The Autofluorescence Patterns of Acanthamoeba castellanii, Pseudomonas aeruginosa and Staphylococcus aureus: Effects of Antibiotics and Tetracaine
Abstract
:1. Introduction
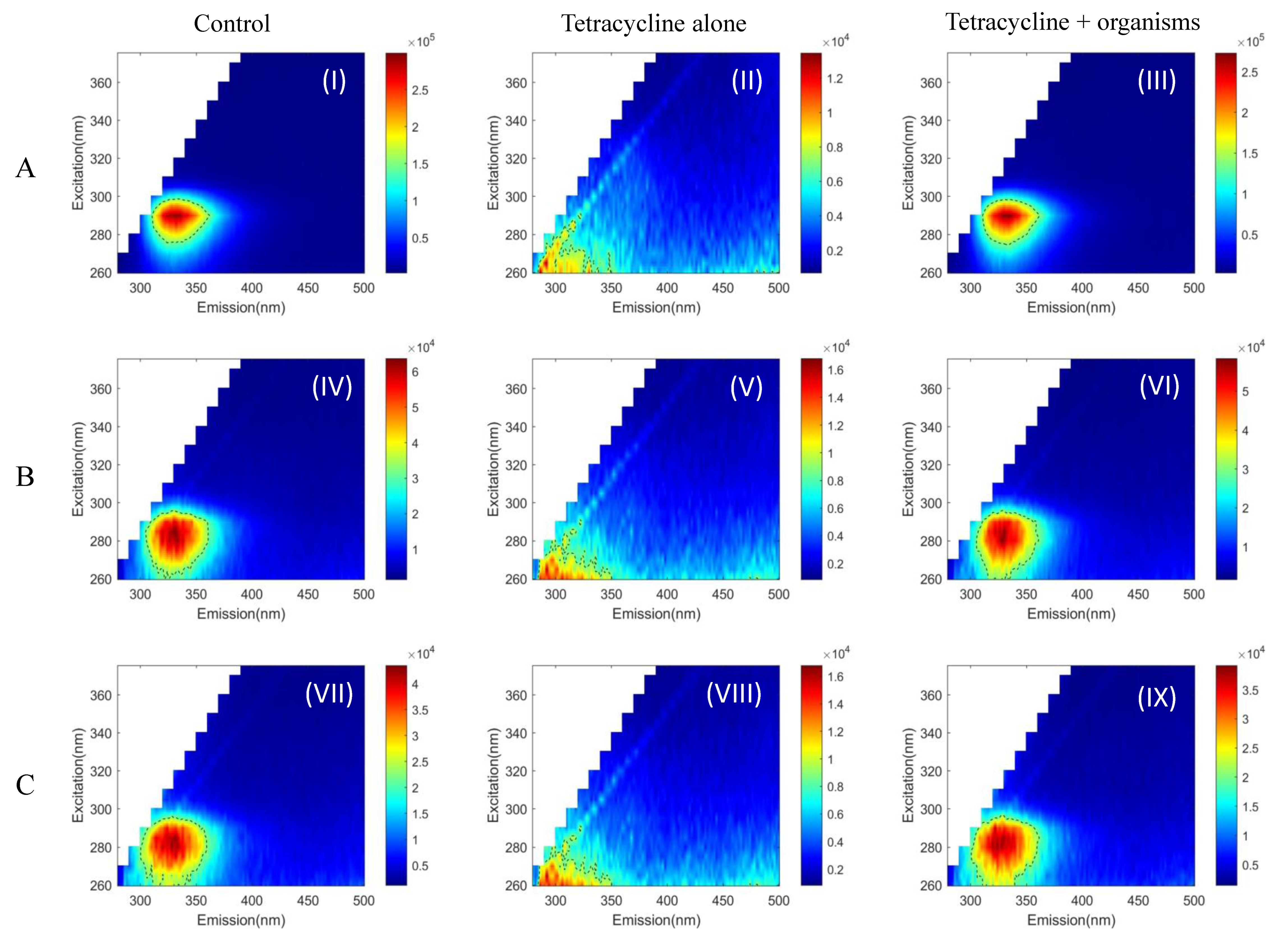
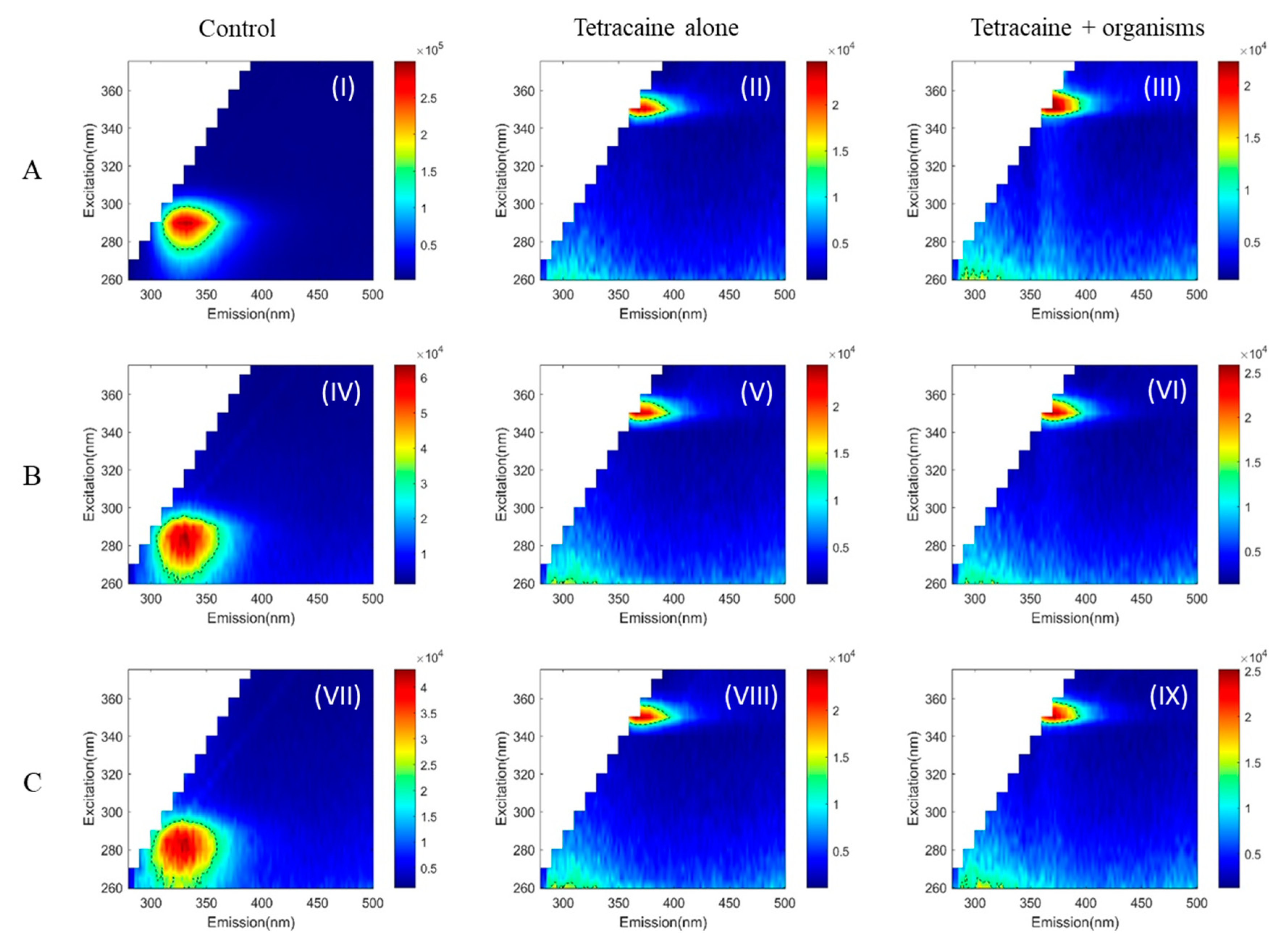
2. Results
3. Discussion
4. Materials and Methods
4.1. Culturing of Acanthamoeba and Bacteria
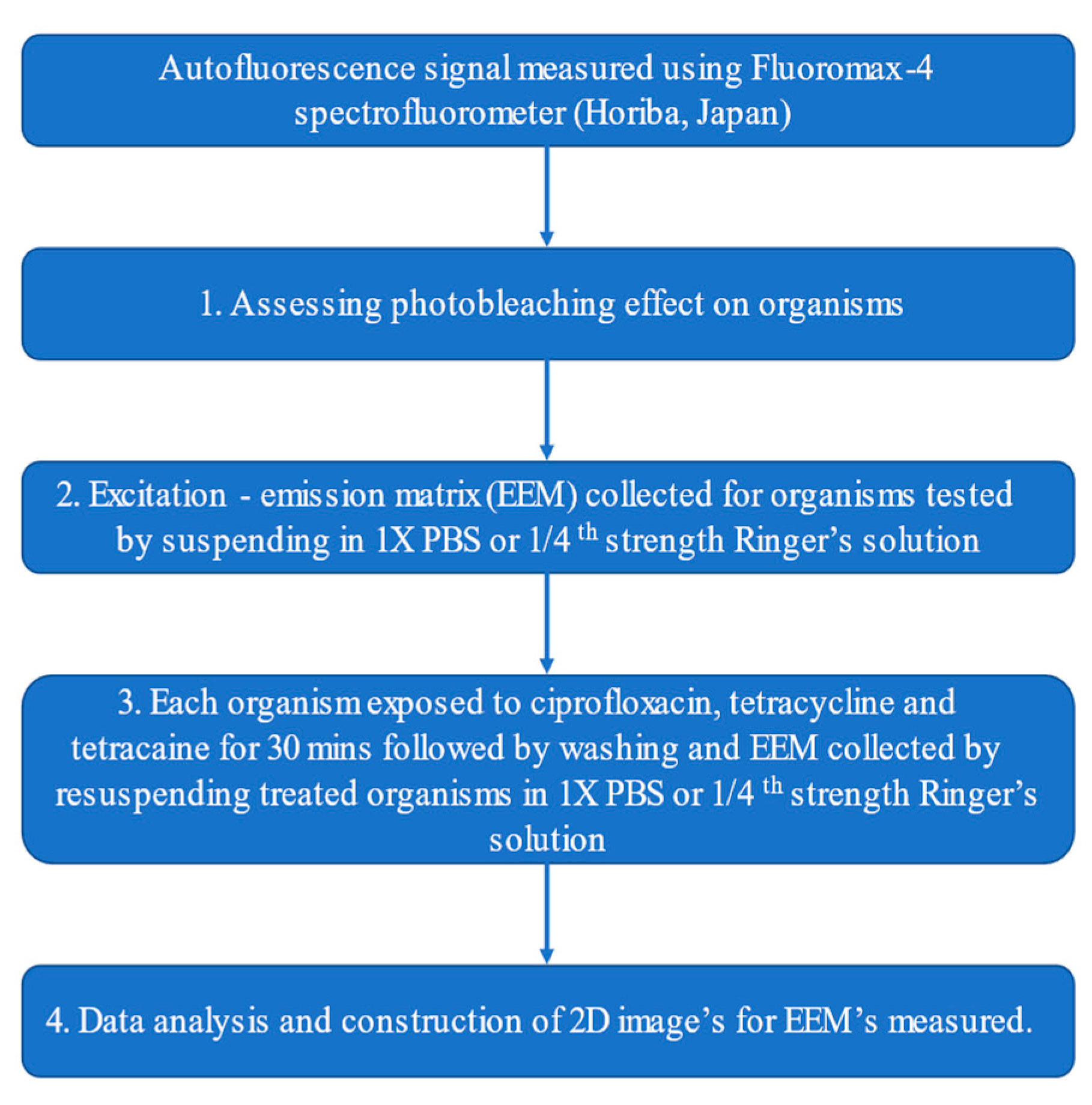
4.2. Measurement of Autofluorescence Signals
4.3. Measurement of Autofluorescence Signal after In Vitro Treatment with Topical Medications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, W.; Wang, Z.; Qu, J.; Zhang, Y.; Sun, X. Acanthamoeba keratitis related to contact lens use in a tertiary hospital in China. BMC Ophthalmol. 2019, 19, 202. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, F.; Ozkan, J.; Jalbert, I.; Holden, B.A.; Petsoglou, C.; McClellan, K. Contact Lens-Related Acanthamoeba Keratitis. Optom. Vis. Sci. 2009, 86, E1196–E1201. [Google Scholar] [CrossRef] [PubMed]
- Bacon, A.S.; Dart, J.K.G.; Ficker, L.A.; Matheson, M.M.; Wright, P. Acanthamoeba Keratitis: The Value of Early Diagnosis. Ophthalmology 1993, 100, 1238–1243. [Google Scholar] [CrossRef]
- Claerhout, I.; Goegebuer, A.; Van Den Broecke, C.; Kestelyn, P. Delay in diagnosis and outcome of Acanthamoeba keratitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 648–653. [Google Scholar] [CrossRef]
- Dart, J.K.; Saw, V.P.; Kilvington, S. Acanthamoeba keratitis: Diagnosis and treatment update 2009. Am. J. Ophthalmol. 2009, 148, 487–499.e2. [Google Scholar] [CrossRef] [PubMed]
- Tu, E.Y.; Joslin, C.E.; Sugar, J.; Booton, G.C.; Shoff, M.E.; Fuerst, P.A. The Relative Value of Confocal Microscopy and Superficial Corneal Scrapings in the Diagnosis of Acanthamoeba Keratitis. Cornea 2008, 27, 764–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, D.T.; Bui, M.-T.; Cavanagh, H.D. Epidemiology and Outcome of Microbial Keratitis: Private University Versus Urban Public Hospital Care. Eye Contact Lens 2018, 44, S82–S86. [Google Scholar] [CrossRef] [PubMed]
- Farias, R.; Pinho, L.; Santos, R. Epidemiological profile of infectious keratitis. J Rev. Bras. Oftalmol. 2017, 76, 116–120. [Google Scholar] [CrossRef]
- Ge, Z.; Qing, Y.; Zicheng, S.; Shiying, S. Rapid and sensitive diagnosis of Acanthamoeba keratitis by loop-mediated isothermal amplification. Clin. Microbiol. Infect. 2013, 19, 1042–1048. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, N.M.; Younis, M.S.; Elhamshary, A.M.; Abd-Elmaboud, A.I.; Kishik, S.M. Acanthamoeba DNA can be directly amplified from corneal scrapings. Parasitol. Res. 2014, 113, 3267–3272. [Google Scholar] [CrossRef]
- Chidambaram, J.D.; Prajna, N.V.; Palepu, S.; Lanjewar, S.; Shah, M.; Elakkiya, S.; Macleod, D.; Lalitha, P.; Burton, M.J. In Vivo Confocal Microscopy Cellular Features of Host and Organism in Bacterial, Fungal, and Acanthamoeba Keratitis. Am. J. Ophthalmol. 2018, 190, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Qian, Q.; Feng, T.; Dong, L. Study of utility PCR amplification of gene fragment of 26s-rDNA in early diagnosis of acanthamoeba keratitis. J. Wenzhou Med. Coll. 2007, 5, 422–424. [Google Scholar]
- Meerwaldt, R.; Links, T.; Graaff, R.; Thorpe, S.R.; Baynes, J.W.; Hartog, J.; Gans, R.; Smit, A. Simple Noninvasive Measurement of Skin Autofluorescence. Ann. N. Y. Acad. Sci. 2005, 1043, 290–298. [Google Scholar] [CrossRef]
- Dhingra, J.K.; Perrault, D.F.; McMillan, K.; Rebeiz, E.E.; Kabani, S.; Manoharan, R.; Itzkan, I.; Feld, M.S.; Shapshay, S.M.; Surgery, N. Early diagnosis of upper aerodigestive tract cancer by autofluorescence. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Rovati, L.L.; Docchio, F. Autofluorescence methods in ophthalmology. J. Biomed. Opt. 2004, 9, 9–22. [Google Scholar] [CrossRef] [PubMed]
- von Rückmann, A.; Fitzke, F.W.; Bird, A.C. In vivo fundus autofluorescence in macular dystrophies. Arch Ophthalmol. 1997, 115, 609–615. [Google Scholar] [CrossRef]
- Molyneux, P.M.; Kilvington, S.; Wakefield, M.J.; Prydal, J.I.; Bannister, N.P. Autofluorescence Signatures of Seven Pathogens: Preliminary in Vitro Investigations of a Potential Diagnostic for Acanthamoeba Keratitis. Cornea 2015, 34, 1588–1592. [Google Scholar] [CrossRef] [Green Version]
- Dartnell, L.R.; Roberts, T.A.; Moore, G.; Ward, J.M.; Muller, J.-P. Fluorescence Characterization of Clinically-Important Bacteria. PLoS ONE 2013, 8, e75270. [Google Scholar] [CrossRef] [Green Version]
- Wolfbeis, O.S. Fluorescence Spectroscopy: New Methods and Applications; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Eyler, R.F.; Shvets, K. Clinical Pharmacology of Antibiotics. Clin. J. Am. Soc. Nephrol. CJASN 2019, 14, 1080–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, Y.-W.; Rawal, B.D. Mechanism of Action of Tetracaine Hydrochloride against Pseudomonas aeruginosa. J. Infect. Dis. 1977, 136, 679–683. [Google Scholar] [CrossRef]
- Rieutord, A.; Vazquez, L.; Soursac, M.; Prognon, P.; Blais, J.; Bourget, P.; Mahuzier, G. Fluoroquinolones as sensitizers of lanthanide fluorescence: Application to the liquid chromatographic determination of ciprofloxacin using terbium. Anal. Chim. Acta 1994, 290, 215–225. [Google Scholar] [CrossRef]
- Iglesias-García, I.; Brandariz, I.; Iglesias, E. Fluorescence study of tetracaine–cyclodextrin inclusion complexes. Supramol. Chem. 2010, 22, 228–236. [Google Scholar] [CrossRef]
- Saint-Ruf, C.; Crussard, S.; Franceschi, C.; Orenga, S.; Ouattara, J.; Ramjeet, M.; Surre, J.; Matic, I. Antibiotic Susceptibility Testing of the Gram-Negative Bacteria Based on Flow Cytometry. Front. Microbiol. 2016, 7, 1121. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, M.M.; Greenwood-Quaintance, K.E.; Patel, R.; Pulido, J.S. Selected Antimicrobial Activity of Topical Ophthalmic Anesthetics. Transl. Vis. Sci. Technol. 2016, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Pelosini, L.; Treffene, S.; Hollick, E. Antibacterial Activity of Preservative-Free Topical Anesthetic Drops in Current Use in Ophthalmology Departments. Cornea 2009, 28, 58–61. [Google Scholar] [CrossRef]
- Labetoulle, M.; Frau, E.; Offret, H.; Nordmann, P.; Naas, T. Non-preserved 1% lidocaine solution has less antibacterial properties than currently available anaesthetic eye-drops. Curr. Eye Res. 2002, 25, 91–97. [Google Scholar] [CrossRef]
- Heaselgrave, W.; Hamad, A.; Coles, S.; Hau, S. In Vitro Evaluation of the Inhibitory Effect of Topical Ophthalmic Agents on Acanthamoeba Viability. Transl. Vis. Sci. Technol. 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullin, G.S.; Rubinfeld, R.S. The Antibacterial Activity of Topical Anesthetics. Cornea 1997, 16, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Tu, E.Y.; Joslin, C.E.; Nijm, L.M.; Feder, R.S.; Jain, S.; Shoff, M.E. Polymicrobial keratitis: Acanthamoeba and infectious crystalline keratopathy. Am. J. Ophthalmol. 2009, 148, 13–19.e12. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Li, X.; Deng, Y.; Chen, L.; Zhou, S.; Huang, W.; Lin, S.; Yuan, J. Associated factors, diagnosis and management of Acanthamoeba keratitis in a referral Center in Southern China. BMC Ophthalmol. 2017, 17, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habibalahi, A.; Moghari, M.D.; Campbell, J.M.; Anwer, A.G.; Mahbub, S.B.; Gosnell, M.; Saad, S.; Pollock, C.; Goldys, E.M. Non-invasive real-time imaging of reactive oxygen species (ROS) using multispectral auto-fluorescence imaging technique: A novel tool for redox biology. Redox Biol. 2020, 34, 101561. [Google Scholar] [CrossRef] [PubMed]
- Habibalahi, A.; Bala, C.; Allende, A.; Anwer, A.G.; Goldys, E.M. Novel automated non invasive detection of ocular surface squamous neoplasia using multispectral autofluorescence imaging. Ocul. Surf. 2019, 17, 540–550. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peguda, H.K.; Mahbub, S.B.; Sherpa, T.D.; Subedi, D.; Habibalahi, A.; Anwer, A.G.; Gu, Z.; Willcox, M.D.P.; Goldys, E.M.; Carnt, N.A. The Autofluorescence Patterns of Acanthamoeba castellanii, Pseudomonas aeruginosa and Staphylococcus aureus: Effects of Antibiotics and Tetracaine. Pathogens 2021, 10, 894. https://doi.org/10.3390/pathogens10070894
Peguda HK, Mahbub SB, Sherpa TD, Subedi D, Habibalahi A, Anwer AG, Gu Z, Willcox MDP, Goldys EM, Carnt NA. The Autofluorescence Patterns of Acanthamoeba castellanii, Pseudomonas aeruginosa and Staphylococcus aureus: Effects of Antibiotics and Tetracaine. Pathogens. 2021; 10(7):894. https://doi.org/10.3390/pathogens10070894
Chicago/Turabian StylePeguda, Hari Kumar, Saabah B. Mahbub, Tashi Doma Sherpa, Dinesh Subedi, Abbas Habibalahi, Ayad G. Anwer, Zi Gu, Mark D. P. Willcox, Ewa M. Goldys, and Nicole A. Carnt. 2021. "The Autofluorescence Patterns of Acanthamoeba castellanii, Pseudomonas aeruginosa and Staphylococcus aureus: Effects of Antibiotics and Tetracaine" Pathogens 10, no. 7: 894. https://doi.org/10.3390/pathogens10070894
APA StylePeguda, H. K., Mahbub, S. B., Sherpa, T. D., Subedi, D., Habibalahi, A., Anwer, A. G., Gu, Z., Willcox, M. D. P., Goldys, E. M., & Carnt, N. A. (2021). The Autofluorescence Patterns of Acanthamoeba castellanii, Pseudomonas aeruginosa and Staphylococcus aureus: Effects of Antibiotics and Tetracaine. Pathogens, 10(7), 894. https://doi.org/10.3390/pathogens10070894








