M.A.; et al. Transboundary tuberculosis: Importation of alpacas infected with Mycobacterium bovis from the United Kingdom to Poland and potential for serodiagnostic assays in detecting tuberculin skin test false-negative animals. Transbound. Emerg. Dis. 2020, 67, 1306–1314. [Google Scholar] [CrossRef]
- Petroff, S.A. Some cultural studies on the tubercle bacillus. Bull. Johns Hopkins Hosp. 1915, 26, 276–279. [Google Scholar]
- Asmar, S.; Drancourt, M. Chlorhexidine decontamination of sputum for culturing Mycobacterium tuberculosis. BMC Microbiol. 2015, 15, 155. [Google Scholar] [CrossRef] [Green Version]
- Chambers, M.A.; Williams, A.; Gavier-Widén, D.; Whelan, A.; Hall, G.; Marsh, P.D.; Bloom, B.R.; Jacobs, W.R.; Hewinson, G. Identification of a Mycobacterium bovis BCG Auxotrophic Mutant That Protects Guinea Pigs against M. bovis and Hematogenous Spread of Mycobacterium tuberculosis without Sensitization to Tuberculin. Infect. Immun. 2000, 68, 7094–7099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, G.S.; Clifford, D.; Whelan, A.O.; Tchilian, E.Z.; Beverley, P.C.; Salguero, F.J.; Xing, Z.; Vordermeier, H.M.; Villarreal-Ramos, B. Protection induced by simultaneous subcutaneous and endobronchial vaccination with BCG/BCG and BCG/adenovirus expressing antigen 85A against Mycobacterium bovis in cattle. PLoS ONE 2015, 10, e0142270. [Google Scholar]
- García-Barragán, Á.; Gutiérrez-Pabello, J.A.; Alfonseca-Silva, E. Calcitriol increases nitric oxide production and modulates microbicidal capacity against Mycobacterium bovis in bovine macrophages. Comp. Immunol. Microbiol. Infect. Dis. 2018, 59, 17–23. [Google Scholar] [CrossRef]
- Angelakis, E.; Roux, V.; Raoult, D.; Rolain, J.-M. Real-time PCR strategy and detection of bacterial agents of lymphadenitis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1363–1368. [Google Scholar] [CrossRef]
- Adékambi, T.; Colson, P.; Drancourt, M. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J. Clin. Microbiol. 2003, 41, 5699–5708. [Google Scholar] [CrossRef] [Green Version]
- Kohl, T.A.; Utpatel, C.; Schleusener, V.; Filippo, M.R.D.; Beckert, P.; Cirillo, D.M.; Niemann, S. MTBseq: A comprehensive pipeline for whole genome sequence analysis of Mycobacterium tuberculosis complex isolates. PeerJ 2018, 6, e5895. [Google Scholar] [CrossRef] [Green Version]
- Coll, F.; McNerney, R.; Preston, M.D.; Guerra-Assunção, J.A.; Warry, A.; Hill-Cawthorne, G.; Mallard, K.; Nair, M.; Miranda, A.; Alves, A.; et al. Rapid determination of anti-tuberculosis drug resistance from whole-genome sequences. Genome Med. 2015, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Hunt, M.; Bradley, P.; Lapierre, S.G.; Heys, S.; Thomsit, M.; Hall, M.B.; Malone, K.M.; Wintringer, P.; Walker, T.M.; Cirillo, D.M.; et al. Antibiotic resistance prediction for Mycobacterium tuberculosis from genome sequence data with Mykrobe. Wellcome Open Res. 2019, 4, 191. [Google Scholar] [CrossRef] [Green Version]
| Variation Factors | Number of Suspect Carcasses | Rate (%) | Total | p | |
|---|---|---|---|---|---|
| Gender | Male | 53 | 8.59 | 617 | 0.038 |
| Female | 41 | 13.18 | 311 | ||
| Age | Young (<2 years) | 42 | 9.83 | 427 | <10−4 |
| Adults (2–5 years) | 26 | 6.51 | 399 | ||
| Elderly (>5 years) | 26 | 25.5 | 102 | ||
| Weight (score) | Lean (1–2) | 31 | 10.83 | 286 | 0.03 |
| Middle (2.5–3) | 60 | 11.19 | 536 | ||
| Fat (3.5–5) | 3 | 2.83 | 106 | ||
| Location | Lymph nodes | 90 | 95.74 | - | - |
| Viscera | 4 (3 lungs, 1 liver) | 4.26 | - | ||
| Total | 94 | 10.13 | 928 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tazerart, F.; Saad, J.; Sahraoui, N.; Yala, D.; Niar, A.; Drancourt, M. Whole Genome Sequence Analysis of Mycobacterium bovis Cattle Isolates, Algeria. Pathogens 2021, 10, 802. https://doi.org/10.3390/pathogens10070802
Tazerart F, Saad J, Sahraoui N, Yala D, Niar A, Drancourt M. Whole Genome Sequence Analysis of Mycobacterium bovis Cattle Isolates, Algeria. Pathogens. 2021; 10(7):802. https://doi.org/10.3390/pathogens10070802
Chicago/Turabian StyleTazerart, Fatah, Jamal Saad, Naima Sahraoui, Djamel Yala, Abdellatif Niar, and Michel Drancourt. 2021. "Whole Genome Sequence Analysis of Mycobacterium bovis Cattle Isolates, Algeria" Pathogens 10, no. 7: 802. https://doi.org/10.3390/pathogens10070802





