Rhipicephalus Tick: A Contextual Review for Southeast Asia
Abstract
:1. Background
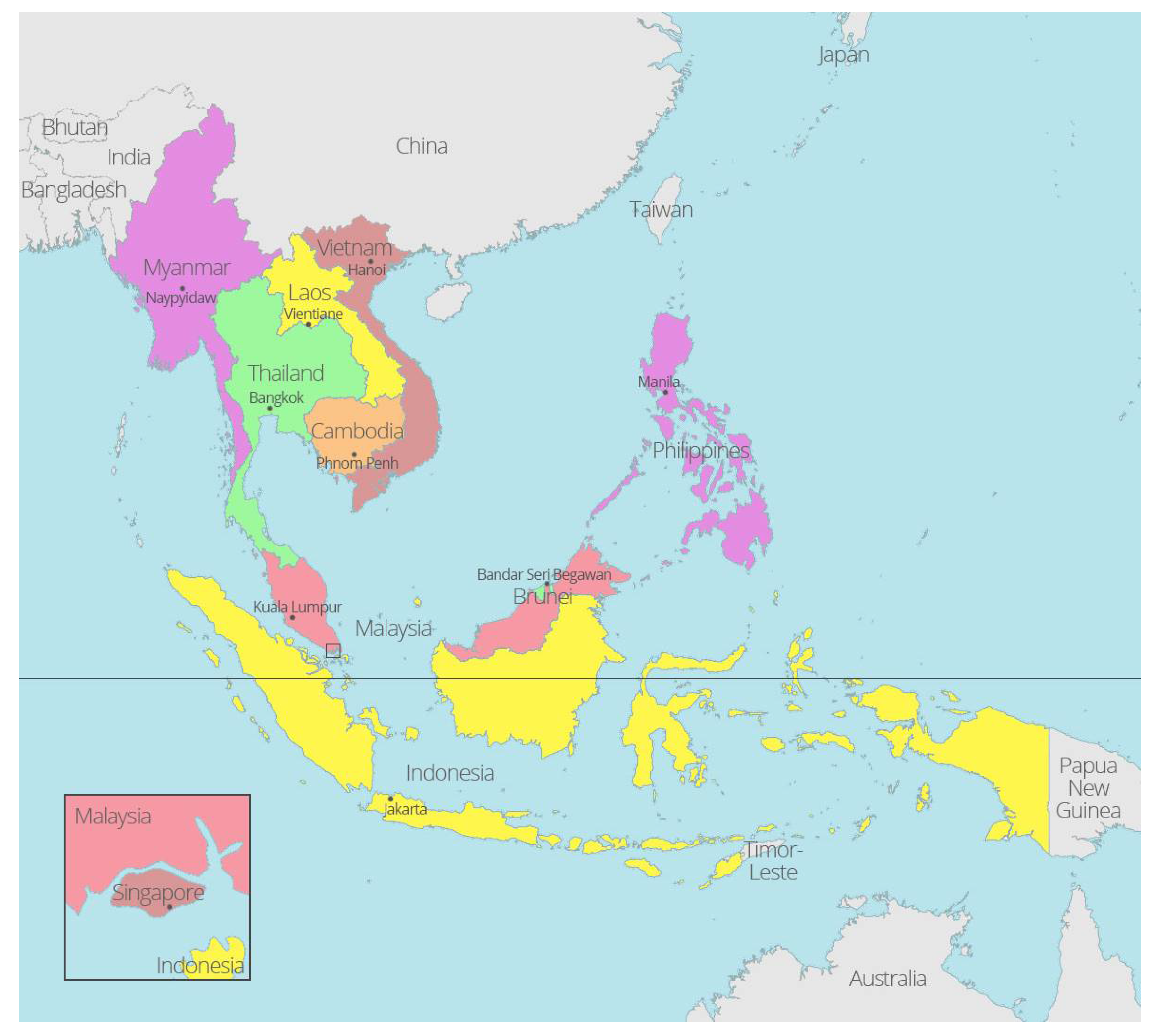
2. Genus Rhipicephalus and Its Common Species in Southeast Asia
3. Host Range of Rhipicephalus Species in Southeast Asia
| Host Type | Country | Tick Species | Host | Reference |
|---|---|---|---|---|
| Livestock | Cambodia | Rhipicephalus microplus | Unknown | [31] |
| Rhipicephalus australis | Unknown | [62] | ||
| Indonesia | Rhipicephalus australis | Unknown | [62] | |
| Rhipicephalus haemaphysaloides | Bos taurus Bubalus bubalis Capra aegagrus hircus | [63] | ||
| Rhipicephalus microplus | Bos taurus Bubalus bubalis Capra aegagrus hircus Equus caballus Sus scrofa | [30,63,64] | ||
| Rhipicephalus pilans | Bos taurus Bubalus bubalis Capra aegagrus hircus Equus caballus Ovis aries | [30,63,64] | ||
| Rhipicephalus sanguineus s.l. | Bos taurus Bubalus bubalis Gallus gallus domesticus Sus scrofa domesticus | [64] | ||
| Rhipicephalus haemaphysaloides | Bos sp. | [32] | ||
| Laos | Rhipicephalus microplus | Bos sp. | [32] | |
| Rhipicephalus australis | Unknown | [62] | ||
| Malaysia | Rhipicephalus microplus | Bos taurus | [23,65] | |
| Rhipicephalus microplus | Bos sp. | [26] | ||
| Myanmar | Rhipicephalus microplus | Bos sp. Sus scrofa | [17] | |
| Singapore | Rhipicephalus microplus | Bos sp. and Bos taurus | [36,66,67] | |
| Thailand | Rhipicephalus australis | Unknown | [62] | |
| The Philippines | Rhipicephalus microplus | Bos sp. and Bos indicus Bubalus bubalis Capra aegagrus hircus | [37,38,68] | |
| Rhipicephalus haemaphysaloides | Bos sp. | [69] | ||
| Timor-Leste | Rhipicephalus microplus | Bos sp. Capra aegagrus hircus | [69] | |
| Rhipicephalus sanguineus s.l. | Bos taurus | [69] | ||
| Rhipicephalus annulatus | Bos sp. | [70] | ||
| Vietnam | Rhipicephalus microplus | Bos sp. | [33] | |
| Rhipicephalus sanguineus s.l. | Bos sp. | [71] | ||
| Rhipicephalus haemaphysaloides | Canis lupus familiaris | [63] | ||
| Companion animals | Indonesia | Rhipicephalus sanguineus s.l. | Canis lupus familiaris Felis catus | [24,63,72] |
| Rhipicephalus haemaphysaloides | Canis lupus familiaris | [32] | ||
| Laos | Rhipicephalus sanguineus s.l. | Canis lupus familiaris | [41,73] | |
| Rhipicephalus sanguineus s.l. | Canis lupus familiaris | [45,54,74,75,76,77,78,79] | ||
| Malaysia | Rhipicephalus sanguineus s.l. | Canis lupus familiaris | [42] | |
| Myanmar | Rhipicephalus sanguineus s.l. | Canis lupus familiaris Felis catus | [24,79,80] | |
| Singapore | Rhipicephalus sanguineus s.l. | Canis lupus familiaris | [28,44,79] | |
| Thailand | Rhipicephalus sanguineus s.l. | Canis lupus familiaris Felis catus | [24,38,79] | |
| The Philippines | Rhipicephalus haemaphysaloides | Canis lupus familiaris | [71] | |
| Vietnam | Rhipicephalus sanguineus s.l. | Canis lupus familiaris | [28,43,71,79] | |
| Rhipicephalus haemaphysaloides | Forest rats * | [63] | ||
| Rodents | Indonesia | Rhipicephalus microplus | Rattus exulans Rattus hoffmanni Rattus rattus | [64] |
| Rhipicephalus pilans | Niviventer fulvescens Rattus argentiventer Rattus exulans Rattus rattus Rattus tiomanicus | [63,64,81] | ||
| Rhipicephalus sp. | Sundamys muelleri | [82] | ||
| Malaysia | Rhipicephalus haemaphysaloides | Pteropus vampirus Rusa unicolor Helarctos malayanus Panthera tigris Varanus salvator Sus scrofa Hylomys suillus | [63,83] | |
| Wild animals | Indonesia | Rhipicephalus microplus | Bos javanicus Manis javanica Rusa timorensis Rusa unicolor | [63,64] |
| Rhipicephalus pilans | Crocidura nigripes Hylomys suillus Rusa timorensis Suncus murinus Sus scrofa | [63,84] | ||
| Rhipicephalus sanguineus s.l. | Bos javanicus Rusa unicolor | [63] | ||
| Rhipicephalus haemaphysaloides | Arctictis binturong Cuon alpinus Martes flavigula Neofelis nebulosi | [85] | ||
| Thailand | Rhipicephalus microplus | - | [64] | |
| Human | Indonesia | Rhipicephalus pilans | - | [64,81] |
| Rhipicephalus sanguineus s.l. | - | [63] | ||
| Rhipicephalus microplus | - | [85] | ||
| Thailand | Rhipicephalus sanguineus s.l. | - | [86] |
4. The Impacts of Ticks and Tick-Borne Diseases
5. Resistant and Susceptibility Host Responses
6. Controlling and Acaricides Resistance
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Worldometers. South-Eastern Asia Population. 2020. Available online: https://www.worldometers.info/world-population/south-eastern-asia-population/ (accessed on 4 January 2021).
- Otte, J.; Pica-Ciamarra, U.; Morzaria, S. A Comparative overview of the livestock-environment interactions in Asia and Sub-saharan Africa. Front. Vet. Sci. 2019, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Coker, R.J.; Hunter, B.M.; Rudge, J.W.; Liverani, M.; Hanvoravongchai, P. Emerging infectious diseases in Southeast Asia: Regional challenges to control. Lancet 2011, 377, 599–609. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Estimates of the Global Burden of Foodborne Diseases. 2015. Available online: https://www.who.int/foodsafety/publications/foodborne_disease/fergreport/en/ (accessed on 4 January 2021).
- Klous, G.; Huss, A.; Heederik, D.J.; Coutinho, R.A. Human–livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Health 2016, 2, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Jeanna, B. 13 Animal-to-Human Diseases Kill 2.2 Million People Each Year. 2012. Available online: https://www.livescience.com/21426-global-zoonoses-diseases-hotspots.html (accessed on 4 January 2021).
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2006. [Google Scholar]
- Ilea, R.C. Intensive livestock farming: Global trends, increased environmental concerns, and ethical solutions. J. Agric. Environ. Ethics. 2009, 22, 153–167. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Agricultural Transformation of Middle-Income Asian Economies: Diversification, Farm Size and Mechanization; Dawe, D., Ed.; ESA Working Paper No. 15-04; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Dantas-Torres, F.; Bruno, B.C.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef]
- de la Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Wang, H.; Wang, T.; Sun, W.; Yang, X.; Liu, J. Tick-borne pathogens and the vector potential of ticks in China. Parasites Vectors 2015, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mediannikov, O.; Diatta, G.; Fenollar, F.; Sokhna, C.; Trape, J.F.; Raoult, D. Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl. Trop. Dis. 2010, 4, e821. [Google Scholar] [CrossRef] [Green Version]
- Moyer, M.W. The growing global battle against blood-sucking ticks. 2015. Available online: https://www.nature.com/news/the-growing-global-battle-against-blood-sucking-ticks-1.18227 (accessed on 4 January 2021).
- Paules, C.I.; Marston, H.D.; Bloom, M.E.; Fauci, A.S. Tickborne diseases—Confronting a growing threat. N. Engl. J. Med. 2018, 379, 701–703. [Google Scholar] [CrossRef]
- Charrel, R.N.; Berenger, J.M.; Laroche, M.; Ayhan, N.; Bitam, I.; Delaunay, P.; Parola, P. Neglected vector-borne bacterial diseases and arboviruses in the Mediterranean area. New Microbes New Infect. 2018, 26, S31–S36. [Google Scholar] [CrossRef]
- Petney, T.N. A preliminary study of the significance of ticks and tick-borne diseases in South-east Asia. Mitt. Österr. Ges. Tropenmed. Parasitol. 1993, 15, 33–42. [Google Scholar]
- Petney, T.N.; Saijuntha, W.; Boulanger, N.; Chitimia-Dobler, L.; Pfeffer, M.; Eamudomkarn, C.; Andrews, R.H.; Ahamad, M.; Putthasorn, N.; Muders, S.V.; et al. Ticks (Argasidae, Ixodidae) and tick-borne diseases of continental Southeast Asia. Zootaxa 2019, 4558, 1–89. [Google Scholar] [CrossRef] [PubMed]
- William, L.N.; Sonenshine, D.E.; Noden, B.H.; Brown, R.N. Ticks (Ixodida). In Medical and Veterinary Entomology; Mullen, G.R., Durden, L.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 603–672. [Google Scholar]
- Ernieenor, F.C.L.; Ernna, G.; Mariana, A. Phenotypic and genotypic identification of hard ticks of the genus Haemaphysalis (Acari: Ixodidae) in Peninsular Malaysia. Exp. Appl. Acarol. 2017, 71, 387–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, P.J.; Jefferies, R. Arthropod-transmitted diseases of companion animals in Southeast Asia. Trends Parasitol. 2004, 20, 27–34. [Google Scholar] [CrossRef]
- Colella, V.; Nguyen, V.L.; Tan, D.Y.; Lu, N.; Fang, F.; Zhijuan, Y.; Wang, J.; Liu, X.; Chen, X.; Dong, J.; et al. Zoonotic vectorborne pathogens and ectoparasites of dogs and cats in Eastern and Southeast Asia. Emerg. Infect. Dis. 2020, 26, 1221–1233. [Google Scholar] [CrossRef]
- Low, V.L.; Tay, S.T.; Kho, K.L.; Koh, F.X.; Tan, T.K.; Lim, Y.A.L.; Ong, B.L.; Panchadcharam, C.; Norma-Rashid, Y.; Sofian-Azirun, M. Molecular characterisation of the tick Rhipicephalus microplus in Malaysia: New insights into the cryptic diversity and distinct genetic assemblages throughout the world. Parasites Vectors 2015, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Murrell, A.; Campbell, N.J.H.; Barker, S.C. A total evidence phylogeny of ticks provides insights into the evolution of life cycles and biogeography. Mol. Phylogenet. Evol. 2001, 21, 244–258. [Google Scholar] [CrossRef]
- Murrell, A.; Barker, S.C. Synonymy of Boophilus curtice, 1891 with Rhipicephalus Koch, 1844 (Acari: Ixodidae). Syst. Parasitol. 2003, 56, 169–172. [Google Scholar] [CrossRef]
- Roy, B.C.; Estrada-Peña, A.; Krücken, J.; Rehman, A.; Nijhof, A.M. Morphological and phylogenetic analyses of Rhipicephalus microplus ticks from Bangladesh, Pakistan and Myanmar. Ticks Tick Borne Dis. 2018, 9, 1069–1079. [Google Scholar] [CrossRef]
- Csordas, B.G.; Garcia, M.V.; Cunha, R.C.; Giachetto, P.F.; Blecha, I.M.Z.; Andreotti, R. New insights from molecular characterization of the tick Rhipicephalus (Boophilus) microplus in Brazil. Rev. Bras. Parasitol. Vet. 2016, 25, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Dantas-Torres, F.; Latrofa, M.S.; Annoscia, G.; Giannelli, A.; Parisi, A.; Otranto, D. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasites Vectors 2013, 6, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifah, N.; Heo, C.C.; Ehlers, J.; Houssaini, J.; Tappe, D. Ticks and tick-borne pathogens in animals and humans in the island nations of Southeast Asia: A review. Acta Trop. 2020, 209, 105527. [Google Scholar] [CrossRef]
- Sahara, A.; Nugraheni, Y.R.; Patra, G.; Prastowo, J.; Priyowidodo, D. Ticks (Acari: Ixodidae) infestation on cattle in various regions in Indonesia. Vet. World 2019, 12, 1755. [Google Scholar] [CrossRef] [Green Version]
- Burger, T.D.; Shao, R.; Barker, S.C. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol. Phylogenet. Evol. 2014, 76, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Vongphayloth, K.; Brey, P.T.; Robbins, R.G.; Sutherland, I.W. First survey of the hard tick (Acari: Ixodidae) fauna of Nakai District, Khammouane Province, Laos, and an updated checklist of the ticks of Laos. Syst. Appl. Acarol. 2016, 21, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Hai, N.T.; Atsushi, M. Evaluation acaricidal efficacy of Camellia sasanqua thumb seed oil against the cattle tick Rhipicephalus (Boophilus) microplus and the dog tick Rhipicephalus sanguineus. Int. J. Med. Plant Res. 2014, 3, 284–289. [Google Scholar]
- Kolonin, G.V. Review of the Ixodid Tick Fauna (Acari: Ixodidae) of Vietnam. J. Med. Entomol. 1995, 32, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, Y.; Usaki, N.; Thongchai, C.; Kramomtong, I.; Kriengsak, P.; Tamura, Y. Seroepidemiologic survey in Thailand of Coxiella burnetii infection in cattle and chickens and presence in ticks attached to dairy cattle. Southeast Asian J. Trop. Med. Public Health 2014, 45, 1167. [Google Scholar] [PubMed]
- Kaewmongkol, S.; Kaewmongkol, G.; Inthong, N.; Lakkitjaroen, N.; Sirinarumitr, T.; Berry, C.M.; Jonsson, N.N.; Stich, R.W.; Jittapalapong, S. Variation among Bm86 sequences in Rhipicephalus (Boophilus) microplus ticks collected from cattle across Thailand. Exp. Appl. Acarol. 2015, 66, 247–256. [Google Scholar] [CrossRef]
- Ybanez, A.P.; Sivakumar, T.; Ybanez, R.H.D.; Ratilla, J.C.; Perez, Z.O.; Gabotero, S.R.; Inokuma, H. First molecular characterization of Anaplasma marginale in cattle and Rhipicephalus (Boophilus) microplus ticks in Cebu, Philippines. J. Vet. Med. Sci. 2013, 75, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Portugaliza, H.P.; Bagot, M.A. Different species of lice (Phthiraptera), fleas (Siphonaptera) and ticks (Ixodida) collected from livestock, poultry, reptile and companion animal in Leyte Island, Philippines. Livest. Res. Rural. 2015, 27, 1–10. [Google Scholar]
- Grisi, L.; Leite, R.C.; Martins, J.R.D.S.; Barros, A.T.M.D.; Andreotti, R.; Cançado, P.H.D.; León, A.A.P.D.; Pereira, J.B.; Villela, H.S. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev. Bras. Parasitol. Vet. 2014, 23, 150–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nava, S.; Estrada-Peña, A.; Petney, T.; Beati, L.; Labruna, M.B.; Szabó, M.P.; Venzal, J.M.; Mastropaolo, M.; Mangold, A.J.; Guglielmone, A.A. The taxonomic status of Rhipicephalus sanguineus (Latreille, 1806). Vet. Parasitol. 2015, 208, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Kernif, T.; Socolovschi, C.; Wells, K.; Lakim, M.B.; Inthalad, S.; Slesak, G.; Boudebouch, N.; Beaucournu, J.C.; Newton, P.N.; Raoult, D.; et al. Bartonella and Rickettsia in arthropods from the Lao PDR and from Borneo, Malaysia. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hmoon, M.M.; Htun, L.L.; Wai, S.S.; Thu, M.J.; Aung, S.T.; Chel, H.M.; Thaw, Y.N.; Win, S.Y.; Soe, N.C.; Bawm, S. Morphological and molecular identification of ticks infested in stray dogs within Nay Pyi Taw Area, Myanmar. South Asian J. Life Sci. 2018, 6, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.L.; Colella, V.; Iatta, R.; Bui, K.L.; Dantas-Torres, F.; Otranto, D. Ticks and associated pathogens from dogs in northern Vietnam. Parasitol. Res. 2019, 118, 139–142. [Google Scholar] [CrossRef]
- Changbunjong, T.; Buddhirongawatr, R.; Suwanpakdee, S.; Siengsanan, J.; Yongyuttawichai, P.; Cheewajorn, K.; Jangjaras, J.; Sangloung, C.; Ratanakorn, P. A survey of ectoparasitic arthropods on domestic animals in Tak Province, Thailand. Southeast Asian J. Trop. Med. Public Health 2009, 40, 435–442. [Google Scholar]
- Low, V.L.; Prakash, B.K.; Lim, Y.A.L.; Tan, T.K.; Vinnie-Siow, W.Y.; Sofian-Azirun, M.; AbuBakar, S. Detection of Anaplasmataceae agents and co-infection with other tick-borne protozoa in dogs and Rhipicephalus sanguineus sensu lato ticks. Exp. Appl. Acarol. 2018, 75, 429–435. [Google Scholar] [CrossRef]
- Koh, F.X.; Panchadcharam, C.; Tay, S.T. Vector-borne diseases in stray dogs in peninsular Malaysia and molecular detection of Anaplasma and Ehrlichia spp. from Rhipicephalus sanguineus (Acari: Ixodidae) ticks. J. Med. Entomol. 2015, 53, 183–187. [Google Scholar] [CrossRef]
- Galay, R.L.; Manalo, A.A.L.; Dolores, S.L.D.; Aguilar, I.P.M.; Sandalo, K.A.C.; Cruz, K.B.; Divina, B.P.; Andoh, M.; Masatani, T.; Tanaka, T. Molecular detection of tick-borne pathogens in canine population and Rhipicephalus sanguineus (sensu lato) ticks from southern Metro Manila and Laguna, Philippines. Parasites Vectors 2018, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Hadi, U.K.; Soviana, S.; Pratomo, I.R.C. Prevalence of ticks and tick-borne diseases in Indonesian dogs. J. Vet. Sci. Technol. 2016, 7, 330. [Google Scholar]
- Bakkes, D.K.; Ropiquet, A.; Chitimia-Dobler, L.; Matloa, D.E.; Apanaskevich, D.A.; Horak, I.G.; Mans, B.J.; Matthee, C.A. Adaptive radiation and speciation in Rhipicephalus ticks: A medley of novel hosts, nested predator-prey food webs, off-host periods and dispersal along temperature variation gradients. Mol. Phylogenetics Evol. 2021, 162, 107178. [Google Scholar] [CrossRef]
- Walker, A.R.; Matthews, J.; Preston, P.M. The development of electronic keys for the identification of ticks. Int. J. Trop. Insect Sci. 2005, 25, 2–5. [Google Scholar] [CrossRef]
- Anastos, G. The scutate ticks, or Ixodidae, of Indonesia. Entomol. Am. 1950, 30, 1–144. [Google Scholar]
- Yamaguti, N.; Tipton, V.J.; Keegan, H.L.; Toshioka, S. Ticks of Japan, Korea, and the Ryukyu islands. Brigh. Young Univ. Sci. Bull. Biol. Ser. 1971, 15, 1. [Google Scholar]
- Keirans, J.E.; Litwak, T.R. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), East of the Mississippi River. J. Med. Entomol. 1989, 26, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Low, V.L.; Prakash, B.K. First genetic characterization of the brown dog tick Rhipicephalus sanguineus sensu lato in Peninsular Malaysia. Exp. Appl. Acarol. 2018, 75, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Alim, M.A.; Tsuji, N.; Mondal, M.M.H. An investigation into the distribution, host-preference and population density of ixodid ticks affecting domestic animals in Bangladesh. Trop. Anim. Health Prod. 2006, 38, 485–490. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Mouillot, D.; Shenbrot, G.I.; Khokhlova, I.S.; Vinarski, M.V.; Korallo-Vinarskaya, N.P.; Poulin, R. Similarity in ectoparasite faunas of Palaearctic rodents as a function of host phylogenetic, geographic or environmental distances: Which matters the most? Int. J. Parasitol. 2010, 40, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Wanapat, M. Current livestock production and protein sources as animal feeds in Thailand. Protein Sources for the Animal Feed Industry. In Expert Consultation and Workshop Bangkok; Food and Agriculture Organization: Rome, Italy, 2004; pp. 199–213. [Google Scholar]
- Lambertz, C.; Chaikong, C.; Maxa, J.; Schlecht, E.; Gauly, M. Characteristics, socioeconomic benefits and household livelihoods of beef buffalo and beef cattle farming in Northeast Thailand. J. Agric. Rural Dev. Trop. 2012, 113, 155–164. [Google Scholar]
- Thompson, J.N. The Geographic Mosaic of Coevolution; University of Chicago Press: Chicago, IL, USA, 2005. [Google Scholar]
- Sadek, T.P. Vector-associated zoonoses in cats. In Feline Internal Medicine Secrets; Lappin, L.R., Ed.; Hanley and Belfus Inc.: Philadelphia, PA, USA, 2001; pp. 444–448. [Google Scholar]
- Hart, B.L. Role of grooming in biological control of ticks. Ann. N. Y. Acad. Sci. 2006, 916, 565–569. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Venzal, J.M.; Nava, S.; Mangold, A.; Guglielmone, A.A.; Labruna, M.B.; de La Fuente, J. Reinstatement of Rhipicephalus (Boophilus) australis (Acari: Ixodidae) with redescription of the adult and larval stages. J. Med. Entomol. 2012, 49, 794–802. [Google Scholar] [CrossRef] [Green Version]
- Munaf, H.B. Keanekaragaman hospes jenis-jenis caplak marga-marga Amblyomma, Boophilus dan Rhipicephalus (Acarina: Ixodidae) yang tercatat memarasit kerbau dan sapi di Indonesia. Ber. Biol. 1986, 3. [Google Scholar] [CrossRef]
- Kadarsan, S. Larval Ixodid Ticks of Indonesia (Acarina: Ixodidae). Ph.D. Dissertation, University of Maryland, College Park, MD, USA, 1971. [Google Scholar]
- Tay, S.T.; Koh, F.X.; Kho, K.L.; Ong, B.L. Molecular survey and sequence analysis of Anaplasma spp. in cattle and ticks in a Malaysian farm. Trop. Biomed. 2014, 31, 769–776. [Google Scholar]
- Jittapalapong, S.; Thanasilp, S.; Kengradomkit, C.; Sirinarukmit, T.; Kaewmongkol, G.; Stich, R.W. Molecular cloning, sequence analysis, and immune recognition of Bm95 from Thai strains of Rhipicephalus (Boophilus) microplus. Ann. N. Y. Acad. Sci. 2008, 1149, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Kaewhom, P.; Stich, R.W.; Needham, G.R.; Jittapalapong, S. Molecular analysis of calreticulin expressed in salivary glands of Rhipicephalus (Boophilus) microplus indigenous to Thailand. Ann. N. Y. Acad. Sci. 2008, 1149, 53–57. [Google Scholar] [CrossRef]
- Swann, P.H.P.; Claveria, F.G. Rhipicephalus (Boophilus) microplus ticks (Family Ixodidae) in goats raised in a small private farm in San Jose del Monte, Bulacan, Central Luzon, Philippines. Philipp. J. Sci. 2017, 146, 493–496. [Google Scholar]
- Silva, H.R.B.C. Prospecção Parasitológica em Timor. Subsídios para o Estudo da Fauna Parasitológica dos Seus Animais Domésticos; Junta de Investigações do Ultramar: Lisboa, Portugal, 1960. [Google Scholar]
- Chien, N.T.H.; Linh, B.K.; Van Tho, N.; Hieu, D.D.; Lan, N.T. Status of cattle ticks infection in yellow and dairy cows in Ba Vi District. In Proceedings of the International Conference on Agriculture Development in the Context of International Integration: Opportunities and Challenges, Hanoi, Vietnam, 7–8 December 2016; pp. 115–119. [Google Scholar]
- Dong, T.L.; Minh, D.B. Determine the presence of pathogens on ticks in the Mekong Delta region. In International Conference on the Development of Biomedical Engineering in Vietnam; Springer: Singapore, 2020; pp. 707–713. [Google Scholar]
- Sinaga, B.V.; Hariani, N. Prevalensi dan Intensitas ektoparasit pada anjing peliharaan (Canis familiaris) di Kalimantan Timur, Indonesia. J. Bioterdidik 2019, 7, 5. [Google Scholar]
- Wilson, N. New distributional records of ticks from Southeast Asia and the Pacific (Metastigmata: Argasidae, Ixodidae). Orient. Insects 1970, 4, 37–46. [Google Scholar] [CrossRef]
- Macadam, I.; Gudan, D.; Timbs, D.V.; Urquhart, H.R.; Sewell, M.M.H. Metazoan parasites of dogs in Sabah, Malaysia. Trop. Anim. Health Prod. 1984, 16, 34–38. [Google Scholar] [CrossRef]
- Latrofa, M.S.; Dantas-Torres, F.; Giannelli, A.; Otranto, D. Molecular detection of tick-borne pathogens in Rhipicephalus sanguineus group ticks. Ticks Tick Borne Dis. 2014, 5, 943–946. [Google Scholar] [CrossRef]
- Watanabe, M.; Nakao, R.; Amin-Babjee, S.M.; Maizatul, A.M.; Youn, J.H.; Qiu, Y.; Watanabe, M. Molecular screening for Rickettsia, Anaplasmataceae and Coxiella burnetii in Rhipicephalus sanguineus ticks from Malaysia. Trop. Biomed. 2015, 32, 390–398. [Google Scholar] [PubMed]
- Prakash, B.K.; Low, V.L.; Tan, T.K.; Vinnie-Siow, W.Y.; Lim, Y.A.L.; Morvarid, A.R.; Sofian-Azirun, M. Detection of Hepatozoon canis in the brown dog tick and domestic dogs in Peninsular Malaysia. J. Med. Entomol. 2018, 55, 1346–1348. [Google Scholar] [CrossRef] [Green Version]
- Prakash, B.K.; Low, V.L.; Vinnie-Siow, W.Y.; Tan, T.K.; Lim, Y.A.L.; Morvarid, A.R.; Sofian-Azirun, M. Detection of Babesia spp. in dogs and their ticks from Peninsular Malaysia: Emphasis on Babesia gibsoni and Babesia vogeli infections in Rhipicephalus sanguineus sensu lato (Acari: Ixodidae). J. Med. Entomol. 2018, 55, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.L.; Colella, V.; Greco, G.; Fang, F.; Nurcahyo, W.; Hadi, U.K.; Venturina, V.; Tong, K.B.Y.; Tsai, Y.L.; Taweethavonsawat, P.; et al. Molecular detection of pathogens in ticks and fleas collected from companion dogs and cats in East and Southeast Asia. Parasites Vectors 2020, 13, 420. [Google Scholar] [CrossRef]
- Theis, J.H.; Franti, C.E. Changing infestation rates of Rhipicephalus sanguineus (Latreille) (Ixodidae) ticks on dogs on Singapore Island, 1965–1966. J. Med. Entomol. 1971, 8, 23–28. [Google Scholar] [CrossRef]
- Hasan, M.B. Tick fauna of Baluran Wildlife Reserve, Indonesia. Hemera Zoa 1978, 70, 37–44. [Google Scholar]
- Adrus, M.; Ahamad, M.; Abdullah, M.T. Detection of rickettsiae in engorged ticks from small mammals in Malaysia. Borneo J. Resour. Sci. Technol. 2014, 4, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Anastos, G. Two new species of ticks from Soembawa Island, Indonesia (Acarina: Ixodidae). J. Parasitol. Res. 1956, 42, 306–310. [Google Scholar] [CrossRef]
- Walker, J.B.; Keirans, J.E.; Horak, I.G. Genus Rhipicephalus (Acari, Ixodidae). A Guide to the Brown Ticks of the World; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Cornet, J.P.; Demoraes, F.; Souris, M.; Kittayapong, P.; Gonzalez, J.P. Spatial distribution of ticks in Thailand: A discussion basis for tick-borne virus spread assessment. Int. J. Geoinformat. 2009, 5, 57–62. [Google Scholar]
- Kitaoka, S.; Suzuki, H. Studies on the parasite fauna of Thailand. Parasitic ticks on mammals and description of Ixodes siamensis sp. n. and Rhipicephalus tetracornus sp. n. (Acarina: Ixodidae). Trop. Med. 1983, 25, 205–219. [Google Scholar]
- Angus, B.M. The history of the cattle tick Boophilus microplusin Australia and achievementsinits control. Int. J. Parasitol. 1996, 26, 1341–1355. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Livestock in South East Asia. Notes on Development and Growth. 2012. Available online: http://www.fao.org/ag/againfo/home/en/news_archive/2011_Livestock_S-Asia.html (accessed on 31 January 2021).
- Rahman, W.A.; Lye, Y.P.; Chandrawathani, P. The seroprevalence of bovine babesiosis in Malaysia. Trop. Biomed. 2010, 27, 301–307. [Google Scholar] [PubMed]
- Ochirkhuu, N.; Konnai, S.; Mingala, C.N.; Okagawa, T.; Villanueva, M.; Pilapil, F.M.I.R.; Murata, S.; Ohashi, K. Molecular epidemiological survey and genetic analysis of vector-borne infections of cattle in Luzon Island, the Philippines. Vet. Parasitol. 2015, 212, 161–167. [Google Scholar] [CrossRef]
- Jirapattharasate, C.; Moumouni, P.F.A.; Cao, S.; Iguchi, A.; Liu, M.; Wang, G.; Zhou, M.; Vudriko, P.; Efstratiou, A.; Changbunjong, T.; et al. Molecular detection and genetic diversity of bovine Babesia spp., Theileria orientalis, and Anaplasma marginale in beef cattle in Thailand. Parasitol. Res. 2017, 116, 751–762. [Google Scholar] [CrossRef]
- Perry, B.; Grace, D. The impacts of livestock diseases and their control on growth and development processes that are pro-poor. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2643–2655. [Google Scholar] [CrossRef] [Green Version]
- Inpankaew, T.; Hii, S.F.; Chimnoi, W.; Traub, R.J. Canine vector-borne pathogens in semi-domesticated dogs residing in northern Cambodia. Parasites Vectors 2016, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Dantrakool, A.; Somboon, P.; Hashimoto, T.; Saito-Ito, A. Identification of a new type of Babesia species in wild rats (Bandicota indica) in Chiang Mai Province, Thailand. J. Clin. Microbiol. 2004, 42, 850–854. [Google Scholar] [CrossRef] [Green Version]
- Lim, P.L.; Chavatte, J.M.; Vasoo, S.; Yang, J. Imported human Babesiosis, Singapore, 2018. Emerg. Infect. Dis. 2020, 26, 826–828. [Google Scholar] [CrossRef]
- Iseki, H.; Zhou, L.; Kim, C.; Inpankaew, T.; Sununta, C.; Yokoyama, N.; Xuenan, X.; Sathaporn, J.; Igarashi, I. Seroprevalence of Babesia infections of dairy cows in northern Thailand. Vet. Parasitol. 2010, 170, 193–196. [Google Scholar] [CrossRef]
- Jaimes-Dueñez, J.; Triana-Chávez, O.; Holguín-Rocha, A.; Tobon-Castaño, A.; Mejía-Jaramillo, A.M. Molecular surveillance and phylogenetic traits of Babesia bigemina and Babesia bovis in cattle (Bos taurus) and water buffaloes (Bubalus bubalis) from Colombia. Parasites Vectors 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Guswanto, A.; Allamanda, P.; Mariamah, E.S.; Sodirun, S.; Wibowo, P.E.; Indrayani, L.; Nugroho, R.H.; Wirata, I.K.; Jannah, N.; Dias, L.P.; et al. Molecular and serological detection of bovine babesiosis in Indonesia. Parasites Vectors 2017, 10, 550. [Google Scholar] [CrossRef] [Green Version]
- Anamika, K.; Morand, S.; Jittapalapong, S.; Carcy, B. Babesia occurrence in rodents in relation to landscapes of mainland Southeast Asia. Vector Borne Zoonotic Dis. 2018, 18, 121–130. [Google Scholar]
- Sukanto, I.P.; Payne, R.C.; Partoutomo, S. Bovine babesiosis in Indonesia. Prev. Vet. Med. 1993, 16, 151–156. [Google Scholar] [CrossRef]
- Nugraha, A.B.; Cahyaningsih, U.; Amrozi, A.; Ridwan, Y.; Agungpriyono, S.; Taher, D.M.; Guswanto, A.; Gantuya, S.; Tayebwa, D.S.; Tuvshintulga, B.; et al. Serological and molecular prevalence of equine piroplasmosis in Western Java, Indonesia. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 1–6. [Google Scholar] [CrossRef]
- Kamyingkird, K.; Yangtara, S.; Desquesnes, M.; Cao, S.; Moumouni, A.; Jittapalapong, S.; Nimsupan, B.; Terkawi, M.A.; Masatani, T.; Nishikawa, Y.; et al. Seroprevalence of Babesia caballi and Theileria equi in horses and mules from Northern Thailand. J. Protozool. Res. 2014, 24, 11–17. [Google Scholar]
- Rothschild, C.M. Equine piroplasmosis. J. Equine Vet. Sci. 2013, 33, 497–508. [Google Scholar] [CrossRef]
- Haron, A.W.; Abdullah, F.F.; Abba, Y.; Mohammed, K.; Adamu, L.; Tijjani, A.; Sadiq, M.A.; Ahmed, S.S.; Lila, M.A. Detection of Theileria spp and hematological profiles of infected cattle from selected farms in Selangor, Malaysia. Alex. J. Vet. Sci. 2015, 44, 9–14. [Google Scholar] [CrossRef]
- Kamio, T.; Rajamanickam, C.; Kawazu, S.I.; Fujisaki, K. Epidemiology and pathogenicity of bovine theileriosis in Malaysia. Jarq-Jpn. Agric. Res. Q. 1990, 24, 231–234. [Google Scholar]
- Agina, O.A.; Shaari, M.R.; Isa, N.M.; Ajat, M.; Zamri-Saad, M.; Mazlan, M.; Muhamad, A.S.; Kassim, A.A.; Lee, C.H.; Rusli, F.H.; et al. First report of bovine anaemia associated Theileria sinensis infection and phylogenetic analyses of partial gene sequences of Theileria and Anaplasma species detected in naturally infected Malaysian cattle. Parasites Vectors 2020, 2020, 1–52. [Google Scholar]
- Ybañez, A.P.; Ybañez, R.H.D.; Claveria, F.G.; Cruz-Flores, M.J.; Xuenan, X.; Yokoyama, N.; Inokuma, H. High genetic diversity of Anaplasma marginale detected from Philippine cattle. J. Vet. Med. Sci. 2014, 76, 1009–1014. [Google Scholar] [CrossRef] [Green Version]
- Faizal, M.D.; Haryanto, A.; Tjahajati, I. Diagnosis and molecular characterization of Anaplasma platys in dog patients in Yogyakarta area, Indonesia. Indones. J. Biotechnol. 2019, 24, 43–50. [Google Scholar]
- Shukri, M.M.; Kho, K.L.; Kisomi, M.G.; Lani, R.; Marlina, S.; Radzi, S.F.M.; Tay, S.T.; Wong, L.P.; Mahmud, A.B.A.; Nizam, Q.N.H.; et al. Seroprevalence report on tick-borne encephalitis virus and Crimean-Congo hemorrhagic fever virus among Malaysian’s farm workers. BMC Public Health 2015, 15, 704. [Google Scholar]
- Skotarczak, B. Canine ehrlichiosis. Ann. Agric. Environ. Med. 2003, 10, 137–142. [Google Scholar]
- Koh, F.X.; Kho, K.L.; Kisomi, M.G.; Wong, L.P.; Bulgiba, A.; Tan, P.E.; Lim, Y.A.L.; Nizam, Q.N.H.; Panchadcharam, C.; Tay, S.T. Ehrlichia and Anaplasma Infections: Serological Evidence and Tick Surveillance in Peninsular Malaysia. J. Med. Entomol. 2017, 55, 269–276. [Google Scholar] [CrossRef]
- Chansiri, L. Tick-borne diseases in Thailand. Trop. Anim. Health Prod. 1997, 29, 52S. [Google Scholar] [CrossRef]
- Ghosh, S.; Azhahianambi, P.; de la Fuente, J. Control of ticks of ruminants, with special emphasis on livestock farming systems in India: Present and future possibilities for integrated control—A review. Exp. Appl. Acarol. 2006, 40, 49–66. [Google Scholar] [CrossRef]
- Kivaria, F.M. Estimated direct economic costs associated with tick-borne diseases on cattle in Tanzania. Trop. Anim. Health Prod. 2006, 38, 291–299. [Google Scholar] [CrossRef]
- Rodriguez-Vivas, R.I.; Grisi, L.; de León, A.A.P.; Villela, H.S.; Torres-Acosta, J.F.J.; Sánchez, H.F.; Salas, D.R.; Cruz, R.R.; Saldierna, F.; García-Carrasco, D. Potential economic impact assessment for cattle parasites in Mexico review. Rev. Mex. Cienc. Pec. 2017, 8, 61–74. [Google Scholar] [CrossRef]
- Ybañez, A.P.; Mingala, C.N.; Ybañez, R.H.D. Historical review and insights on the livestock tick-borne disease research of a developing country: The Philippine scenario. Parasitol. Int. 2018, 67, 262–266. [Google Scholar] [CrossRef]
- Mcleod, R.; Kristjanson, P. Final Report of Joint Esys/ILRI/ACIAR TickCost Project—Economic Impact of Ticks and Tick-Borne Diseases to Livestock in Africa, Asia and Australia; International Livestock Research Institute: Nairobi, Kenya, 1999. [Google Scholar]
- Rodrigues, D.S.; Leite, R.C. Economic impact of Rhipicephalus (Boophilus) microplus: Estimate of decreased milk production on a dairy farm. Arq. Bras. Med. Vet. Zootec. 2013, 65, 1570–1572. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations (FAO). Module 1. Ticks: Acaricide Resistance: Diagnosis Management and Prevention. Guidelines Resistance Management and Integrated Parasite Control in Ruminants. 2004. Available online: http://webcache.googleusercontent.com/search?q=cache:WmUrUjIYXB0J:www.fao.org/tempref/docrep/fao/010/ag014e/ag014e00.pdf+&cd=2&hl=en&ct=clnk&gl=my&client=firefox-b-d (accessed on 27 February 2021).
- Tuvshintulga, B.; Sivakumar, T.; Yokoyama, N.; Igarashi, I. Development of unstable resistance to diminazene aceturate in Babesia bovis. Int. J. Parasitol. Drugs Drug Resist. 2019, 9, 87–92. [Google Scholar] [CrossRef]
- Jonsson, N.N.; Piper, E.K.; Constantinoiu, C.C. Host resistance in cattle to infestation with the cattle tick Rhipicephalus microplus. Parasite Immunol. 2014, 36, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G. Variations between and within breeds of cattle in resistance to field infestations of the cattle tick (Boophilus microplus). Aust. J. Agric. Res. 1971, 22, 159–168. [Google Scholar] [CrossRef]
- Kongsuwan, K.; Josh, P.; Colgrave, M.L.; Bagnall, N.H.; Gough, J.; Burns, B.; Pearson, R. Activation of several key components of the epidermal differentiation pathway in cattle following infestation with the cattle tick, Rhipicephalus (Boophilus) microplus. Int. J. Parasitol. 2010, 40, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, M.A. Effects of Boophilus microplus Larval Infestation on Kedah-Kelantan Cattle and Their Bos Taurus Crosses. Ph.D. Dissertation, Universiti Putra Malaysia, Serdang, Selangor, Malaysia, 1997. [Google Scholar]
- Ferreira, L.L.; Soares, S.F.; de Oliveira Filho, J.G.; Oliveira, T.T.; de León, A.A.P.; Borges, L.M.F. Role of Rhipicephalus microplus cheliceral receptors in gustation and host differentiation. Ticks Tick Borne Dis. 2015, 6, 228–233. [Google Scholar] [CrossRef]
- Tabor, A.E.; Ali, A.; Rehman, G.; Rocha Garcia, G.; Zangirolamo, A.F.; Malardo, T.; Jonsson, N.N. Cattle tick Rhipicephalus microplus-Host Interface: A review of resistant and susceptible host responses. Front. Cell. Infect. Microbiol. 2017, 7, 506. [Google Scholar] [CrossRef] [Green Version]
- Mattioli, R.C.; Pandey, V.S.; Murray, M.; Fitzpatrick, J.L. Immunogenetic influences on tick resistance in African cattle with particular reference to trypanotolerant N’Dama (Bos taurus) and trypanosusceptible Gobra zebu (Bos indicus) cattle. Acta Trop. 2000, 75, 263–277. [Google Scholar] [CrossRef]
- Bennett, G.F. Boophilus microplus (Acarina: Ixodidae): Experimental infestations on cattle restrained from grooming. Exp. Parasitol. 1969, 26, 323–328. [Google Scholar] [CrossRef]
- Kemp, D.H.; Bourne, A. Boophilus microplus: The effect of histamine on the attachment of cattle-tick larvae-studies in vivo and in vitro. Parasitology 1980, 80, 487–496. [Google Scholar] [CrossRef]
- de Castro, J.J.; Newson, R.M. Host resistance in cattle tick control. Parasitol. Today 1993, 9, 13–17. [Google Scholar] [CrossRef]
- Schleger, A.V.; Lincoln, D.T.; Bourne, A.S. Arteriovenous anastomoses in the dermal vasculature of the skin of Bos taurus cattle, and their relationship with resistance to the tick, Boophilus microplus. Aust. J. Biol. Sci. 1981, 34, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.R. Immunology of interactions between ticks and laboratory animals. Exp. Appl. Acarol. 1989, 7, 5–13. [Google Scholar] [CrossRef]
- Garcia, G.R.; Maruyama, S.R.; Nelson, K.T.; Ribeiro, J.M.C.; Gardinassi, L.G.; Maia, A.A.M.; Ferreira, B.R.; Kooyman, F.N.J.; de Miranda Santos, I.K. Immune recognition of salivary proteins from the cattle tick Rhipicephalus microplus differs according to the genotype of the bovine host. Parasites Vectors 2017, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Vivas, R.I.; Jonsson, N.N.; Bhushan, C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Res. 2018, 117, 3–29. [Google Scholar] [CrossRef] [Green Version]
- Basripuzi, H.B.; Sani, R.A.; Ariff, O.M. Anthelmintic resistance in selected goat farms in Kelantan. Mal. J. Anim. Sci. 2012, 15, 47–56. [Google Scholar]
- Puspitasari, S.; Farajallah, A.; Erni Sulistiawati, M. Effectiveness of Ivermectin and Albendazole against Haemonchus contortus in Sheep in West Java, Indonesia. Trop. Life Sci. Res. 2016, 27, 135–144. [Google Scholar]
- Kochapakdee, S.; Pandey, V.S.; Pralomkarn, W.; Choldumrongkul, S.; Ngampongsai, W.; Lawpetchara, A. Anthelmintic resistance in goats in southern Thailand. Vet. Rec. 1995, 137, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, N.N.; Miller, R.J.; Kemp, D.H.; Knowles, A.; Ardila, A.E.; Verrall, R.G.; Rothwel, J.T. Rotation of treatments between spinosad and amitraz for the control of Rhipicephalus (Boophilus) microplus populations with amitraz resistance. Vet. Parasitol. 2010, 169, 157–164. [Google Scholar] [CrossRef]
- Bouyer, J.; Culbert, N.J.; Dicko, A.H.; Pacheco, M.G.; Virginio, J.; Pedrosa, M.C.; Garziera, L.; Pinto, A.T.M.; Klaptocz, A.; Germann, J.; et al. Field performance of sterile male mosquitoes released from an uncrewed aerial vehicle. Sci. Robot. 2020, 5, eaba6251. [Google Scholar] [CrossRef]
- Osburn, R.L.; Knipling, E.F. The potential use of sterile hybrid Boophilus ticks (Acari: Ixodidae) as a supplemental eradication technique. J. Med. Entomol. 1982, 19, 637–644. [Google Scholar] [CrossRef]
- FAOPMA. News Items. Israel: Problems of sterile—Male tick control. Pest Artic. News Summ. Sect. A. Insect. Control 1968, 14, 423. [Google Scholar]
- Loong, S.K.; Lim, F.S.; Khoo, J.J.; Lee, H.Y.; Suntharalingam, C.; Ishak, S.N.; Mohd-Taib, F.S.; AbuBakar, S. Culturable pathogenic bacteria in ticks parasitizing farm animals and rodents in Malaysia. Trop. Biomed. 2020, 37, 803–811. [Google Scholar] [PubMed]
- Wong, C.C.; Moog, F.; Chen, C.P. Forage and ruminant livestock integration in tree crop plantations of Southeast Asia. In Grasslands: Developments Opportunities Perspectives; Reynolds, S., Frame, J., Eds.; Taylor and Francis: London, UK, 2019; pp. 403–431. [Google Scholar]
- Phouyyavong, K.; Tomita, S.; Yokoyama, S. Impact of forage introduction on cattle grazing practices and crop—Livestock systems: A case study in an upland village in northern Laos. Rangel. J. 2019, 41, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Nicaretta, J.E.; Santos, J.B.D.; Couto, L.F.M.; Heller, L.M.; Cruvinel, L.B.; de Melo Júnior, R.D.; de Assis Cavalcante, A.S.; Zapa, D.M.B.; Ferreira, L.L.; de Oliveira Monteiro, C.M.; et al. Evaluation of rotational grazing as a control strategy for Rhipicephalus microplus in a tropical region. Res. Vet. Sci. 2020, 131, 92–97. [Google Scholar] [CrossRef]
- Ndawula, C., Jr.; Tabor, A.E. Cocktail anti-tick vaccines: The unforeseen constraints and approaches toward enhanced efficacies. Vaccines 2020, 8, 457. [Google Scholar] [CrossRef]
- de la Fuente, J.; Almazán, C.; Canales, M.; de la Lastra, J.M.P.; Kocan, K.M.; Willadsen, P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim. Health Res. Rev. 2007, 8, 23–28. [Google Scholar] [CrossRef]
- Garcia-Garcia, J.C.; Montero, C.; Redondo, M.; Vargas, M.; Canales, M.; Boue, O.; Rodríguez, M.; Joglar, M.; Machado, H.; González, I.L.; et al. Control of tick resistant to immunization with Bm86 in cattle vaccinated with the recombinant antigen Bm95 isolated from the cattle tick, Boophilus microplus. Vaccine 2000, 8, 2275–2287. [Google Scholar] [CrossRef]
- Lim, F.S.; Loong, S.K.; Khoo, J.J.; Tan, K.K.; Zainal, N.; Abdullah, M.F.; Khor, C.S.; AbuBakar, S. Identification and characterization of Corynebacterium lactis isolated from Amblyomma testudinarium of Sus scrofa in Malaysia. Syst. Appl. Acarol. 2018, 23, 1838–1844. [Google Scholar] [CrossRef]
- Frisch, J.E. Towards a permanent solution for controlling cattle ticks. Int. J. Parasitol. 1999, 29, 57–71. [Google Scholar] [CrossRef]
- Burrow, H.M. Genetic aspects of cattle adaptation in the tropics. In The Genetics of Cattle; Garrick, D.J., Ruvinsky, A., Eds.; CAB International: Oxfordshire, UK, 2014; pp. 571–597. [Google Scholar]
- Burrow, H.M.; Mans, B.J.; Cardoso, F.F.; Birkett, M.A.; Kotze, A.C.; Hayes, B.J.; Mapholi, N.; Dzama, K.; Marufu, M.C.; Githaka, N.W.; et al. Towards a new phenotype for tick resistance in beef and dairy cattle: A review. Anim. Prod. Sci. 2019, 59, 1401–1427. [Google Scholar] [CrossRef] [Green Version]
- Berthouly, C.; Maillard, J.C.; Doan, L.P.; Van, T.N.; Bed’Hom, B.; Leroy, G.; Thanh, H.H.; Laloë, D.; Bruneau, N.; Chi, C.V.; et al. Revealing fine scale subpopulation structure in the Vietnamese H’Mong cattle breed for conservation purposes. BMC Genet. 2010, 11, 45. [Google Scholar] [CrossRef] [Green Version]
- Hartati, H.; Utsunomiya, Y.T.; Sonstegard, T.S.; Garcia, J.F.; Jakaria, J.; Muladno, M. Evidence of Bos javanicus x Bos indicus hybridization and major QTLs for birth weight in Indonesian Peranakan Ongole cattle. BMC Genet. 2015, 16, 75. [Google Scholar] [CrossRef] [Green Version]
- Burrow, H.M. Importance of adaptation and genotype× environment interactions in tropical beef breeding systems. Animal 2012, 6, 729–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Molano, C.E.; Torres, S.U.; Monrroy, L.N. Background on the control of the cattle tick R. (B.) microplus and the use of coumarin substances as an alternative. Pharm. Pharmacol. Int. J. 2020, 8, 215–232. [Google Scholar] [CrossRef]
- Sanusi, S.B.; Abu Bakar, M.F.; Mohamed, M.; Sabran, S.F.; Mainasara, M.M. Southeast Asian medicinal plants as a potential source of antituberculosis agent. Evid. Based Complement. Altern. Med. 2017, 2017, 7185649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosado-Aguilar, J.A.; Aguilar-Caballero, A.; Rodriguez-Vivas, R.I.; Borges-Argaez, R.; Garcia-Vazquez, Z.; Mendez-Gonzalez, M. Acaricidal activity of extracts from Petiveria alliacea (Phytolaccaceae) against the cattle tick, Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Vet. Parasitol. 2010, 168, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Goode, P.; Ellse, L.; Wall, R. Preventing tick attachment to dogs using essential oils. Ticks Tick Borne Dis. 2018, 9, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Shezryna, S.; Anisah, N.; Saleh, I.; Syamsa, R.A. Acaricidal activity of the essential oils from Citrus hystrix (Rutaceae) and Cymbopogon citratus (Poaceae) on the cattle tick Rhipicephalus (Boophilus) microplus larvae (Acari: Ixodidae). Trop. Biomed. 2020, 37, 433–442. [Google Scholar] [PubMed]
- Kwanmuang, K.; Pongputhinan, T.; Jabri, A.; Chitchumnung, P. Small-scale farmers under Thailand’s smart farming system. FFTC-AP 2020, 2647. Available online: https://ap.fftc.org.tw/article/2647 (accessed on 4 March 2021).
- Tran, C.T. Overview of agricultural policies in Vietnam. FFTC-AP 2014, 629. Available online: https://ap.fftc.org.tw/article/629 (accessed on 5 March 2021).
- Kustiari, R. Livestock development policy in Indonesia. FFTC-AP 2014, 728. Available online: https://ap.fftc.org.tw/article/728 (accessed on 5 March 2021).
- Hashim, F.A.H. Strategies to strengthen livestock industry in Malaysia. FFTC-AP 2015, 911. Available online: https://ap.fftc.org.tw/article/911 (accessed on 5 March 2021).
- Altieri, M.A.; Nichols, C.I. Agroecology scaling up for food sovereignty and resiliency. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Berlin, Germany, 2012; pp. 1–29. [Google Scholar]
- Lambertz, C.; Chongkasikit, N.; Jittapalapong, S.; Gauly, M. Immune response of Bos indicus cattle against the anti-tick antigen BM91 derived from local Rhipicephalus (Boophilus) microplus ticks and its effects on tick reproduction under natural infestation. J. Parasitol. Res. 2012, 2012, 907607. [Google Scholar] [CrossRef] [PubMed]
- Artchayasawat, A.; Boueroy, P.; Boonmars, T.; Pumhirunroj, B.; Sriraj, P.; Aukkanimart, R.; Boonjaraspinyo, S.; Pitaksakulrat, O.; Ratanasuwan, P.; Suwannatrai, A.; et al. The effects of water submersion on cattle ticks. Thai J. Vet. Med. 2020, 50, 371–379. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, L.P.; Hamdan, R.H.; Hassan, B.N.H.; Reduan, M.F.H.; Okene, I.A.-A.; Loong, S.K.; Khoo, J.J.; Samsuddin, A.S.; Lee, S.H. Rhipicephalus Tick: A Contextual Review for Southeast Asia. Pathogens 2021, 10, 821. https://doi.org/10.3390/pathogens10070821
Tan LP, Hamdan RH, Hassan BNH, Reduan MFH, Okene IA-A, Loong SK, Khoo JJ, Samsuddin AS, Lee SH. Rhipicephalus Tick: A Contextual Review for Southeast Asia. Pathogens. 2021; 10(7):821. https://doi.org/10.3390/pathogens10070821
Chicago/Turabian StyleTan, Li Peng, Ruhil Hayati Hamdan, Basripuzi Nurul Hayyan Hassan, Mohd Farhan Hanif Reduan, Ibrahim Abdul-Azeez Okene, Shih Keng Loong, Jing Jing Khoo, Ahmad Syazwan Samsuddin, and Seng Hua Lee. 2021. "Rhipicephalus Tick: A Contextual Review for Southeast Asia" Pathogens 10, no. 7: 821. https://doi.org/10.3390/pathogens10070821
APA StyleTan, L. P., Hamdan, R. H., Hassan, B. N. H., Reduan, M. F. H., Okene, I. A.-A., Loong, S. K., Khoo, J. J., Samsuddin, A. S., & Lee, S. H. (2021). Rhipicephalus Tick: A Contextual Review for Southeast Asia. Pathogens, 10(7), 821. https://doi.org/10.3390/pathogens10070821







