Simvastatin Reduces Protection and Intestinal T Cell Responses Induced by a Norovirus P Particle Vaccine in Gnotobiotic Pigs
Abstract
:1. Introduction
2. Results
2.1. Simvastatin Feeding Significantly Reduced Serum Cholesterol in Gn Pigs
2.2. Protective Efficacy Conferred by the P Particle Vaccine in Simvastatin-Fed Pigs
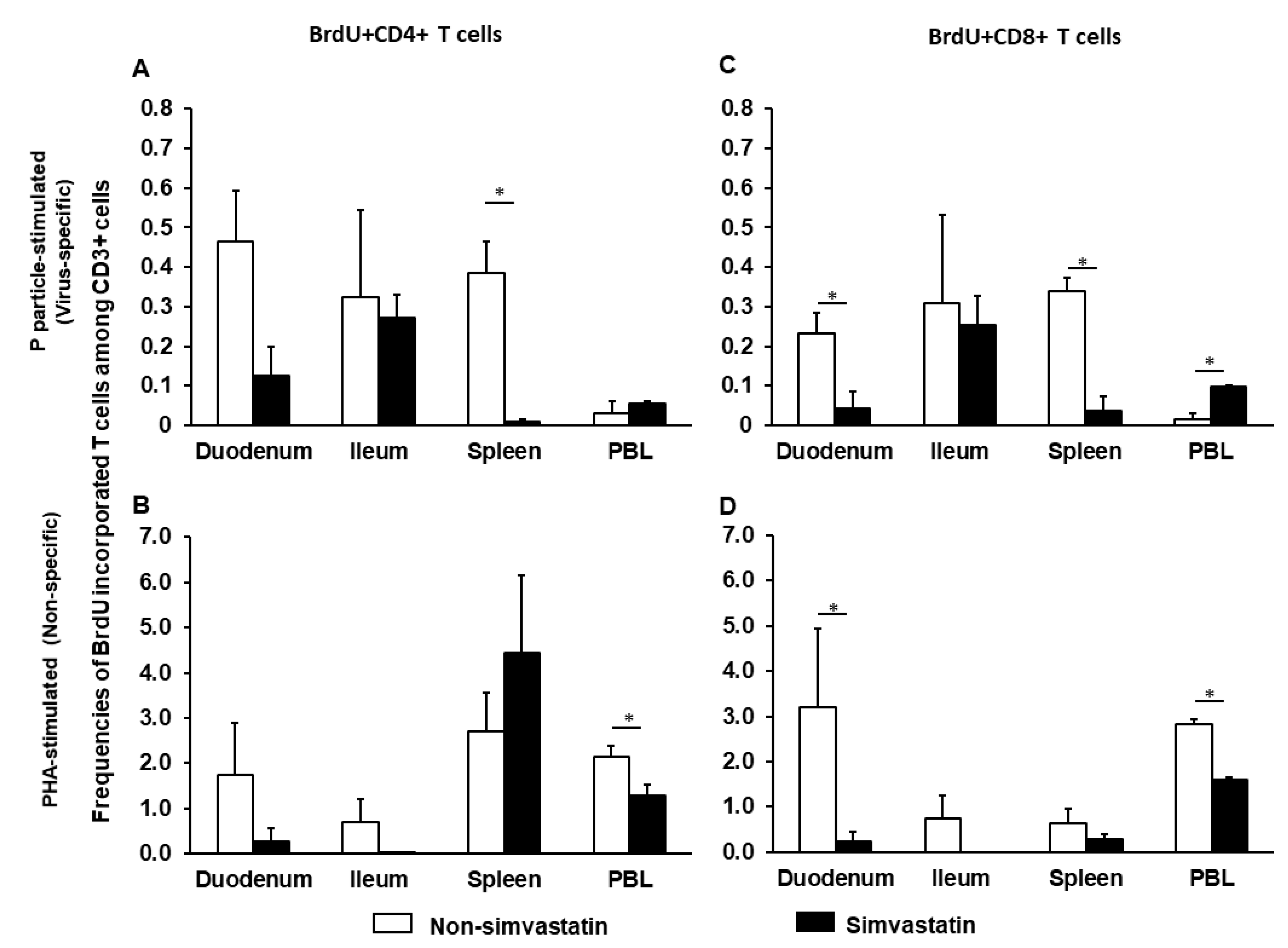
2.3. Simvastatin Decreases Frequencies of Proliferating Intestinal CD8+ T Cells after Infection
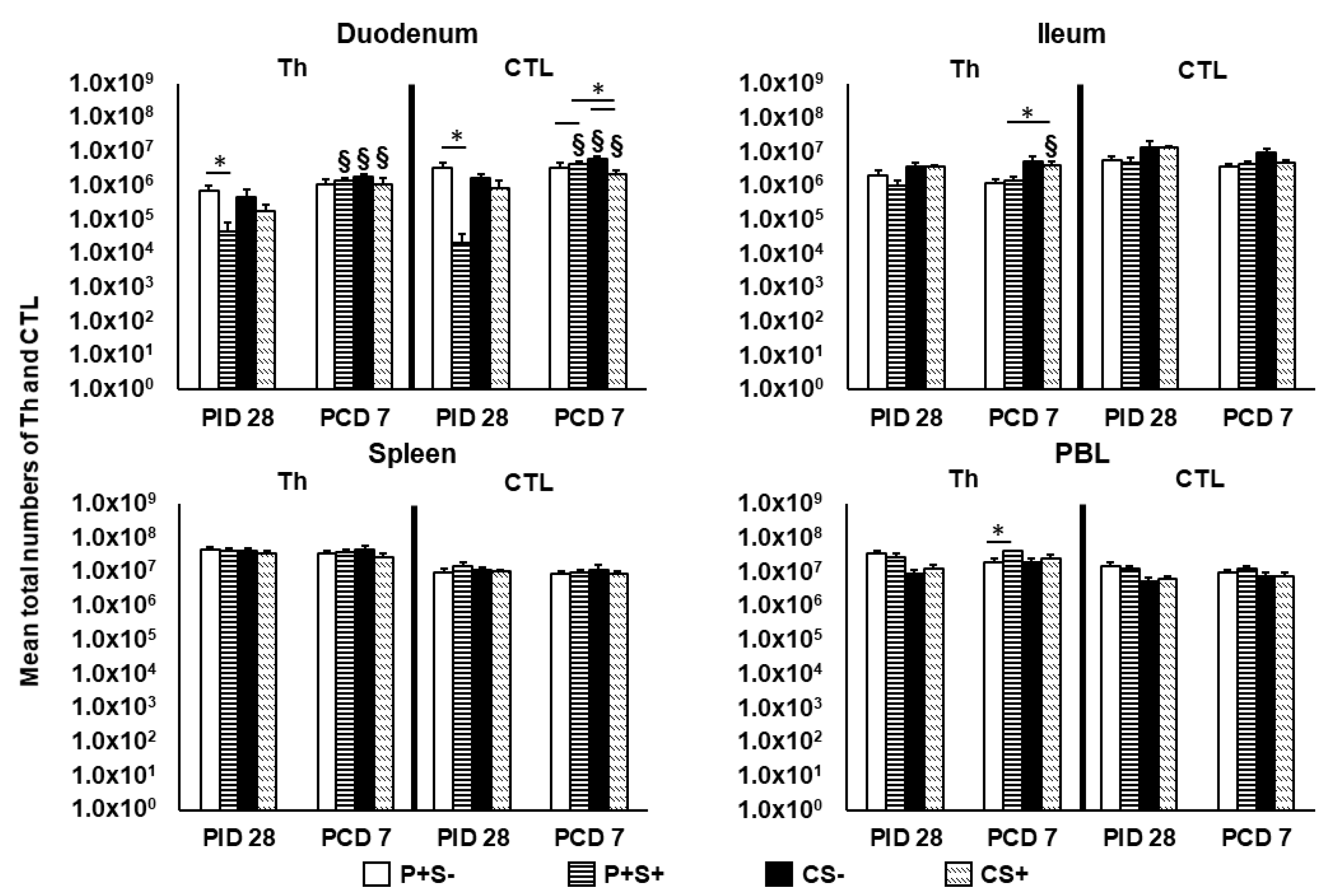
2.4. Simvastatin Feeding Decreased Total MNCs Isolated from Duodenum Pre-Challenge and PBL Post-Challenge
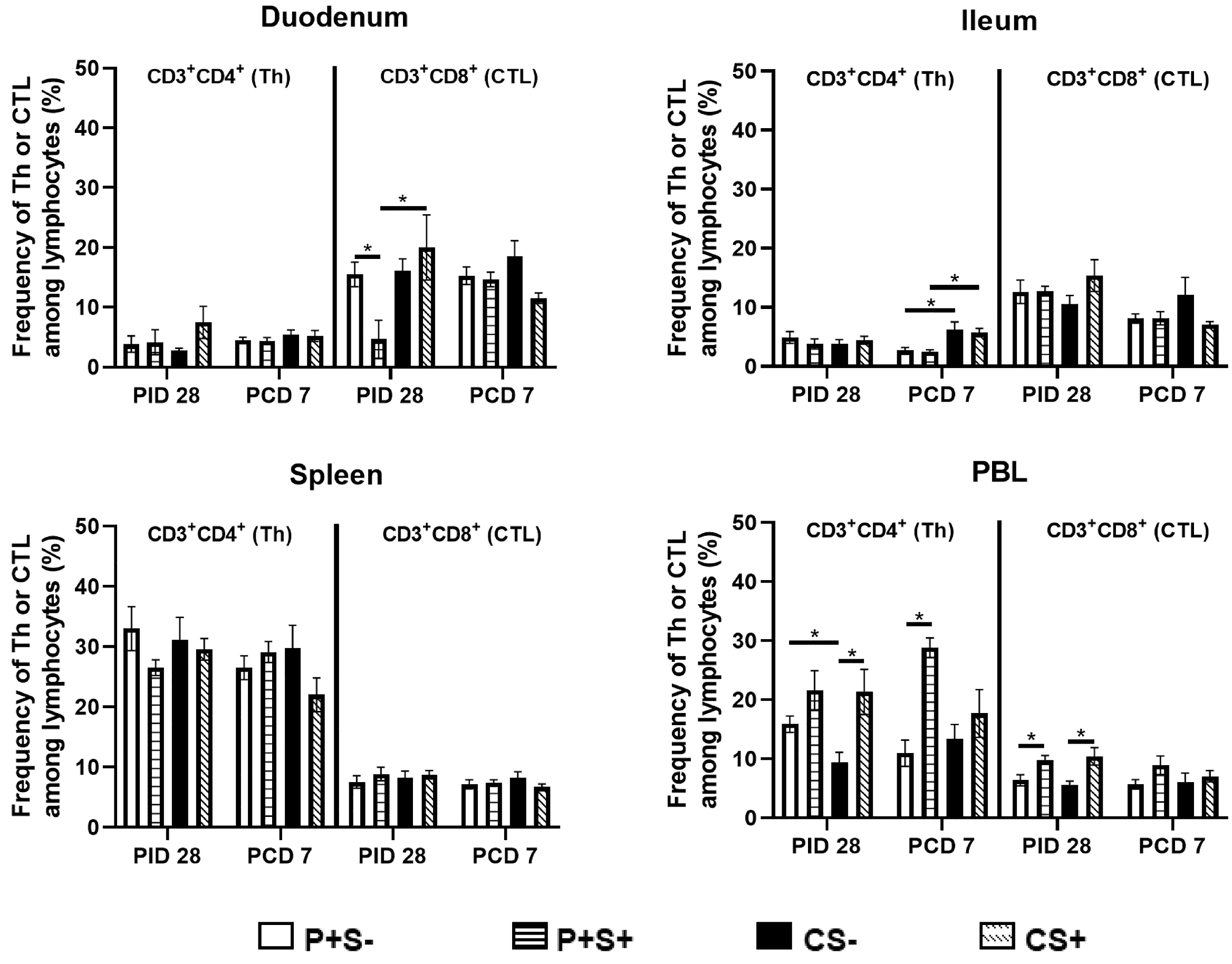
2.5. Simvastatin Decreased Total Number and Frequency of CTLs in Duodenum of Vaccinated Pigs Pre-Challenge but Increased Th in PBL Post-Challenge
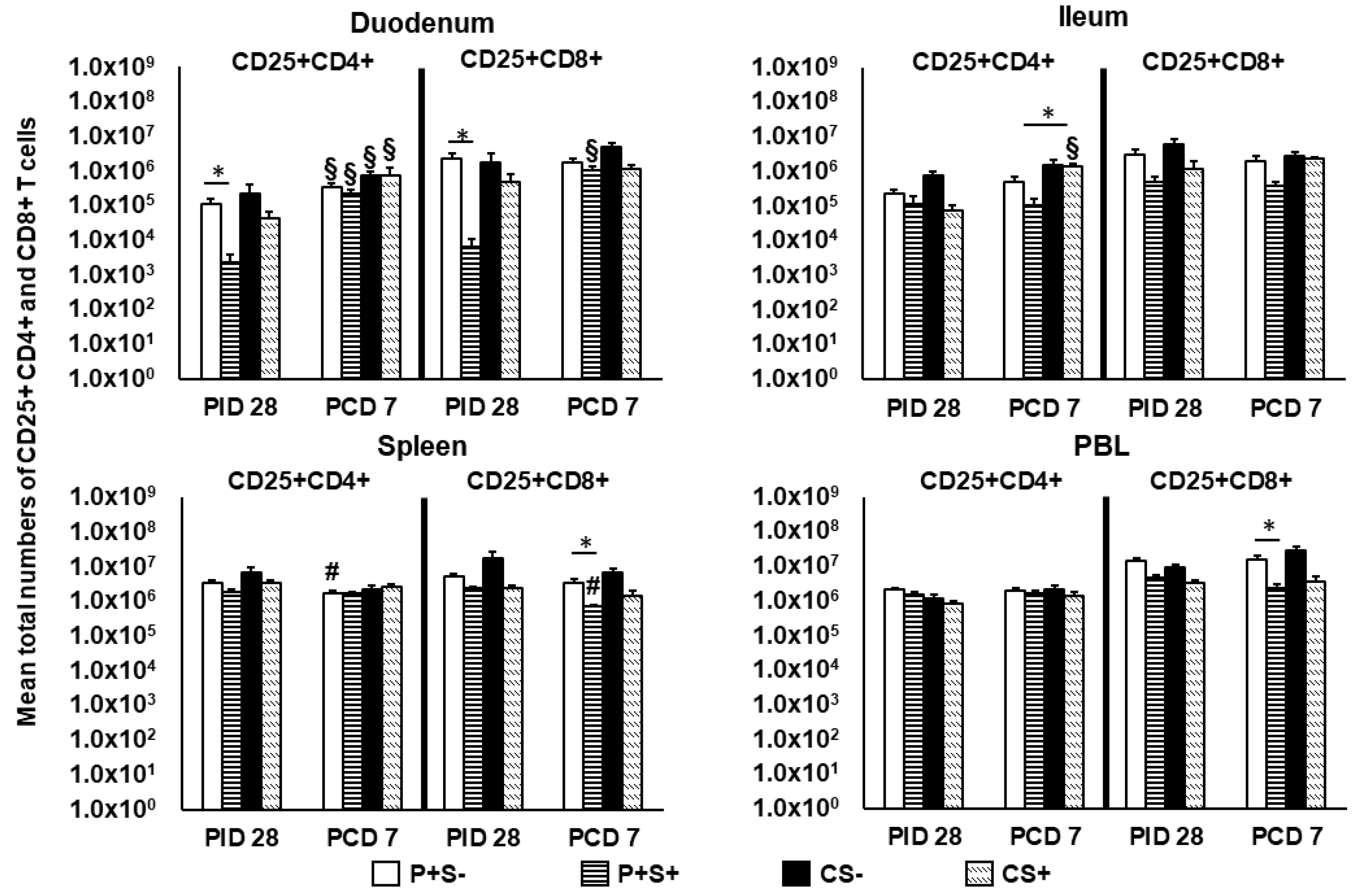
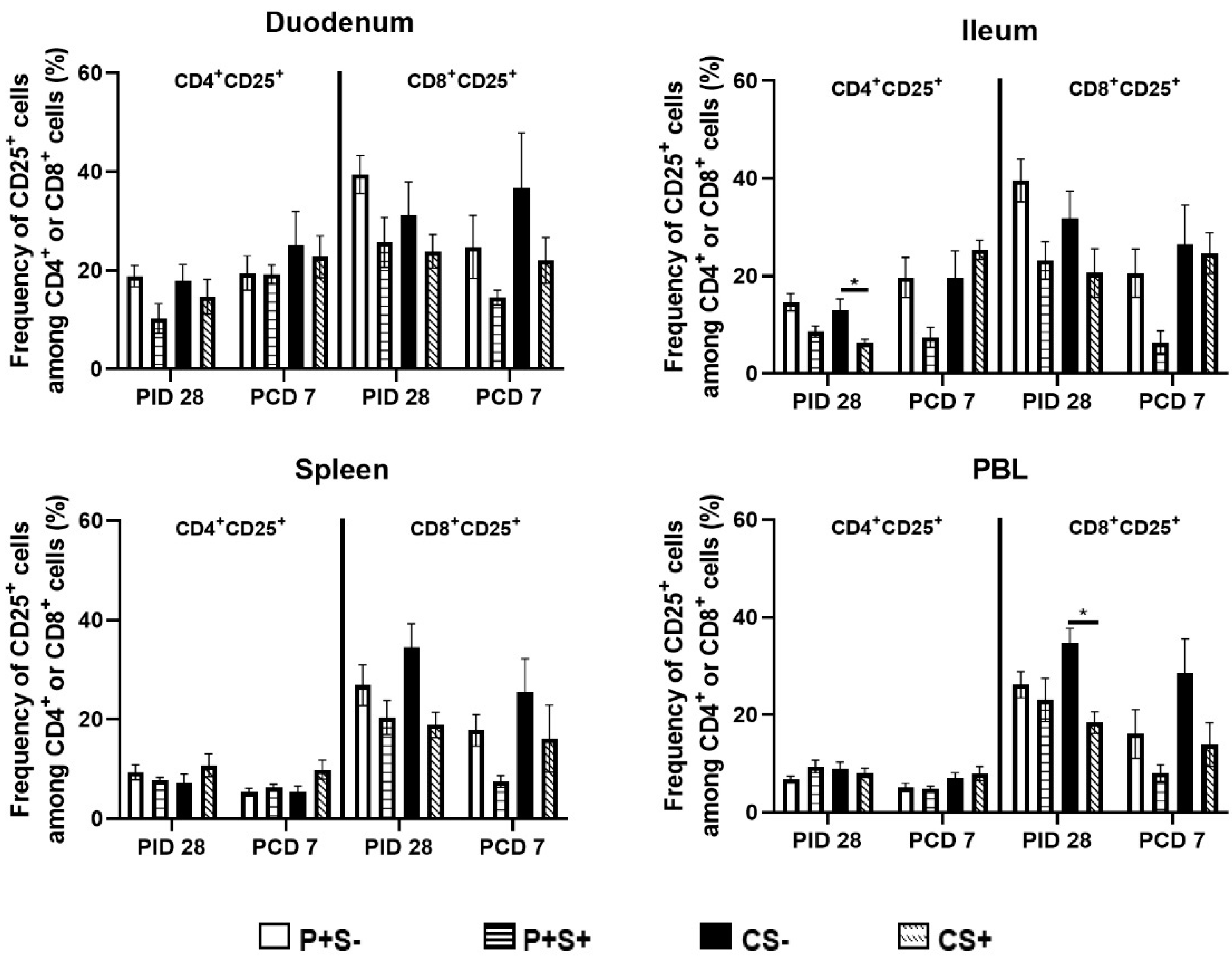
2.6. Simvastatin Reduced Numbers and/or Frequencies of Activated CD4+ and CD8+ T Cells in the Intestinal Tissues and Blood Pre-Challenge and CD8+ T Cells in Spleen and Blood Post-Challenge
2.7. Simvastatin Feeding Reduced Numbers of CD8+IFN-γ+ T Cells in Duodenum and PBL at PID 28
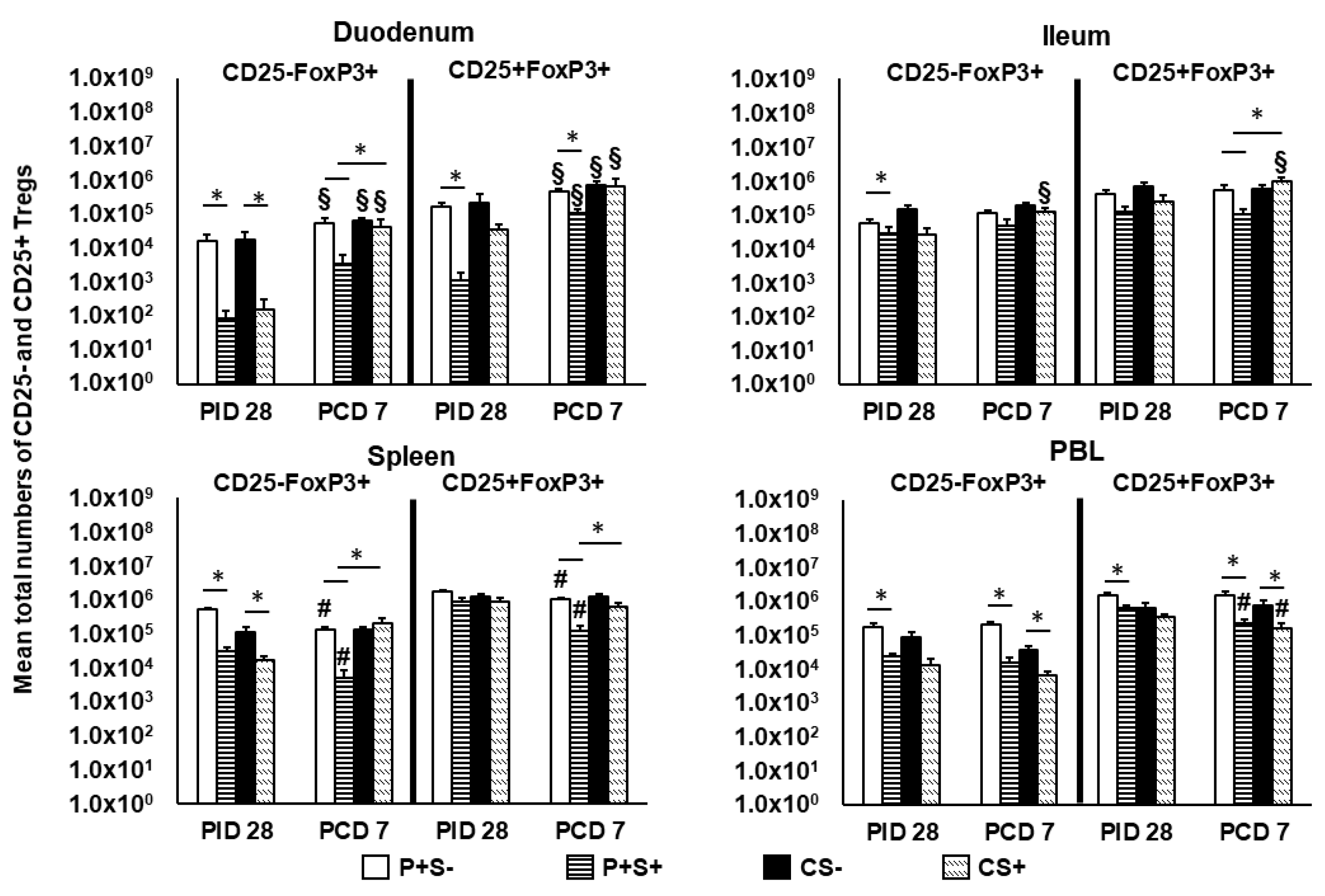
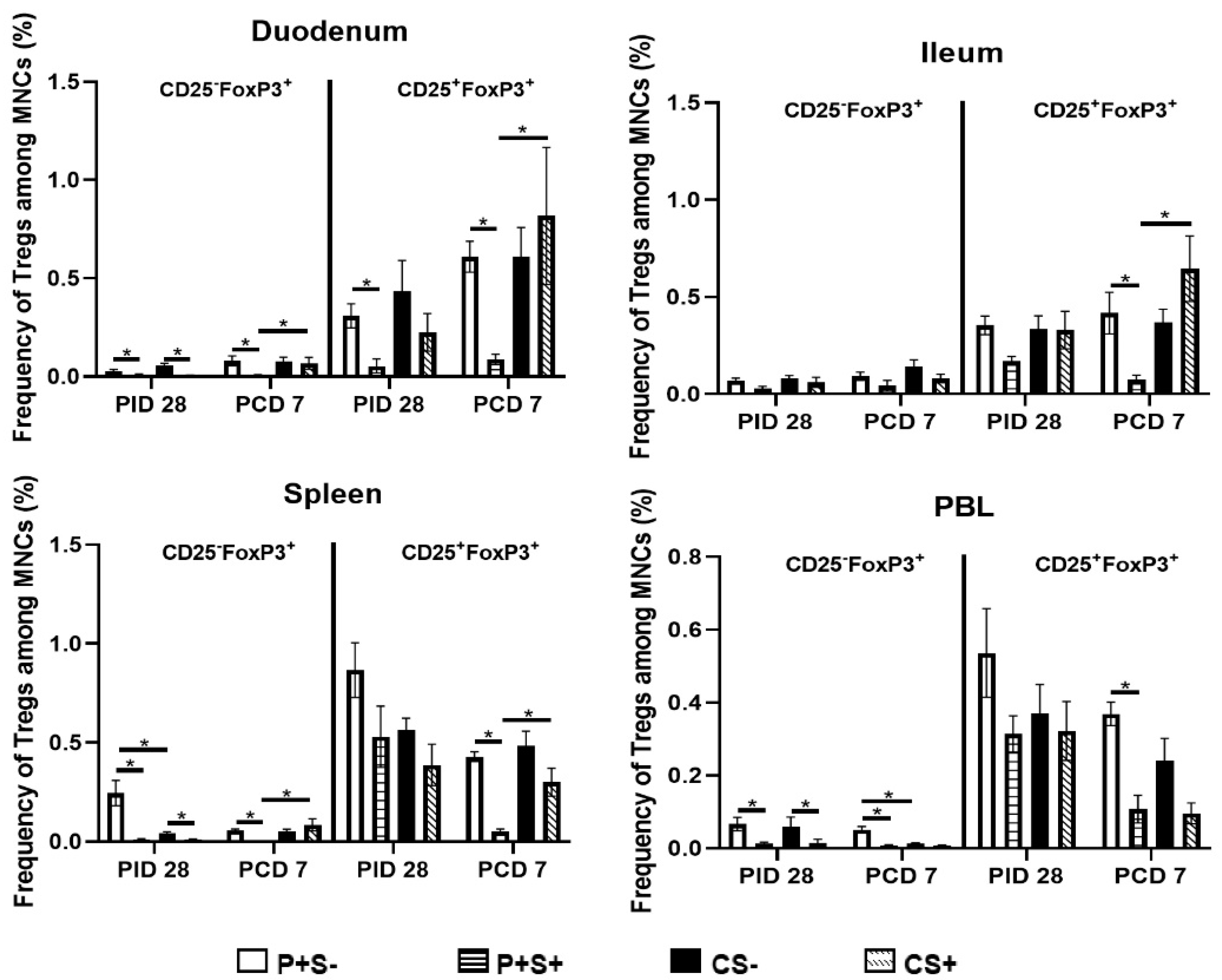
2.8. Simvastatin-Fed Pigs Had Reduced CD25−FoxP3+ and CD25+FoxP3+ Tregs in Duodenum
3. Discussion
4. Materials and Methods
4.1. Virus
4.2. Vaccine
4.3. Simvastatin Preparation
4.4. Simvastatin Treatment, Vaccination and Virus Inoculation of Gn Pigs
4.5. Assessment of NoV Shedding and Diarrhea
4.6. In Vitro MNC Proliferation Assay
4.7. Flow Cytometry Analysis of Total Th, CTL and IFN-γ Producing CD4+ and CD8+ T Cells
4.8. Flow Cytometry Analysis of Activated Non-Regulatory (FoxP3−) and IL-10 and TGF-β Producing Tregs (FoxP3+) Cells
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cannon, J.L.; Bonifacio, J.; Bucardo, F.; Buesa, J.; Bruggink, L.; Chan, M.C.; Fumian, T.M.; Giri, S.; Gonzalez, M.D.; Hewitt, J.; et al. Global Trends in norovirus genotype distribution among children with acute gastroenteritis. Emerg. Infect. Dis. 2021, 27, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Kazama, S.; Miura, T.; Masago, Y.; Konta, Y.; Tohma, K.; Manaka, T.; Liu, X.; Nakayama, D.; Tanno, T.; Saito, M.; et al. Environmental surveillance of norovirus genogroups I and II for sensitive detection of epidemic variants. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Wu, S.; Kong, X.; Xie, H.; Fu, J.; He, Y.; Feng, W.; Liu, N.; Li, J.; Rainey, J.J.; et al. Norovirus outbreak surveillance, China, 2016–2018. Emerg. Infect. Dis. 2020, 26, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farahmand, M.; Moghoofei, M.; Dorost, A.; Shoja, Z.; Ghorbani, S.; Kiani, S.J.; Khales, P.; Esteghamati, A.; Sayyahfar, S.; Jafarzadeh, M.; et al. Global prevalence and genotype distribution of norovirus infection in children with gastroenteritis: A meta-analysis on 6 years of research from 2015 to 2020. Rev. Med. Virol. 2021, e2237. [Google Scholar] [CrossRef]
- Bartsch, S.M.; O’Shea, K.J.; Lee, B.Y. The clinical and economic burden of norovirus gastroenteritis in the United States. J. Infect. Dis. 2020, 222, 1910–1919. [Google Scholar] [CrossRef]
- Ramesh, A.K.; Parreno, V.; Schmidt, P.J.; Lei, S.; Zhong, W.; Jiang, X.; Emelko, M.B.; Yuan, L. Evaluation of the 50% infectious dose of human norovirus Cin-2 in gnotobiotic pigs: A comparison of classical and contemporary methods for endpoint estimation. Viruses 2020, 12, 955. [Google Scholar] [CrossRef]
- Barclay, L.; Cannon, J.L.; Wikswo, M.E.; Phillips, A.R.; Browne, H.; Montmayeur, A.M.; Tatusov, R.L.; Burke, R.M.; Hall, A.J.; Vinjé, J. Emerging novel GII.P16 noroviruses associated with multiple capsid genotypes. Viruses 2019, 11, 535. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Guan, H.; Zhang, Y.; Wang, C.; Yang, G.; Ruan, S.; Zhao, H.; Han, X. The prevalence of non-GII.4 norovirus genotypes in acute gastroenteritis outbreaks in Jinan, China. PLoS ONE 2018, 13, e0209245. [Google Scholar] [CrossRef]
- Belliot, G.; Lopman, B.A.; Ambert-Balay, K.; Pothier, P. The burden of norovirus gastroenteritis: An important foodborne and healthcare-related infection. Clin. Microbiol. Infect. 2014, 20, 724–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, A.J.; Curns, A.T.; McDonald, L.C.; Parashar, U.D.; Lopman, B.A. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin. Infect. Dis. 2012, 55, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Atmar, R.L.; Baehner, F.; Cramer, J.P.; Song, E.; Borkowski, A.; Mendelman, P.M.; on behalf of the NOR-201 Study Group. Rapid Responses to 2 virus-like particle norovirus vaccine candidate formulations in healthy adults: A randomized controlled trial. J. Infect. Dis. 2016, 214, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, D.I.; Atmar, R.L.; Lyon, G.M.; Treanor, J.J.; Chen, W.H.; Jiang, X.; Vinje, J.; Gregoricus, N.; Frenck, R.W., Jr.; Moe, C.L.; et al. Norovirus vaccine against experimental human GII.4 virus illness: A challenge study in healthy adults. J. Infect. Dis. 2015, 211, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Fang, P.; Chachiyo, T.; Xia, M.; Huang, P.; Fang, Z.; Jiang, W.; Jiang, X. Noroviral P particle: Structure, function and applications in virus-host interaction. Virology 2008, 382, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, M.; Huang, P.; Xia, M.; Fang, P.A.; Zhong, W.; McNeal, M.; Wei, C.; Jiang, W.; Jiang, X. Norovirus P particle, a novel platform for vaccine development and antibody production. J. Virol. 2011, 85, 753–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, M.; Zhong, W.; Song, D.; Thornton, S.; Jiang, X.E. coli-expressed recombinant norovirus capsid proteins maintain authentic antigenicity and receptor binding capability. J. Med. Virol. 2004, 74, 641–649. [Google Scholar] [CrossRef]
- Kocher, J.; Bui, T.; Giri-Rachman, E.; Wen, K.; Li, G.; Yang, X.; Liu, F.; Tan, M.; Xia, M.; Zhong, W.; et al. Intranasal P particle vaccine provided partial cross-variant protection against human GII.4 norovirus diarrhea in gnotobiotic pigs. J. Virol. 2014, 88, 9728–9743. [Google Scholar] [CrossRef] [Green Version]
- Atmar, R.L.; Bernstein, D.I.; Harro, C.D.; Al-Ibrahim, M.S.; Chen, W.H.; Ferreira, J.; Estes, M.K.; Graham, D.Y.; Opekun, A.R.; Richardson, C.; et al. Norovirus vaccine against experimental human Norwalk Virus illness. N. Engl. J. Med. 2011, 365, 2178–2187. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, J.L.; Brown, M.S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Heart Protection Study Collaborative, G.; Bulbulia, R.; Bowman, L.; Wallendszus, K.; Parish, S.; Armitage, J.; Peto, R.; Collins, R. Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: A randomised controlled trial. Lancet 2011, 378, 2013–2020. [Google Scholar] [CrossRef] [Green Version]
- Cholesterol Treatment Trialists, C.; Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [CrossRef]
- Cojocaru, L.; Rusali, A.C.; Suta, C.; Radulescu, A.M.; Suta, M.; Craiu, E. The role of simvastatin in the therapeutic approach of rheumatoid arthritis. Autoimmune Dis. 2013, 2013, 326258. [Google Scholar] [CrossRef]
- Kanda, H.; Yokota, K.; Kohno, C.; Sawada, T.; Sato, K.; Yamaguchi, M.; Komagata, Y.; Shimada, K.; Yamamoto, K.; Mimura, T. Effects of low-dosage simvastatin on rheumatoid arthritis through reduction of Th1/Th2 and CD4/CD8 ratios. Mod. Rheumatol. Jpn. Rheum. Assoc. 2007, 17, 364–368. [Google Scholar] [CrossRef]
- Leung, B.P.; Sattar, N.; Crilly, A.; Prach, M.; McCarey, D.W.; Payne, H.; Madhok, R.; Campbell, C.; Gracie, J.A.; Liew, F.Y.; et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J. Immunol. 2003, 170, 1524–1530. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tao, Y.; Wang, J.; Garcia-Mata, R.; Markovic-Plese, S. Simvastatin inhibits secretion of Th17-polarizing cytokines and antigen presentation by DCs in patients with relapsing remitting multiple sclerosis. Eur. J. Immunol. 2013, 43, 281–289. [Google Scholar] [CrossRef]
- Dalcico, R.; de Menezes, A.M.; Deocleciano, O.B.; Oria, R.B.; Vale, M.L.; Ribeiro, R.A.; Brito, G.A. Protective mechanisms of simvastatin in experimental periodontal disease. J. Periodontol. 2013, 84, 1145–1157. [Google Scholar] [CrossRef]
- Nassar, C.A.; Battistetti, G.D.; Nahsan, F.P.; Olegario, J.; Marconato, J.; Marin, C.F.; Faccioni, D.M.; da Costa, K.F.; Kottwitz, L.B.; Nassar, P.O. Evaluation of the effect of simvastatin on the progression of alveolar bone loss in experimental periodontitis—An animal study. J. Int. Acad. Periodontol. 2014, 16, 2–7. [Google Scholar]
- Kwak, B.; Mulhaupt, F.; Myit, S.; Mach, F. Statins as a newly recognized type of immunomodulator. Nat. Med. 2000, 6, 1399–1402. [Google Scholar] [CrossRef]
- Kwak, B.; Mulhaupt, F.; Veillard, N.; Pelli, G.; Mach, F. The HMG-CoA reductase inhibitor simvastatin inhibits IFN-gamma induced MHC class II expression in human vascular endothelial cells. Swiss Med. Wkly. 2001, 131, 41–46. [Google Scholar] [PubMed]
- Hillyard, D.Z.; Nutt, C.D.; Thomson, J.; McDonald, K.J.; Wan, R.K.; Cameron, A.J.; Mark, P.B.; Jardine, A.G. Statins inhibit NK cell cytotoxicity by membrane raft depletion rather than inhibition of isoprenylation. Atherosclerosis 2007, 191, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Hillyard, D.Z.; Cameron, A.J.; McDonald, K.J.; Thomson, J.; MacIntyre, A.; Shiels, P.G.; Panarelli, M.; Jardine, A.G. Simvastatin inhibits lymphocyte function in normal subjects and patients with cardiovascular disease. Atherosclerosis 2004, 175, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Okopien, B.; Krysiak, R.; Kowalski, J.; Madej, A.; Belowski, D.; Zielinski, M.; Labuzek, K.; Herman, Z.S. The effect of statins and fibrates on interferon-gamma and interleukin-2 release in patients with primary type II dyslipidemia. Atherosclerosis 2004, 176, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Mausner-Fainberg, K.; Luboshits, G.; Mor, A.; Maysel-Auslender, S.; Rubinstein, A.; Keren, G.; George, J. The effect of HMG-CoA reductase inhibitors on naturally occurring CD4+CD25+ T cells. Atherosclerosis 2008, 197, 829–839. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, K.; Li, J.; Dong, M.; Yang, J.; An, G.; Qin, W.; Gao, F.; Zhang, C.; Zhang, Y. Statins induce the accumulation of regulatory T cells in atherosclerotic plaque. Mol. Med. 2012, 18, 598–605. [Google Scholar] [CrossRef]
- Ulivieri, C.; Fanigliulo, D.; Benati, D.; Pasini, F.L.; Baldari, C.T. Simvastatin impairs humoral and cell-mediated immunity in mice by inhibiting lymphocyte homing, T-cell activation and antigen cross-presentation. Eur. J. Immunol. 2008, 38, 2832–2844. [Google Scholar] [CrossRef]
- Gerondopoulos, A.; Jackson, T.; Monaghan, P.; Doyle, N.; Roberts, L.O. Murine norovirus-1 cell entry is mediated through a non-clathrin-, non-caveolae-, dynamin- and cholesterol-dependent pathway. J. Gen. Virol. 2010, 91, 1428–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, J.W.; Wobus, C.E. Endocytosis of murine norovirus 1 into murine macrophages is dependent on dynamin II and cholesterol. J. Virol. 2010, 84, 6163–6176. [Google Scholar] [CrossRef] [Green Version]
- Mauroy, A.; Gillet, L.; Mathijs, E.; Vanderplasschen, A.; Thiry, E. Alternative attachment factors and internalization pathways for GIII.2 bovine noroviruses. J. Gen. Virol. 2011, 92, 1398–1409. [Google Scholar] [CrossRef]
- Chang, K.O. Role of cholesterol pathways in norovirus replication. J. Virol. 2009, 83, 8587–8595. [Google Scholar] [CrossRef] [Green Version]
- Shawli, G.T.; Adeyemi, O.O.; Stonehouse, N.J.; Herod, M.R. The Oxysterol 25-Hydroxycholesterol Inhibits Replication of Murine Norovirus. Viruses 2019, 11, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glitscher, M.; Martin, D.H.; Woytinek, K.; Schmidt, B.; Tabari, D.; Scholl, C.; Stingl, J.C.; Seelow, E.; Choi, M.; Hildt, E. Targeting cholesterol metabolism as efficient antiviral strategy against the hepatitis E Virus. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 159–180. [Google Scholar] [CrossRef]
- Jung, K.; Wang, Q.; Kim, Y.; Scheuer, K.; Zhang, Z.; Shen, Q.; Chang, K.O.; Saif, L.J. The effects of simvastatin or interferon-alpha on infectivity of human norovirus using a gnotobiotic pig model for the study of antivirals. PLoS ONE 2012, 7, e41619. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.; Kocher, J.; Li, Y.; Wen, K.; Li, G.; Liu, F.; Yang, X.; LeRoith, T.; Tan, M.; Xia, M.; et al. Median infectious dose of human norovirus GII.4 in gnotobiotic pigs is decreased by simvastatin treatment and increased by age. J. Gen. Virol. 2013, 94, 2005–2016. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, K.K.; Shevach, E.M. Simvastatin induces Foxp3+ T regulatory cells by modulation of transforming growth factor-beta signal transduction. Immunology 2010, 130, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Moon, J.Y.; Choi, H.K.; Kim, H.O.; Hur, G.Y.; Jung, K.H.; Lee, S.Y.; Kim, J.H.; Shin, C.; Shim, J.J.; et al. Immune regulatory effects of simvastatin on regulatory T cell-mediated tumour immune tolerance. Clin. Exp. Immunol. 2010, 161, 298–305. [Google Scholar] [CrossRef]
- Aliabadi, N.; Lopman, B.A.; Parashar, U.D.; Hall, A.J. Progress toward norovirus vaccines: Considerations for further development and implementation in potential target populations. Expert. Rev. Vaccines 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Pattekar, A.; Mayer, L.S.; Lau, C.W.; Liu, C.; Palko, O.; Bewtra, M.; Consortium, H.; Lindesmith, L.C.; Brewer-Jensen, P.D.; Baric, R.S.; et al. Norovirus-specific CD8(+) T cell responses in human blood and tissues. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 1267–1289. [Google Scholar] [CrossRef] [PubMed]
- Chachu, K.A.; LoBue, A.D.; Strong, D.W.; Baric, R.S.; Virgin, H.W. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog. 2008, 4, e1000236. [Google Scholar] [CrossRef] [Green Version]
- Tomov, V.T.; Osborne, L.C.; Dolfi, D.V.; Sonnenberg, G.F.; Monticelli, L.A.; Mansfield, K.; Virgin, H.W.; Artis, D.; Wherry, E.J. Persistent enteric murine norovirus infection is associated with functionally suboptimal virus-specific CD8 T cell responses. J. Virol. 2013, 87, 7015–7031. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Regev, D.; Watanabe, M.; Hickman, D.; Moussatche, N.; Jesus, D.M.; Kahan, S.M.; Napthine, S.; Brierley, I.; Hunter, R.N., 3rd; et al. Identification of immune and viral correlates of norovirus protective immunity through comparative study of intra-cluster norovirus strains. PLoS Pathog. 2013, 9, e1003592. [Google Scholar] [CrossRef] [Green Version]
- Lindesmith, L.; Moe, C.; Lependu, J.; Frelinger, J.A.; Treanor, J.; Baric, R.S. Cellular and humoral immunity following Snow Mountain virus challenge. J. Virol. 2005, 79, 2900–2909. [Google Scholar] [CrossRef] [Green Version]
- Reeck, A.; Kavanagh, O.; Estes, M.K.; Opekun, A.R.; Gilger, M.A.; Graham, D.Y.; Atmar, R.L. Serological correlate of protection against norovirus-induced gastroenteritis. J. Infect. Dis. 2010, 202, 1212–1218. [Google Scholar] [CrossRef] [Green Version]
- Karst, S.M.; Wobus, C.E.; Lay, M.; Davidson, J.; Virgin, H.W.T. STAT1-dependent innate immunity to a Norwalk-like virus. Science 2003, 299, 1575–1578. [Google Scholar] [CrossRef]
- Wickert, L.E.; Karta, M.R.; Audhya, A.; Gern, J.E.; Bertics, P.J. Simvastatin attenuates rhinovirus-induced interferon and CXCL10 secretion from monocytic cells in vitro. J. Leukoc. Biol. 2014, 95, 951–959. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Jiang, X. The p domain of norovirus capsid protein forms a subviral particle that binds to histo-blood group antigen receptors. J. Virol. 2005, 79, 14017–14030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.; Patton, J.T.; McDonald, S.M. Culturing, storage, and quantification of rotaviruses. Curr. Protoc. Microbiol. 2009, 15, 15C-13. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.J.; Jobst, P.M.; Weiss, M. Gnotobiotic pigs: From establishing facility to modeling human infectious diseases. In Gnotobiotics; Schoeb, T.R., Eaton, K.A., Eds.; Acamedic Press: Waltham, MA, USA, 2017; pp. 349–368. [Google Scholar] [CrossRef]
- Yuan, L.; Ward, L.A.; Rosen, B.I.; To, T.L.; Saif, L.J. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Virol. 1996, 70, 3075–3083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, K.; Li, G.; Bui, T.; Liu, F.; Li, Y.; Kocher, J.; Lin, L.; Yang, X.; Yuan, L. High dose and low dose Lactobacillus acidophilus exerted differential immune modulating effects on T cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs. Vaccine 2012, 30, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Wen, K.; Azevedo, M.S.; Gonzalez, A.M.; Zhang, W.; Saif, L.J. Virus-specific intestinal IFN-gamma producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine 2008. [Google Scholar] [CrossRef] [Green Version]


| Diarrhea b | Virus Shedding | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group * | n | Percent of pigs with diarrhea (no. of pigs with diarrhea/total no.) | Mean no. of days with diarrhea c (SEM) | Mean AUC (SEM) ** | Fold reduction in AUC | Percent of pigs shed virus (no. of pigs with shedding/total no.) | Mean no. of days with shedding c (SEM) ** | Mean AUC | Fold reduction in AUC |
| P+S+ | 6 | 100% (6/6) | 3.0 (0.7) | 8.8 (1.1) A | 0.0 | 83% (5/6) | 1.7 (0.4) B | 1.87 × 104 | −2.3 |
| CS+ | 6 | 83% (5/6) | 2.5 (0.6) | 8.8 (0.7) A | NA | 83% (5/6) | 4.2 (1.3) A | 4.22 × 104 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocher, J.; Castellucci, T.B.; Wen, K.; Li, G.; Yang, X.; Lei, S.; Jiang, X.; Yuan, L. Simvastatin Reduces Protection and Intestinal T Cell Responses Induced by a Norovirus P Particle Vaccine in Gnotobiotic Pigs. Pathogens 2021, 10, 829. https://doi.org/10.3390/pathogens10070829
Kocher J, Castellucci TB, Wen K, Li G, Yang X, Lei S, Jiang X, Yuan L. Simvastatin Reduces Protection and Intestinal T Cell Responses Induced by a Norovirus P Particle Vaccine in Gnotobiotic Pigs. Pathogens. 2021; 10(7):829. https://doi.org/10.3390/pathogens10070829
Chicago/Turabian StyleKocher, Jacob, Tammy Bui Castellucci, Ke Wen, Guohua Li, Xingdong Yang, Shaohua Lei, Xi Jiang, and Lijuan Yuan. 2021. "Simvastatin Reduces Protection and Intestinal T Cell Responses Induced by a Norovirus P Particle Vaccine in Gnotobiotic Pigs" Pathogens 10, no. 7: 829. https://doi.org/10.3390/pathogens10070829
APA StyleKocher, J., Castellucci, T. B., Wen, K., Li, G., Yang, X., Lei, S., Jiang, X., & Yuan, L. (2021). Simvastatin Reduces Protection and Intestinal T Cell Responses Induced by a Norovirus P Particle Vaccine in Gnotobiotic Pigs. Pathogens, 10(7), 829. https://doi.org/10.3390/pathogens10070829







