Zika Virus Potential Vectors among Aedes Mosquitoes from Hokkaido, Northern Japan: Implications for Potential Emergence of Zika Disease
Abstract
:1. Introduction
2. Results
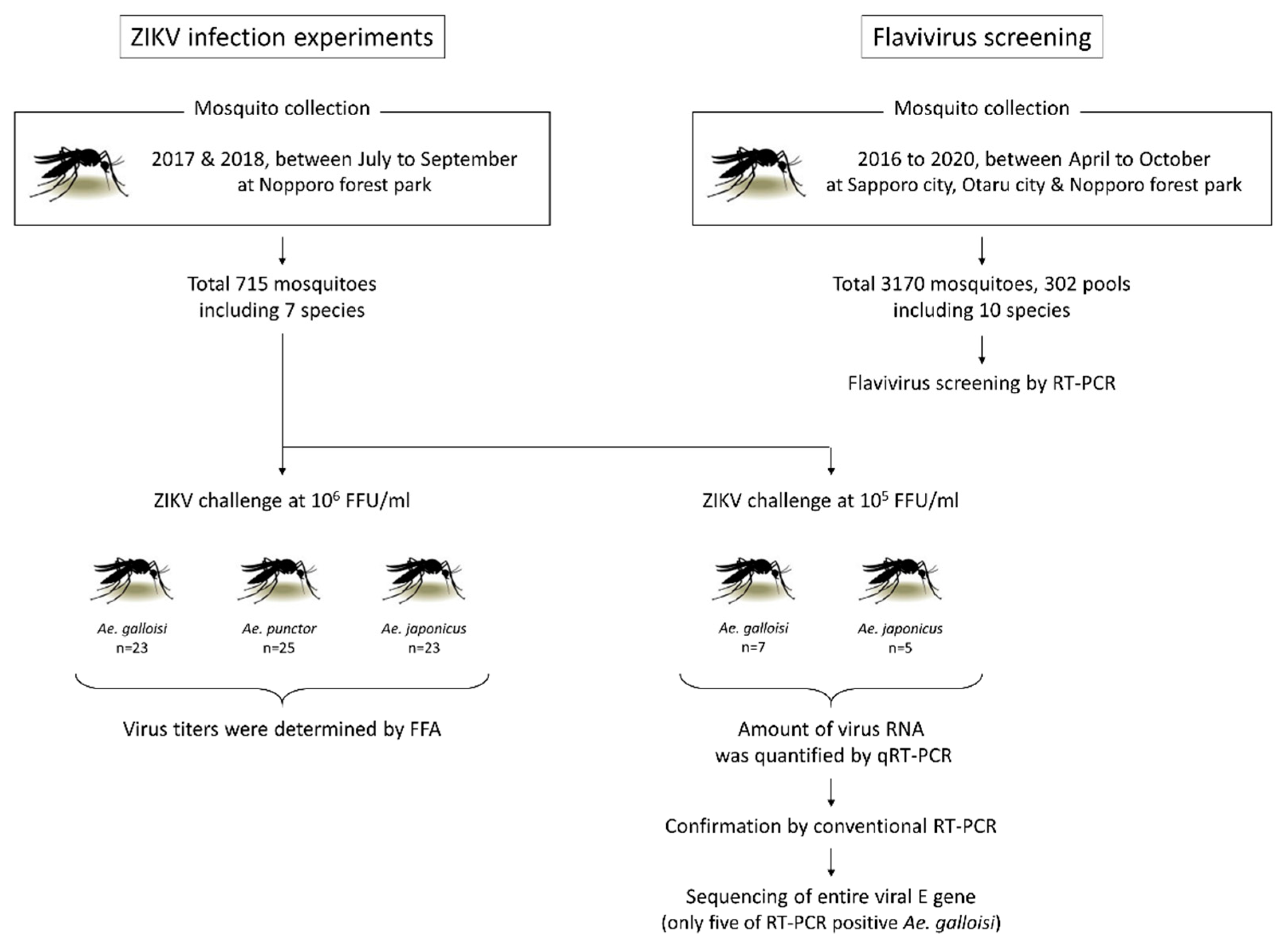
2.1. Species of Collected Mosquitoes and Flavivirus Screening
2.2. Ae. galloisi and Ae. japonicus Demonstrate Susceptibility to ZIKV Strain PRVABC59
2.3. Ae. galloisi Shows Higher Susceptibility to a Smaller amount of ZIKV Challenge When Compared with Ae. japonicus
2.4. ZIKV Could Propagate in the Abdomen of Ae. galloisi
3. Discussion
4. Materials and Methods
4.1. Cells and Viruses
4.2. Mosquito Collection, Maintenance and Species Identification
4.3. Experimental ZIKV Infection of Mosquitoes
4.4. Virus Titration and Viral RNA Evaluation in the Mosquitoes
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elbers, A.R.; Koenraadt, C.J.; Meiswinkel, R. Mosquitoes and culicoides biting midges: Vector range and the influence of climate change. Rev. Sci. Tech. 2015, 34, 123–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamimura, K. Distribution and Habitats of Japanese Mosquitoes (Diptera: Culicidae), 2nd ed.; Matsuoka, H., Ed.; Mie University Press: Mie, Japan, 2016; pp. 21–42. ISBN 978-4-903866-34-5. (In Japanese) [Google Scholar]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika virus and birth defects—Reviewing the evidence for causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef]
- Faria, N.R.; Azevedo, R.; Kraemer, M.U.G.; Souza, R.; Cunha, M.S.; Hill, S.C.; Theze, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, E., Jr.; Carvalho, F.H.; Tonni, G.; Werner, H. Prenatal imaging findings in fetal Zika virus infection. Curr. Opin. Obstet. Gynecol. 2017, 29, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.H.; Cordeiro, K.M.; Peixoto, A.B.; Tonni, G.; Moron, A.F.; Feitosa, F.E.; Feitosa, H.N.; Araujo, E., Jr. Associated ultrasonographic findings in fetuses with microcephaly because of suspected Zika virus (ZIKV) infection during pregnancy. Prenat. Diagn. 2016, 36, 882–887. [Google Scholar] [CrossRef]
- Werner, H.; Fazecas, T.; Guedes, B.; Lopes Dos Santos, J.; Daltro, P.; Tonni, G.; Campbell, S.; Araujo, E., Jr. Intrauterine Zika virus infection and microcephaly: Correlation of perinatal imaging and three-dimensional virtual physical models. Ultrasound Obstet. Gynecol. 2016, 47, 657–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baud, D.; Gubler, D.J.; Schaub, B.; Lanteri, M.C.; Musso, D. An update on Zika virus infection. Lancet 2017, 390, 2099–2109. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, B.A.; Wilson, A.E.; Zohdy, S. Aedes albopictus is a competent vector of Zika virus: A meta-analysis. PLoS ONE 2019, 14, e0216794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on yap island, federated states of micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.M. Rapid spread of emerging Zika virus in the pacific area. Clin. Microbiol. Infect. 2014, 20, O595–O596. [Google Scholar] [CrossRef] [Green Version]
- Epelboin, Y.; Talaga, S.; Epelboin, L.; Dusfour, I. Zika virus: An updated review of competent or naturally infected mosquitoes. PLoS Negl. Trop. Dis. 2017, 11, e0005933. [Google Scholar] [CrossRef] [Green Version]
- Diallo, D.; Sall, A.A.; Diagne, C.T.; Faye, O.; Faye, O.; Ba, Y.; Hanley, K.A.; Buenemann, M.; Weaver, S.C.; Diallo, M. Zika virus emergence in mosquitoes in southeastern senegal, 2011. PLoS ONE 2014, 9, e109442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Althouse, B.M.; Hanley, K.A.; Diallo, M.; Sall, A.A.; Ba, Y.; Faye, O.; Diallo, D.; Watts, D.M.; Weaver, S.C.; Cummings, D.A. Impact of climate and mosquito vector abundance on sylvatic arbovirus circulation dynamics in senegal. Am. J. Trop. Med. Hyg. 2015, 92, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Heitmann, A.; Luhken, R.; Jost, H.; Helms, M.; Vapalahti, O.; Schmidt-Chanasit, J.; Tannich, E. Experimental transmission of Zika virus by Aedes japonicus japonicus from southwestern Germany. Emerg. Microbes Infect. 2018, 7, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, C.E.; Roundy, C.M.; Azar, S.R.; Huang, J.H.; Yun, R.; Reynolds, E.; Leal, G.; Nava, M.R.; Vela, J.; Stark, P.M.; et al. Zika virus vector competency of mosquitoes, gulf coast, united states. Emerg Infect Dis 2017, 23, 559–560. [Google Scholar] [CrossRef] [Green Version]
- Hall-Mendelin, S.; Pyke, A.T.; Moore, P.R.; Mackay, I.M.; McMahon, J.L.; Ritchie, S.A.; Taylor, C.T.; Moore, F.A.; van den Hurk, A.F. Assessment of local mosquito species incriminates Aedes aegypti as the potential vector of Zika virus in Australia. PLoS Negl. Trop. Dis. 2016, 10, e0004959. [Google Scholar] [CrossRef]
- Quam, M.B.; Sessions, O.; Kamaraj, U.S.; Rocklov, J.; Wilder-Smith, A. Dissecting Japan’s dengue outbreak in 2014. Am. J. Trop. Med. Hyg. 2016, 94, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Kutsuna, S.; Kato, Y.; Moi, M.L.; Kotaki, A.; Ota, M.; Shinohara, K.; Kobayashi, T.; Yamamoto, K.; Fujiya, Y.; Mawatari, M.; et al. Autochthonous dengue fever, Tokyo, Japan, 2014. Emerg. Infect. Dis. 2015, 21, 517–520. [Google Scholar] [CrossRef] [Green Version]
- Kutsuna, S.; Kato, Y. First three imported cases of Zika virus infection in Japan. Uirusu 2016, 66, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes aegypti vector competence studies: A review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef]
- Abbo, S.R.; Visser, T.M.; Wang, H.; Goertz, G.P.; Fros, J.J.; Abma-Henkens, M.H.C.; Geertsema, C.; Vogels, C.B.F.; Koopmans, M.P.G.; Reusken, C.; et al. The invasive Asian bush mosquito Aedes japonicus found in The Netherlands can experimentally transmit Zika virus and Usutu virus. PLoS Negl. Trop. Dis. 2020, 14, e0008217. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Luna, S.M.; Weger-Lucarelli, J.; Ruckert, C.; Murrieta, R.A.; Young, M.C.; Byas, A.D.; Fauver, J.R.; Perera, R.; Flores-Suarez, A.E.; Ponce-Garcia, G.; et al. Variation in competence for ZIKV transmission by Aedes aegypti and Aedes albopictus in Mexico. PLoS Negl. Trop. Dis. 2018, 12, e0006599. [Google Scholar] [CrossRef]
- Calvez, E.; Mousson, L.; Vazeille, M.; O’Connor, O.; Cao-Lormeau, V.M.; Mathieu-Daude, F.; Pocquet, N.; Failloux, A.B.; Dupont-Rouzeyrol, M. Zika virus outbreak in the pacific: Vector competence of regional vectors. PLoS Negl. Trop. Dis. 2018, 12, e0006637. [Google Scholar] [CrossRef] [PubMed]
- Roundy, C.M.; Azar, S.R.; Rossi, S.L.; Huang, J.H.; Leal, G.; Yun, R.; Fernandez-Salas, I.; Vitek, C.J.; Paploski, I.A.; Kitron, U.; et al. Variation in Aedes aegypti mosquito competence for Zika virus transmission. Emerg. Infect. Dis. 2017, 23, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.G.; Fonseca, D.M. Invasion biology of Aedes japonicus japonicus (diptera: Culicidae). Annu. Rev. Entomol. 2014, 59, 31–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubry, F.; Dabo, S.; Manet, C.; Filipovic, I.; Rose, N.H.; Miot, E.F.; Martynow, D.; Baidaliuk, A.; Merkling, S.H.; Dickson, L.B.; et al. ; et al. Enhanced Zika virus susceptibility of globally invasive Aedes aegypti populations. Science 2020, 370, 991–996. [Google Scholar] [CrossRef]
- Van Boheemen, S.; Tas, A.; Anvar, S.Y.; van Grootveld, R.; Albulescu, I.C.; Bauer, M.P.; Feltkamp, M.C.; Bredenbeek, P.J.; van Hemert, M.J. Quasispecies composition and evolution of a typical Zika virus clinical isolate from Suriname. Sci. Rep. 2017, 7, 2368. [Google Scholar] [CrossRef]
- Chakraborty, S. Computational analysis of perturbations in the post-fusion dengue virus envelope protein highlights known epitopes and conserved residues in the Zika virus. F1000Research 2016, 5, 1150. [Google Scholar] [CrossRef] [PubMed]
- Sukupolvi-Petty, S.; Austin, S.K.; Purtha, W.E.; Oliphant, T.; Nybakken, G.E.; Schlesinger, J.J.; Roehrig, J.T.; Gromowski, G.D.; Barrett, A.D.; Fremont, D.H.; et al. Type- and subcomplex-specific neutralizing antibodies against domain iii of dengue virus type 2 envelope protein recognize adjacent epitopes. J. Virol. 2007, 81, 12816–12826. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.H.; Chiang, C.C.; Ng, M.L. Immunization of flavivirus west Nile recombinant envelope domain iii protein induced specific immune response and protection against west Nile virus infection. J. Immunol. 2007, 178, 2699–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Dent, M.; Lai, H.; Sun, H.; Chen, Q. Immunization of Zika virus envelope protein domain iii induces specific and neutralizing immune responses against Zika virus. Vaccine 2017, 35, 4287–4294. [Google Scholar] [CrossRef]
- Sota, T.; Mogi, M. Origin of pitcher plant mosquitoes in Aedes (Stegomyia): A molecular phylogenetic analysis using mitochondrial and nuclear gene sequences. J. Med. Entomol. 2006, 43, 795–800. [Google Scholar] [CrossRef]
- Kauffman, E.B.; Kramer, L.D. Zika virus mosquito vectors: Competence, biology, and vector control. J. Infect. Dis. 2017, 216, S976–S990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musso, D.; Gubler, D.J. Zika virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthet, N.; Nakoune, E.; Kamgang, B.; Selekon, B.; Descorps-Declere, S.; Gessain, A.; Manuguerra, J.C.; Kazanji, M. Molecular characterization of three Zika flaviviruses obtained from sylvatic mosquitoes in the Central African republic. Vector Borne Zoonotic Dis. 2014, 14, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, J.B.; Mee, P.T.; Lynch, S.E.; Vedururu, R.; Trinidad, L.; Paradkar, P. Zika vector transmission risk in temperate Australia: A vector competence study. Virol. J. 2017, 14, 108. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Lambert, A.J.; Holodniy, M.; Saavedra, S.; Signor Ldel, C. Phylogeny of Zika virus in western hemisphere, 2015. Emerg. Infect. Dis. 2016, 22, 933–935. [Google Scholar] [CrossRef]
- Uchida, L.; Espada-Murao, L.A.; Takamatsu, Y.; Okamoto, K.; Hayasaka, D.; Yu, F.; Nabeshima, T.; Buerano, C.C.; Morita, K. The dengue virus conceals double-stranded rna in the intracellular membrane to escape from an interferon response. Sci. Rep. 2014, 4, 7395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Mizusawa, K.; Saugstad, E.S. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu archipelago and the Ogasawara Islands) and Korea (diptera: Culicidae). Contrib. Am. Entomol. Inst. 1979, 16, 1–987. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Scaramozzino, N.; Crance, J.M.; Jouan, A.; DeBriel, D.A.; Stoll, F.; Garin, D. Comparison of flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-pcr assay for detection of flaviviruses targeted to a conserved region of the ns5 gene sequences. J. Clin. Microbiol. 2001, 39, 1922–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, yap state, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘ezr’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Species | No. of Collection | No. of IBM-Feeding | Feeding Rate |
|---|---|---|---|
| Ae. bekkui | 5 | 5 | 100.0% |
| Ae. galloisi | 99 | 30 | 30.3% |
| Ae. punctor | 127 | 29 | 22.8% |
| Ae. japonicus | 311 | 79 | 25.4% |
| Ae. vexans | 27 | 15 | 55.6% |
| Cx. orientalis | 4 | 0 | 0.0% |
| Tr. bambusa | 7 | 0 | 0.0% |
| Unidentified | 135 | 0 | 0.0% |
| Total | 715 | 158 | 22.1% |
| Species | No. of Collection | No. of Pool | Detected Flavivirus (No. of Positive Pools) |
|---|---|---|---|
| Ae. ezoensis | 183 | 21 | 0 |
| Ae. galloisi | 1 | 1 | 0 |
| Ae. japonicus | 2619 | 198 | 0 |
| Ae. nipponicus | 18 | 5 | 0 |
| Ae. punctor | 1 | 1 | 0 |
| Ae. togoi | 134 | 22 | 0 |
| Ae. vexans | 74 | 10 | 0 |
| An. sineroides | 1 | 1 | 0 |
| Cx. orientalis | 37 | 14 | 0 |
| Cx. pipiens group | 86 | 19 | CxFV (2) * |
| Unidentified | 16 | 10 | 0 |
| Total | 3170 | 302 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, L.; Shibuya, M.; Morales-Vargas, R.E.; Hagiwara, K.; Muramatsu, Y. Zika Virus Potential Vectors among Aedes Mosquitoes from Hokkaido, Northern Japan: Implications for Potential Emergence of Zika Disease. Pathogens 2021, 10, 938. https://doi.org/10.3390/pathogens10080938
Uchida L, Shibuya M, Morales-Vargas RE, Hagiwara K, Muramatsu Y. Zika Virus Potential Vectors among Aedes Mosquitoes from Hokkaido, Northern Japan: Implications for Potential Emergence of Zika Disease. Pathogens. 2021; 10(8):938. https://doi.org/10.3390/pathogens10080938
Chicago/Turabian StyleUchida, Leo, Miki Shibuya, Ronald Enrique Morales-Vargas, Katsuro Hagiwara, and Yasukazu Muramatsu. 2021. "Zika Virus Potential Vectors among Aedes Mosquitoes from Hokkaido, Northern Japan: Implications for Potential Emergence of Zika Disease" Pathogens 10, no. 8: 938. https://doi.org/10.3390/pathogens10080938






