Asymptomatic COVID-19 Individuals Tend to Establish Relatively Balanced Innate and Adaptive Immune Responses
Abstract
:1. Introduction
2. Results
2.1. Demographic Characteristics
2.2. Clinical Characteristics
2.3. The Characteristics of Blood Routine Examination, Routine Coagulation Tests and Acute Phase Reactants for the Asymptomatic COVID-19 Individuals
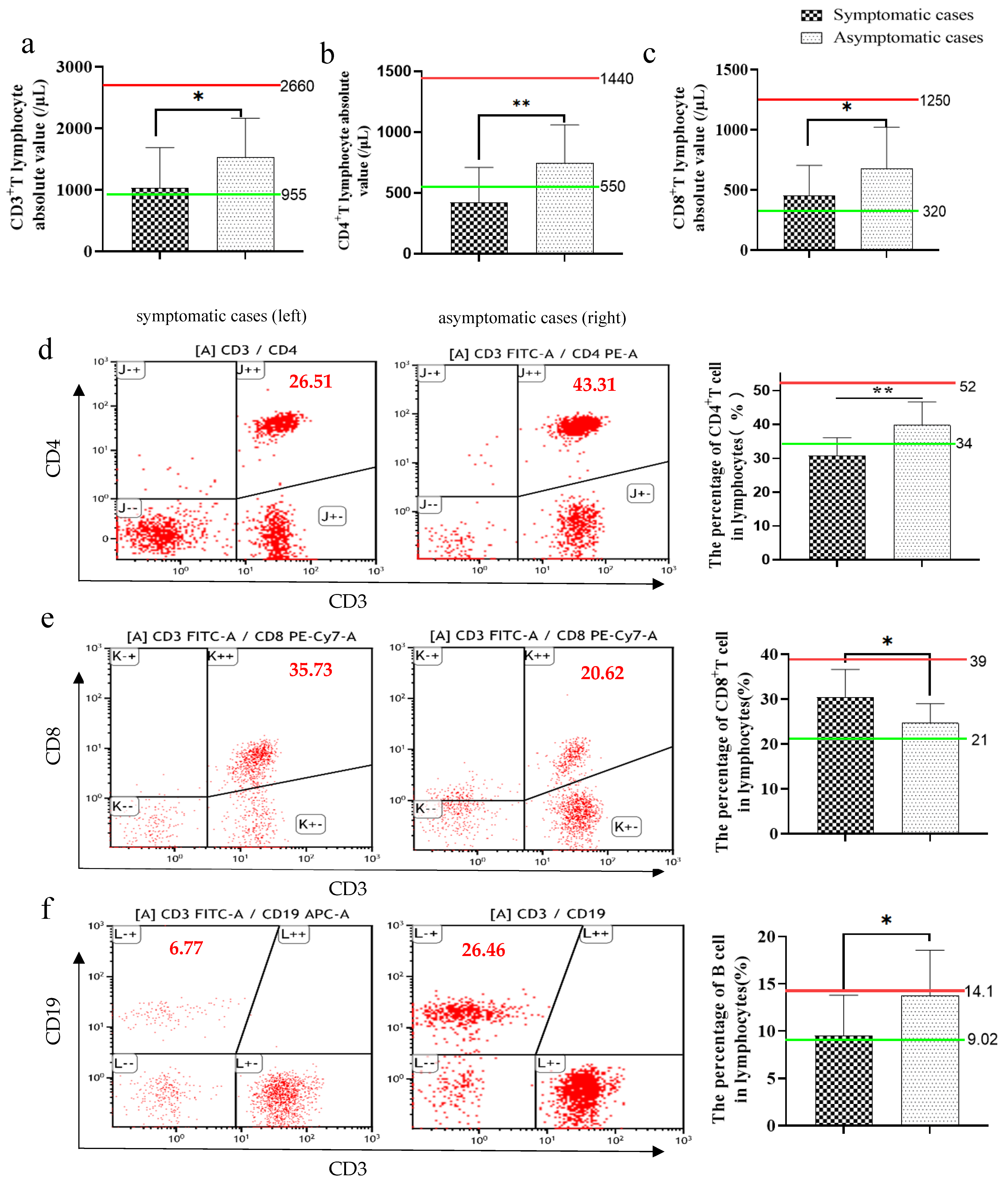
2.4. Immunophenotyping of Lymphocytes, NK and Monocytes
2.5. Immunophenotyping of some Subsets of CD4+ T Lymphocytes
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Flow Cytometry
4.3. RNA Extraction and Quantitative Real-Time PCR (qPCR) to Detect SARS-CoV-2 RNA
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The General Office of National Health Commission Office, National TCM Administration. Diagnosis and Treatment Protocol for COVID-19 Patients (Tentative 8th Edition). Infect. Dis. Immun. 2021, 1, 8–16. [Google Scholar]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Zhang, Y.; Li, Q.; McGoogan, J.; Feng, Z.; Gao, G.; Wu, Z. Asymptomatic SARS-CoV-2 Infections Among Persons Entering China From April 16 to October 12, 2020. JAMA 2021, 325, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, D.; Helgason, A.; Jonsson, H.; Magnusson, O.; Melsted, P.; Norddahl, G.; Saemundsdottir, J.; Sigurdsson, A.; Sulem, P.; Agustsdottir, A.; et al. Spread of SARS-CoV-2 in the Icelandic Population. N. Engl. J. Med. 2020, 382, 2302–2315. [Google Scholar] [CrossRef]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Z.D.; Tang, A.; Li, K.F.; Li, P.; Yan, J.B. Potential Presymptomatic Transmission of SARS-CoV-2, Zhejiang Province, China, 2020. Emerg. Infect. Dis. 2020, 26, 1052–1054. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.E.; Li, Z.; Chiew, C.J.; Yong, S.E.; Toh, M.P.; Lee, V.J. Presymptomatic transmission of SARS-CoV-2—Singapore, January 23–March 16, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 411–415. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, M.; Yokoe, D.S.; Havlir, D.V. Asymptomatic Transmission, the Achilles’ Heel of Current Strategies to Control CO%vid-19. N. Engl. J. Med. 2020, 382, 2158–2160. [Google Scholar] [CrossRef]
- Esmon, C.T. Reprint of Crosstalk between inflammation and thrombosis. Maturitas 2008, 61, 122–131. [Google Scholar] [CrossRef]
- Luyendyk, J.; Schoenecker, J.; Flick, M. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef]
- Notarbartolo, S.; Ranzani, V.; Bandera, A.; Gruarin, P.; Bevilacqua, V.; Putignano, A.R.; Gobbini, A.; Galeota, E.; Manara, C.; Bombaci, M.; et al. Integrated longitudinal immunophenotypic, transcriptional and repertoire analyses delineate immune responses in COVID-19 patients. Sci. Immunol. 2021, 6, eabg5021. [Google Scholar] [CrossRef]
- Davies, N.; Klepac, P.; Liu, Y.; Prem, K.; Jit, M.E. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020, 26, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.; Lau, E.; Wong, J.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.R. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020, 20, 400–402. [Google Scholar] [CrossRef]
- Zhu, Z.; Cai, T.; Fan, L.; Lou, K.; Hua, X.; Huang, Z.; Gao, G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. Soc. Infect. Dis. 2020, 95, 332–339. [Google Scholar] [CrossRef]
- Groeneveld, A. Vascular pharmacology of acute lung injury and acute respiratory distress syndrome. Vasc. Pharmacol. 2002, 39, 247–256. [Google Scholar] [CrossRef]
- Rokni, M.; Ghasemi, V.; Tavakoli, Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: Comparison with SARS and MERS. Rev. Med. Virol. 2020, 30, e2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baraz, L.; Khazanov, E.; Condiotti, R.; Kotler, M.; Nagler, A. Natural killer (NK) cells prevent virus production in cell culture. Bone Marrow Transpl. 1999, 24, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.; Netea, M.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef] [PubMed]
- Girija, A.; Shankar, E.; Larsson, M. Could SARS-CoV-2-Induced Hyperinflammation Magnify the Severity of Coronavirus Disease (COVID-19) Leading to Acute Respiratory Distress Syndrome? Front. Immunol. 2020, 11, 1206. [Google Scholar] [CrossRef]
- Vanderbeke, L.; van Mol, P.; van Herck, Y.; de Smet, F.; Humblet-Baron, S.; Martinod, K.; Antoranz, A.; Arijs, I.; Boeckx, B.; Bosisio, F.; et al. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat. Commun. 2021, 12, 4117. [Google Scholar] [CrossRef]
- Taghizadieh, A.; Mikaeili, H.; Ahmadi, M.; Valizadeh, H. Acute kidney injury in pregnant women following SARS-CoV-2 infection: A case report from Iran. Respir. Med. Case Rep. 2020, 30, 101090. [Google Scholar] [CrossRef]
- Carsetti, R.; Zaffina, S.; Piano Mortari, E.; Terreri, S.; Corrente, F.; Capponi, C.; Palomba, P.; Mirabella, M.; Cascioli, S.; Palange, P.; et al. Different Innate and Adaptive Immune Responses to SARS-CoV-2 Infection of Asymptomatic, Mild, and Severe Cases. Front. Immunol. 2020, 11, 610300. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target. Ther. 2020, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Deng, Y.; Weng, Z.; Yang, L. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int. J. Infect. Dis. 2020, 96, 131–135. [Google Scholar] [CrossRef]
- Mahmoud Salehi Khesht, A.; Karpisheh, V.; Qubais Saeed, B.; Olegovna Zekiy, A.; Yapanto, L.; Nabi Afjadi, M.; Aksoun, M.; Nasr Esfahani, M.; Aghakhani, F.; Movahed, M.; et al. Different T cell related immunological profiles in COVID-19 patients compared to healthy controls. Int. Immunopharmacol. 2021, 97, 107828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; et al. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front. Mol. Biosci. 2020, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Woodland, R.; Jeffrey, H.; Zhong, W.M. Cellular immunity and memory to respiratory virus infections. Immunol. Res. 2001, 24, 53–67. [Google Scholar]
- Sergio, R. T-cell subsets (Th1 versus Th2). Ann. Allergy Asthma Immunol. 2000, 85, 9–18. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Cao, B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Orlov, M.; Wander, P.; Morrell, E.; Mikacenic, C.; Wurfel, M. A Case for Targeting Th17 Cells and IL-17A in SARS-CoV-2 Infections. J. Immunol. 2020, 205, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.; Brown, M.; Sanchez, E.; Tattersall, R.; Manson, J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Chan, Y.; Fong, S.; Poh, C.; Carissimo, G.; Yeo, N.; Amrun, S.; Goh, Y.; Lim, J.; Xu, W.; Chee, R.; et al. Asymptomatic COVID-19: Disease tolerance with efficient anti-viral immunity against SARS-CoV-2. EMBO Mol. Med. 2021, 13, e14045. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Huang, F.; Yang, Y.; Wang, F.; Yuan, J.; Zhang, Z.; Qin, Y.; Li, X.; Zhao, D. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Nat. Sci. Rev. 2020, 7, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Bautista, J.F.; Rodriguez-Nicolas, A.; Rosales-Castillo, A.; Jiménez, P.; Garrido, F.; Anderson, P.; Ruiz-Cabello, F.; López-Ruz, M.Á. Negative Clinical Evolution in COVID-19 Patients Is Frequently Accompanied With an Increased Proportion of Undifferentiated Th Cells and a Strong Underrepresentation of the Th1 Subset. Front. Immunol. 2020, 11, 596553. [Google Scholar] [CrossRef]
- Xla, B.; Mga, B.; Ypa, B.; Lma, B.; Sla, B. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. [Google Scholar]
- Carli, G.; Cecchi, L.; Stebbing, J.; Parronchi, P.; Farsi, A. Is asthma protective against COVID? Allergy 2020, 76, 866–868. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T.; Rington, L.H.; Mruphy, T.; Harringtom, L.D.; et al. Interleukin17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immnol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Jadidi-Niaragh, F.; Mirshafiey, A. The deviated balance between regulatory T cell and Th17 in autoimmunity. Immunopharmacol. Immunotoxicol. 2012, 34, 727–739. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Sadeghi, A.; Tahmasebi, S.; Mahmood, A.; Kuznetsova, M.; Valizadeh, H.; Taghizadieh, A.; Nazemiyeh, M.; Aghebati-Maleki, L.; Jadidi-Niaragh, F.; Abbaspour-Aghdam, S.; et al. Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. J. Cell. Physiol. 2021, 236, 2829–2839. [Google Scholar] [CrossRef]
- André, P.; Groettrup, M.; Klenerman, P.; de Giuli, R.; Booth, B.; Cerundolo, V.; Bonneville, M.; Jotereau, F.; Zinkernagel, R.; Lotteau, V. An inhibitor of HIV-1 protease modulates proteasome activity, antigen presentation, and T cell responses. Proc. Natl. Acad. Sci. USA 1998, 95, 13120–13124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Equils, O.; Shapiro, A.; Madak, Z.; Liu, C.; Lu, D. Human immunodeficiency virus type 1 protease inhibitors block toll-like receptor 2 (TLR2)- and TLR4-Induced NF-kappaB activation. Antimicrob. Agents Chemother. 2004, 48, 3905–3911. [Google Scholar] [CrossRef] [Green Version]
- Sloand, E.M.; Kumar, P.N.; Kim, S.; Chaudhuri, A.; Young, N.S. Human immunodeficiency virus type 1 protease inhibitor modulates activation of peripheral blood CD4(+) T cells and decreases their susceptibility to apoptosis in vitro and in vivo. Blood 1999, 94, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.; Maini, M.; Wack, A. Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. J. Interferon Cytokine Res. 2015, 35, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Controlprevention, C. Technical Guidelines for COVID-19 Laboratory Testing. China CDC Wkly. 2020, 2, 332–336. [Google Scholar]


| Characteristics | Symptomatic Cases (n = 17) | Asymptomatic Cases (n = 24) |
|---|---|---|
| Age (years) | ||
| Mean ± S.D. | 44.9 ± 18.4 | 27.4 ± 9.6 |
| Median | 44 | 23.5 |
| Sex | ||
| Male | 64.7% (11) | 41.7% (10) |
| Female | 35.3% (6) | 58.3% (14) |
| Smoking habit | ||
| Non-smokers | 76.5% (13) | 95.8% (23) |
| Smokers | 23.5% (4) | 4.2% (1) |
| Source | ||
| Mainland | 47.1% (8) | 29.2% (7) |
| Overseas | 52.9% (9) | 70.8% (17) |
| Been to the epidemic area | ||
| Yes | 29.4% (5) | 41.7% (10) |
| No | 70.6% (12) | 58.3% (14) |
| Exposure to confirmed cases | ||
| Yes | 29.4% (5) | 33.3% (8) |
| No | 70.6% (12) | 41.7% (16) |
| Age | Asymptomatic Case (n = 24) | Symptomatic Cases (n = 17) | χ2 | p |
|---|---|---|---|---|
| The Older group (≤40 years) | 2 (15.4%) | 11 (84.6%) | 14.604 | <0.01 |
| The younger group (>40 years) | 22 (78.6%) | 6 (21.4%) |
| Gender | Asymptomatic Case (n = 24) | Symptomatic Cases (n = 17) | χ2 | p |
|---|---|---|---|---|
| Male | 10 (47.6%) | 11 (52.4%) | 2.114 | >0.05 |
| Female | 14 (70%) | 6 (30%) |
| Smoking Habits | Asymptomatic Case (n = 24) | Symptomatic Cases (n = 17) | χ2 | p |
|---|---|---|---|---|
| Smokers | 1 (20%) | 4 (80%) | 3.484 | >0.05 |
| Non-smokers | 23 (63.9%) | 13 (36.1%) |
| Case Source | Asymptomatic Cases (n = 24) | Symptomatic Cases (n = 17) | χ2 | p |
|---|---|---|---|---|
| Local cases | 7 (46.7%) | 8 (53.3%) | 1.373 | >0.05 |
| Overseas cases | 17 (65.4%) | 9 (34.6%) |
| Clinical Characteristics | Symptomatic Cases (n = 17) | Asymptomatic Cases (n = 24) |
|---|---|---|
| Symptoms | ||
| Fever | 41.2% (7) | 0 |
| Headache | 17.6% (3) | 0 |
| Cough | 17.6% (3) | 0 |
| Pharyngalgia | 5.9% (1) | 0 |
| Runny Nose | 11.8% (2) | 0 |
| Digestive tract symptoms | 17.6% (3) | 0 |
| Time to diagnosis (days) | 6.1 ± 8.1 | 2.0 ± 0.6 |
| Nucleic acid testing | ||
| Throat swab | 82.4% (14) | 62.5% (15) |
| Nose swab | 47.1% (8) | 50.0% (12) |
| Anal swab | 29.4% (5) | 37.5% (9) |
| Coronavirus antibodies | ||
| IgG | 71.4% (5/7) | 84.2% (16/19) |
| IgM | 14.3% (1/7) | 21.1%(4/19) |
| complications | ||
| Hypertension | 17.6% (3) | 4.2% (1) |
| Cardiovascular disease | 17.6% (3) | 4.2% (1) |
| Respiratory disease | 5.9% (1) | 0 |
| Cancer | 0 | 0 |
| Diabetes | 0 | 0 |
| Kidney disease | 5.9% (1) | 0 |
| The history of blood transfusion | 11.8% (2) | 0 |
| Allergic history | 5.9% (1) | 8.3% (2) |
| Disease classification | ||
| Asymptomatic infection | 0% (0) | 100% (24) |
| Mild | 88.2% (15) | 0% (0) |
| Severe | 11.8% (2) | 0% (0) |
| Lung CT | ||
| Inflammation | 70.6% (12) | 20.8% (5) |
| Pneumatocele | 11.8% (2) | 4.2% (1) |
| Other (nodule, cord, pleural thickening, pleural thickening) | 35.3% (6) | 12.5% (3) |
| Length of stay | ||
| Mean ± S.D. | 21.9 ± 8.4 | 16.8 ± 5.2 |
| Treatment | ||
| Antibiotic | 0 | 12.5% (3) |
| Antiviral | ||
| Arbidol | 41.7% (10) | 95.8% (23) |
| Traditional Chinese medicine | 76.5% (13) | 95.8% (23) |
| Lopinavir/ Ritonavir | 35.3% (6) | 0 |
| Human Immunoglobulin (I.V) | 0 | 0 |
| Glucocorticoid | 0 | 0 |
| Hydroxychloroquine | 23.5% (4) | 4.2% (1) |
| IFN-α (INH) | 94.1% (16) | 100% (24) |
| Oxygen intracavitary | ||
| low-flow nasal oxygen | 23.5% (4) | 0 |
| Noninvasive mechanical ventilation | 0 | 0 |
| Invasive mechanical ventilation | 0 | 0 |
| Clinical outcome | ||
| Death | 0 | 0 |
| Re-positive | 23.5% (4) | 20.8% (5) |
| Complications | Asymptomatic Cases (n = 24) | Symptomatic Cases (n = 17) | χ2 | p |
|---|---|---|---|---|
| With chronic diseases | 2 (20%) | 8 (80%) | 8.092 | <0.01 |
| Without chronic diseases | 22 (71.0%) | 9 (29%) |
| The type of Infection | Non-Re-Positives | Re-Positives | χ2 | p |
|---|---|---|---|---|
| Asymptomatic cases (n = 24) | 19 (79.2%) | 5 (20.8%) | 0.042 | >0.05 |
| Symptomatic cases (n = 17) | 13 (76.5%) | 4 (23.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhang, Y.; Lu, J.; Li, L.; Gao, H.; Ma, C.; Dai, E.; Wei, L. Asymptomatic COVID-19 Individuals Tend to Establish Relatively Balanced Innate and Adaptive Immune Responses. Pathogens 2021, 10, 1105. https://doi.org/10.3390/pathogens10091105
Li M, Zhang Y, Lu J, Li L, Gao H, Ma C, Dai E, Wei L. Asymptomatic COVID-19 Individuals Tend to Establish Relatively Balanced Innate and Adaptive Immune Responses. Pathogens. 2021; 10(9):1105. https://doi.org/10.3390/pathogens10091105
Chicago/Turabian StyleLi, Miao, Yue Zhang, Jianhua Lu, Li Li, Huixia Gao, Cuiqing Ma, Erhei Dai, and Lin Wei. 2021. "Asymptomatic COVID-19 Individuals Tend to Establish Relatively Balanced Innate and Adaptive Immune Responses" Pathogens 10, no. 9: 1105. https://doi.org/10.3390/pathogens10091105
APA StyleLi, M., Zhang, Y., Lu, J., Li, L., Gao, H., Ma, C., Dai, E., & Wei, L. (2021). Asymptomatic COVID-19 Individuals Tend to Establish Relatively Balanced Innate and Adaptive Immune Responses. Pathogens, 10(9), 1105. https://doi.org/10.3390/pathogens10091105





