Retrospective Study of the Seroprevalence of HIV, HCV, and HBV in Blood Donors at a Blood Bank of Western Mexico
Abstract
:1. Introduction
2. Results
Seroprevalence of HIV, HCV, and HBV in Blood Donors
3. Discussion
4. Materials and Methods
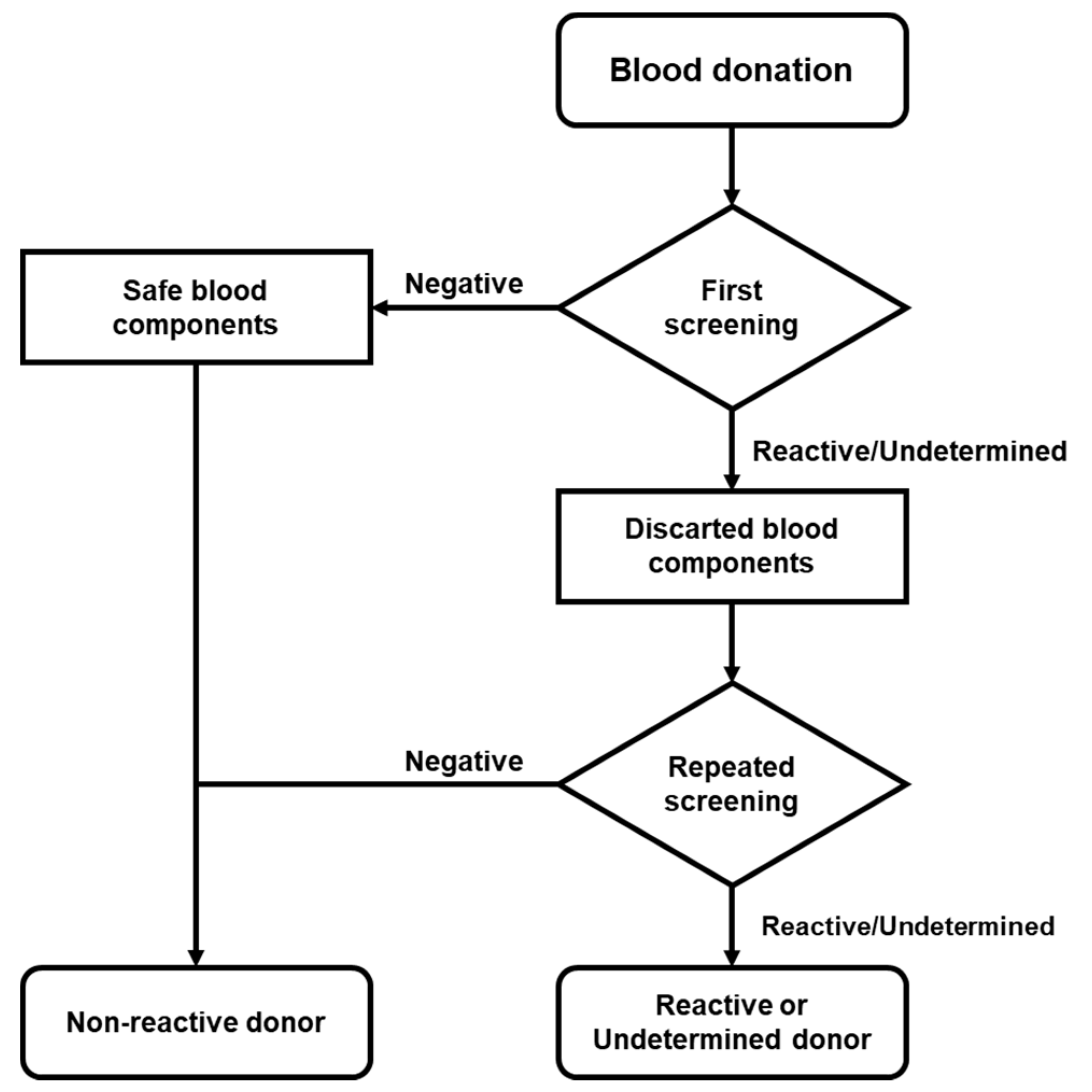
4.1. Study Population
4.2. Blood Group Typing
4.3. Serological and NAT Screening for HIV, HCV, and HBV
4.4. Reactive Donor’s Follow-up and Confirmatory Analysis
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, D.J.; Field, S.; Delaney, M.; Bates, I. Problems and Approaches for Blood Transfusion in the Developing Countries. Hematol. Oncol. Clin. North. Am. 2016, 30, 477–495. [Google Scholar] [CrossRef]
- World Health Organization. Blood Transfusion. Available online: https://www.who.int/news-room/facts-in-pictures/detail/blood-transfusion (accessed on 10 July 2021).
- World Health Organization. Screening Donated Blood for Transfusion-Transmissible Infections: Recommendations. In WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Dwyre, D.M.; Fernando, L.P.; Holland, P.V. Hepatitis B, hepatitis C and HIV transfusion-transmitted infections in the 21st century. Vox Sang. 2011, 100, 92–98. [Google Scholar] [CrossRef]
- Novelo-Garza, B.; Duque-Rodriguez, J.; Mejia-Dominguez, A.M.; Rivas-Gonzalez, M.R.; Torres-Torres, O. Blood safety in Mexico and a perspective on Latin America. Transfus. Apher. Sci. 2019, 58, 102661. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Blood Safety and Availability. Available online: https://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability (accessed on 10 July 2021).
- Busch, M.P.; Bloch, E.M.; Kleinman, S. Prevention of transfusion-transmitted infections. Blood 2019, 133, 1854–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavoie, S.; Caswell, D.; Gill, M.J.; Kadkhoda, K.; Charlton, C.L.; Levett, P.N.; Hatchette, T.; Garceau, R.; Maregmen, J.; Mazzulli, T.; et al. Heterophilic interference in specimens yielding false-reactive results on the Abbott 4th generation ARCHITECT HIV Ag/Ab Combo assay. J. Clin. Virol. 2018, 104, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Sommese, L.; Sabia, C.; Paolillo, R.; Parente, D.; Capuano, M.; Iannone, C.; Cavalca, F.; Schiano, C.; Vasco, M.; De Pascale, M.R.; et al. Screening tests for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus in blood donors: Evaluation of two chemiluminescent immunoassay systems. Scand. J. Infect. Dis. 2014, 46, 660–664. [Google Scholar] [CrossRef]
- IBRD-IDA. The World Bank Data-Mexico. Available online: https://data.worldbank.org/country/MX (accessed on 10 July 2021).
- Jenny, H.E.; Saluja, S.; Sood, R.; Raykar, N.; Kataria, R.; Tongaonkar, R.; Roy, N. Access to safe blood in low-income and middle-income countries: Lessons from India. BMJ Glob. Health 2017, 2, e000167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Status Report on Blood Safety and Availability 2016; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Dodd, R.Y.; Notari, E.P.; Nelson, D.; Foster, G.A.; Krysztof, D.E.; Kaidarova, Z.; Milan-Benson, L.; Kessler, D.A.; Shaz, B.H.; Vahidnia, F.; et al. Development of a multisystem surveillance database for transfusion-transmitted infections among blood donors in the United States. Transfusion 2016, 56, 2781–2789. [Google Scholar] [CrossRef]
- Dodd, R.Y.; Crowder, L.A.; Haynes, J.M.; Notari, E.P.; Stramer, S.L.; Steele, W.R. Screening Blood Donors for HIV, HCV, and HBV at the American Red Cross: 10-Year Trends in Prevalence, Incidence, and Residual Risk, 2007 to 2016. Transfus. Med. Rev. 2020, 34, 81–93. [Google Scholar] [CrossRef]
- Offergeld, R.; Ritter, S.; Hamouda, O. HIV, HCV, HBV and syphilis surveillance among blood donors in Germany 2008–2010. Bundesgesundheitsblatt Gesundh. Gesundh. 2012, 55, 907–913. [Google Scholar] [CrossRef] [Green Version]
- Negash, M.; Ayalew, M.; Geremew, D.; Workineh, M. Seroprevalence and associated risk factors for HIV, Hepatitis B and C among blood Donors in South Gondar District blood Bank, Northwest Ethiopia. BMC Infect. Dis. 2019, 19, 430. [Google Scholar] [CrossRef]
- Yang, S.; Jiao, D.; Liu, C.; Lv, M.; Li, S.; Chen, Z.; Deng, Y.; Zhao, Y.; Li, J. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses, and Treponema pallidum infections among blood donors at Shiyan, Central China. BMC Infect. Dis. 2016, 16, 531. [Google Scholar] [CrossRef] [Green Version]
- Jary, A.; Dienta, S.; Leducq, V.; Le Hingrat, Q.; Cisse, M.; Diarra, A.B.; Fofana, D.B.; Ba, A.; Baby, M.; Achenbach, C.J.; et al. Seroprevalence and risk factors for HIV, HCV, HBV and syphilis among blood donors in Mali. BMC Infect. Dis. 2019, 19, 1064. [Google Scholar] [CrossRef]
- Lopez-Balderas, N.; Hernandez-Romano, J.; Camara-Contreras, M.; Bravo-Sarmiento, E.; Hernandez-Romano, P.A. Trends in prevalence of HIV and syphilis in a central blood bank of Veracruz, Mexico. Transfus. Apher. Sci. 2019, 58, 94–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DOF. NORMA Oficial Mexicana NOM-253-SSA1-2012, Para la Disposición de Sangre Humana y sus Componentes con Fines Terapéuticos; Diario Oficial de la Federación; México; 2012; pp. 1–112. Available online: httpsf://www.gob.mx/cnts/documentos/norma-oficial-mexicana-nom-253-ssa1-2012-para-la-disposicion-de-sangre-humana-y-sus-componentes-con-fines-terapeuticos (accessed on 10 July 2021).
- Yonemura, S.; Doane, S.; Keil, S.; Goodrich, R.; Pidcoke, H.; Cardoso, M. Improving the safety of whole blood-derived transfusion products with a riboflavin-based pathogen reduction technology. Blood Transfus. 2017, 15, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Mundt, J.M.; Rouse, L.; Van den Bossche, J.; Goodrich, R.P. Chemical and biological mechanisms of pathogen reduction technologies. Photochem. Photobiol. 2014, 90, 957–964. [Google Scholar] [CrossRef] [Green Version]
- Valerio-Ureña, J.; Vásquez-Fernández, F.; Pérez-Sosa, J.A.; Cortazar-Benítez, L.F.; Chávez-Tapia, N.C.; Ruvalcaba-Rojas, O.A.; Torres-Medina, V.; Ocejo-Rodríguez, A. Prevalencia de marcadores serológicos de VHB y VHC en donadores de sangre de la ciudad de Veracruz. Gac. Méd. Méx. 2009, 145, 183–187. [Google Scholar] [PubMed]
- Sangrador-Deitos, M.V.; Cruz-Hernández, A.; González-Olvera, J.A.; Rodríguez-Hernández, L.A.; Sánchez-Cárdenas, C.D.; Torres-Salgado, F.G. Prevalencia de serología de enfermedades infecciosas en donadores de sangre durante 17 años en Guanajuato, México. Med. Int. Méx. 2020, 36, 15–20. [Google Scholar] [CrossRef]
- García-Montalvo, B.M. Seropositividad de VIH, VHB, VHC y Treponema pallidum en donadores de sangre en el Sureste de México. Rev. Investig. Clín. 2006, 58, 567–572. [Google Scholar]
- Baltaro, R.J.; Malenie, R.; Melbourne, H.; Garcia, F.; Gould, E.W.; Renshaw, A.A. Risk stratification of HIV infection for patients needing molecular confirmation with the Abbott 4th generation Architect System. J. Clin. Virol. 2019, 113, 31–34. [Google Scholar] [CrossRef]
- Deng, X.; Zang, L.; Wang, X.; Chen, H.; Liu, J.; Gao, Y.; Xu, S.; Wang, L.; Fan, Y.; Candotti, D.; et al. Follow-up program for blood donors with unconfirmed screening results reveals a high false-positive rate in Dalian, China. Transfusion 2020, 60, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Chacon, L.; Mateos, M.L.; Holguin, A. Relevance of cutoff on a 4th generation ELISA performance in the false positive rate during HIV diagnostic in a low HIV prevalence setting. J. Clin. Virol. 2017, 92, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Arredondo, S.; Dmytraczenko, T.; Kombe, G.; Bertozzi, S.M. Costing of scaling up HIV/AIDS treatment in Mexico. Salud Publica Mex. 2008, 50 (Suppl. 4), S437–S444. [Google Scholar] [CrossRef] [PubMed]

| Number | (%) | ||
|---|---|---|---|
| Gender | Female | 26,399 | 32.8 |
| Male | 53,992 | 67.2 | |
| Total | 80,391 | 100 | |
| Age groups (years) | 18–30 | 33,938 | 43.4 |
| 31–40 | 23,278 | 29.7 | |
| 41–50 | 15,006 | 19.2 | |
| 51–65 | 6054 | 7.7 | |
| Total | 78,276 | 100 | |
| Location | Metropolitan area | 60,961 | 77.8 |
| Adjacent regions | 17,356 | 22.2 | |
| Total | 78,317 | 100 | |
| Blood group typing (ABO) | O | 48,175 | 59.9 |
| A | 22,933 | 28.5 | |
| A1 | 45 | 0.056 | |
| A1B | 8 | 0.010 | |
| A2 | 23 | 0.029 | |
| A2B | 2 | 0.002 | |
| AB | 1599 | 2.0 | |
| B | 7606 | 9.5 | |
| Total | 80,391 | 100 | |
| Rh (D antigen) | Negative (−) | 4624 | 5.8 |
| Positive (+) | 75,767 | 94.2 | |
| Total | 80,391 | 100 |
| TTIs | Seroreactive | Percentage (%) | Rate | CI (95%) | Total |
|---|---|---|---|---|---|
| HIV | 152 | 0.1891 | 18.91 | 15.90–21.91 | 80,391 |
| HCV | 385 | 0.4789 | 47.89 | 43.12–52.66 | 80,391 |
| HBV | 181 | 0.2251 | 22.51 | 19.24–2579 | 80,391 |
| HIV | HCV | HBV | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seroreactive | Rate | CI (95%) | Seroreactive | Rate | CI (95%) | Seroreactive | Rate | CI (95%) | |||
| Gender | Female | 44 | 5.47 | 3.86–7.09 | 145 | 18.04 | 15.10–20.97 | 84 | 10.45 | 8.22–12.68 | 80,391 |
| Male | 108 | 13.43 | 10.90–15.97 | 240 | 29.85 | 26.08–33.63 | 97 | 12.07 | 9.67–14.47 | ||
| Age range | 18–30 | 69 | 8.81 | 6.74–10.89 | 124 | 15.84 | 13.06–18.63 | 78 | 9.96 | 7.75–12.18 | 78,276 |
| 31–40 | 52 | 6.64 | 4.84–8.45 | 109 | 13.93 | 11.31–16.54 | 48 | 6.13 | 4.40–7.87 | ||
| 41–50 | 26 | 3.32 | 2.05–4.60 | 87 | 11.11 | 8.78–13.45 | 35 | 4.47 | 2.99–5.95 | ||
| 51–65 | 4 | 0.51 | 0.01–1.01 | 51 | 6.52 | 4.73–8.30 | 14 | 1.79 | 0.85–2.73 | ||
| TTIs | Seroreactive + NAT-Reactive | Percentage (%) | Rate | CI (95%) | Seronegative + NAT-Reactive | Rate | CI (95%) | Total (Positive-Confirmed Seroreactive) | Percentage (%) | Rate | CI (95%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV | 32 | 0.0398 | 3.98 | 2.60–5.36 | 1 | 0.12 | 0.00–0.37 | 33 | 0.041 | 4.10 | 2.70–5.51 |
| HCV | 45 | 0.056 | 5.6 | 3.96–7.23 | 4 | 0.5 | 0.01–0.99 | 49 | 0.061 | 6.10 | 4.39–7.80 |
| HBV | 20 | 0.0249 | 2.49 | 1.40–3.58 | 2 | 0.25 | 0.10–0.59 | 22 | 0.027 | 2.74 | 1.59–3.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-García, J.d.J.; Zúñiga-Magaña, A.G.; Barrera-De León, J.C.; Magaña-Duarte, R.; Ortuño-Sahagún, D. Retrospective Study of the Seroprevalence of HIV, HCV, and HBV in Blood Donors at a Blood Bank of Western Mexico. Pathogens 2021, 10, 878. https://doi.org/10.3390/pathogens10070878
Guerrero-García JdJ, Zúñiga-Magaña AG, Barrera-De León JC, Magaña-Duarte R, Ortuño-Sahagún D. Retrospective Study of the Seroprevalence of HIV, HCV, and HBV in Blood Donors at a Blood Bank of Western Mexico. Pathogens. 2021; 10(7):878. https://doi.org/10.3390/pathogens10070878
Chicago/Turabian StyleGuerrero-García, José de Jesús, Alejandra Guadalupe Zúñiga-Magaña, Juan Carlos Barrera-De León, Rafael Magaña-Duarte, and Daniel Ortuño-Sahagún. 2021. "Retrospective Study of the Seroprevalence of HIV, HCV, and HBV in Blood Donors at a Blood Bank of Western Mexico" Pathogens 10, no. 7: 878. https://doi.org/10.3390/pathogens10070878
APA StyleGuerrero-García, J. d. J., Zúñiga-Magaña, A. G., Barrera-De León, J. C., Magaña-Duarte, R., & Ortuño-Sahagún, D. (2021). Retrospective Study of the Seroprevalence of HIV, HCV, and HBV in Blood Donors at a Blood Bank of Western Mexico. Pathogens, 10(7), 878. https://doi.org/10.3390/pathogens10070878







