Antibody Profile Comparison against MSP1 Antigens of Multiple Plasmodium Species in Human Serum Samples from Two Different Brazilian Populations Using a Multiplex Serological Assay
Abstract
:1. Introduction
2. Results
2.1. Analysis of Coupling Efficiency of Glutathione S-Transferase (GST)-Fusion Proteins to Bio-Plex Carboxylated Magnetic Beads
2.2. Cut-Off Determinations
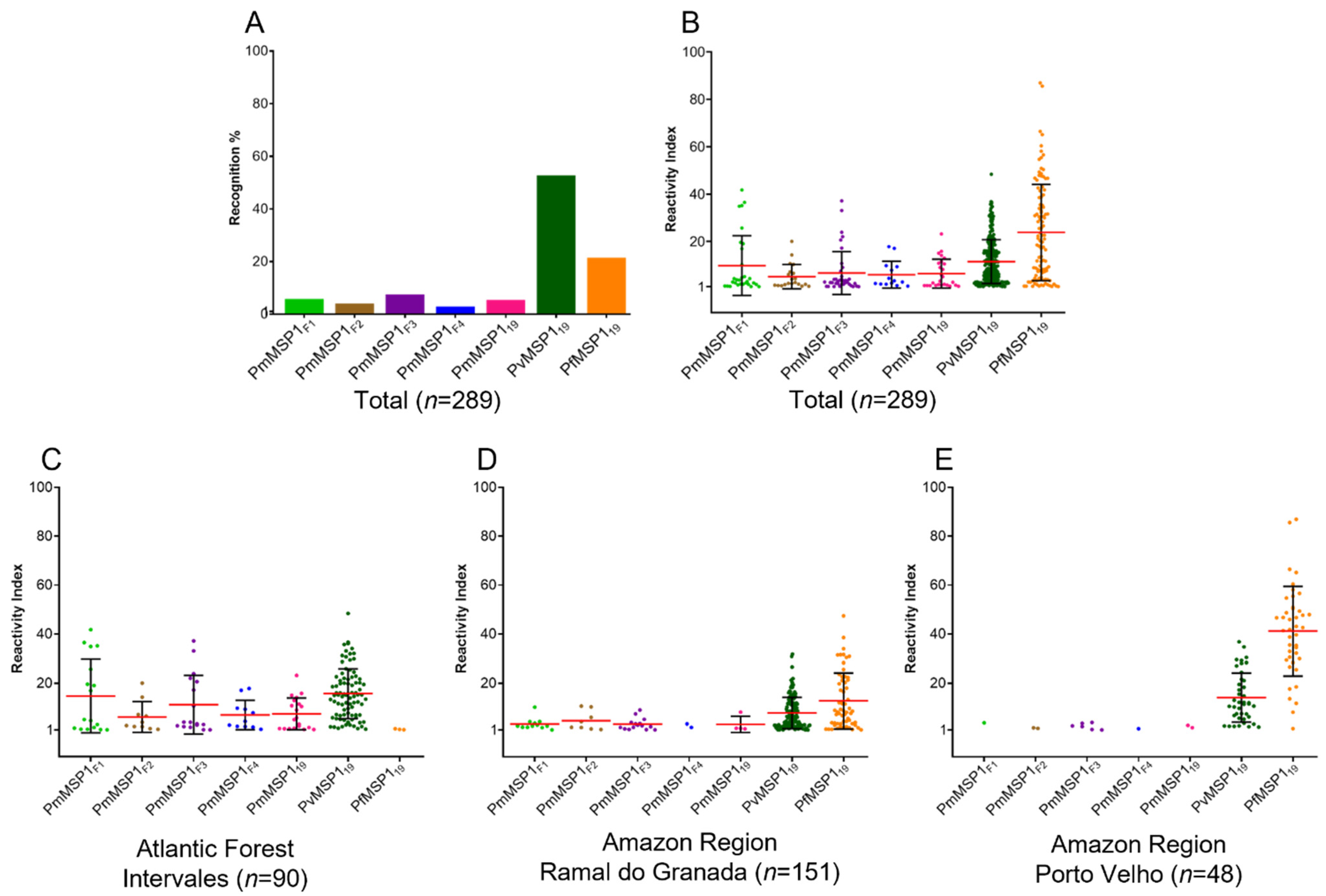
2.3. Naturally Acquired Antibody Responses
3. Discussion
4. Materials and Methods
4.1. Collection of Serum Samples
4.2. Recombinant Antigens
4.3. Coupling Efficiency Assessment
4.4. Recombinant Protein Multiplex Bead Assay (MBA)
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Grande, R.; Antinori, S.; Meroni, L.; Menegon, M.; Severini, C. A Case of Plasmodium malariae recurrence: Recrudescence or reinfection? Malar J. 2019, 18, 169. [Google Scholar] [CrossRef] [PubMed]
- Collins, W.E.; Jeffery, G.M. Plasmodium malariae: Parasite and disease. C.M.R. 2007, 20, 579–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawadak, J.; Dongang Nana, R.R.; Singh, V. Global Trend of Plasmodium malariae and Plasmodium ovale spp. malaria infections in the last two decades (2000–2020): A systematic review and meta-sanalysis. Parasites Vectors 2021, 14, 297. [Google Scholar] [CrossRef] [PubMed]
- Oriero, E.C.; Amenga-Etego, L.; Ishengoma, D.S.; Amambua-Ngwa, A. Plasmodium malariae, current knowledge and future research opportunities on a neglected malaria parasite species. Crit. Rev. Microbiol. 2021, 47, 44–56. [Google Scholar] [CrossRef]
- Scopel, K.K.G.; Fontes, C.J.F.; Nunes, Á.C.; Horta, M.F.; Braga, É.M. High Prevalence of Plamodium malariae infections in a brazilian amazon endemic area (Apiacás—Mato Grosso State) as detected by polymerase chain reaction. Acta Trop. 2004, 90, 61–64. [Google Scholar] [CrossRef]
- Curado, I.; Duarte, A.M.R.; Lal, A.A.; Oliveira, S.G.; Kloetzel, J.K. Antibodies anti bloodstream and circumsporozoite antigens (Plasmodium vivax and Plasmodium malariae/P. brasilianum) in areas of very low malaria endemicity in Brazil. Mem. Inst. Oswaldo Cruz 1997, 92, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Oriero, E.C.; Olukosi, A.Y.; Oduwole, O.A.; Djimde, A.; D’Alessandro, U.; Meremikwu, M.M.; Amambua-Ngwa, A. Seroprevalence and parasite rates of Plasmodium malariae in a high malaria transmission setting of Southern Nigeria. Am. J. Trop. Med. Hyg. 2020, 103, 2208–2216. [Google Scholar] [CrossRef]
- Labadie-Bracho, M.Y.; van Genderen, F.T.; Adhin, M.R. Malaria serology data from the guiana shield: First insight in igg antibody responses to Plasmodium falciparum, Plasmodium vivax and Plasmodium malariae antigens in Suriname. Malar. J. 2020, 19, 360. [Google Scholar] [CrossRef]
- McCaffery, J.N.; Singh, B.; Nace, D.; Moreno, A.; Udhayakumar, V.; Rogier, E. Natural infections with different Plasmodium species induce antibodies reactive to a chimeric Plasmodium vivax recombinant protein. Malar. J. 2021, 20, 86. [Google Scholar] [CrossRef]
- Vinetz, J.M.; Li, J.; McCutchan, T.F.; Kaslow, D.C. Plasmodium malariae infection in an asymptomatic 74-year-old greek woman with splenomegaly. N. Engl. J. Med. 1998, 338, 367–371. [Google Scholar] [CrossRef]
- Gilles, H.M.; Warrell, D.A. Bruce-Chwatt’s Essential Malariology, 3rd ed.; Bruce-Chwatt, L.J., Bruce-Chwatt, L.J., Eds.; Arnold: London, UK; Revista Do Instituto De Medicina Tropical De São Paulo: São Paulo, Brazil, 1993. [Google Scholar]
- Garnham, P.C.C. Malaria Parasites and Other Haemosporidia; Blackwell Scientific: Oxford, UK, 1966; ISBN 978-0-632-01770-6. [Google Scholar]
- Cheaveau, J.; Mogollon, D.C.; Mohon, M.A.N.; Golassa, L.; Yewhalaw, D.; Pillai, D.R. Asymptomatic malaria in the clinical and public health context. Expert Rev. Anti Infect. Ther. 2019, 17, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.; Kinyanjui, S. Immune effector mechanisms in malaria. Parasite Immunol. 2006, 28, 51–60. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-156572-1. [Google Scholar]
- Yman, V.; Wandell, G.; Mutemi, D.D.; Miglar, A.; Asghar, M.; Hammar, U.; Karlsson, M.; Lind, I.; Nordfjell, C.; Rooth, I.; et al. Persistent transmission of Plasmodium malariae and Plasmodium ovale species in an area of declining Plasmodium falciparum transmission in Eastern Tanzania. PLoS Negl. Trop. Dis. 2019, 13, e0007414. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.M.; Le Menach, A.; Pothin, E.; Eisele, T.P.; Gething, P.W.; Eckhoff, P.A.; Moonen, B.; Schapira, A.; Smith, D.L. Mapping multiple components of malaria risk for improved targeting of elimination interventions. Malar. J. 2017, 16, 459. [Google Scholar] [CrossRef]
- Fernandez-Becerra, C.; Sanz, S.; Brucet, M.; Stanisic, D.I.; Alves, F.P.; Camargo, E.P.; Alonso, P.L.; Mueller, I.; del Portillo, H.A. Naturally-acquired humoral immune responses against the N- and C-termini of the Plasmodium vivax MSP1 protein in endemic regions of Brazil and Papua New Guinea using a multiplex assay. Malar. J. 2010, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holder, A.A. The carboxy-terminus of merozoite surface protein 1: Structure, specific antibodies and immunity to Malaria. Parasitol. 2009, 136, 1445–1456. [Google Scholar] [CrossRef]
- Monteiro, E.F.; Fernandez-Becerra, C.; Araujo, M.D.S.; Messias, M.R.; Ozaki, L.S.; Duarte, A.M.R.D.C.; Bueno, M.G.; Catao-Dias, J.L.; Chagas, C.R.F.; Mathias, B.D.S.; et al. Naturally acquired humoral immunity against malaria parasites in non-human primates from the Brazilian Amazon, Cerrado and Atlantic Forest. Pathogens 2020, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Ferreira, J.; Lacerda, M.V.; Brasil, P.; Ladislau, J.L.; Tauil, P.L.; Daniel-Ribeiro, C.T. Malaria in Brazil: An overview. Malar. J. 2010, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Plucinski, M.M.; Candrinho, B.; Chambe, G.; Muchanga, J.; Muguande, O.; Matsinhe, G.; Mathe, G.; Rogier, E.; Doyle, T.; Zulliger, R.; et al. Multiplex serology for impact evaluation of bed net distribution on burden of lymphatic filariasis and four species of human malaria in Northern Mozambique. PLoS Negl. Trop. Dis. 2018, 12, e0006278. [Google Scholar] [CrossRef] [Green Version]
- Assefa, A.; Ali Ahmed, A.; Deressa, W.; Sime, H.; Mohammed, H.; Kebede, A.; Solomon, H.; Teka, H.; Gurrala, K.; Matei, B.; et al. Multiplex serology demonstrate cumulative prevalence and spatial distribution of malaria in Ethiopia. Malar. J. 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- Steinhardt, L.C.; Ravaoarisoa, E.; Wiegand, R.; Harimanana, A.; Hedje, J.; Cotte, A.H.; Zigirumugabe, S.; Kesteman, T.; Rasoloharimanana, T.L.; Rakotomalala, E.; et al. School-based serosurveys to assess the validity of using routine health facility data to target malaria interventions in the Central Highlands of Madagascar. J. Infect. Dis. 2021, 223, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.F.; Amador, E.C.C.; Silva, E.S.; Alvarenga, C.O.; Pereira, P.E.; Póvoa, M.M.; Cunha, M.G. Malaria transmission and individual variability of the naturally acquired IgG antibody against the Plasmodium vivax blood-stage antigen in an endemic area in Brazil. Acta Trop. 2020, 209, 105537. [Google Scholar] [CrossRef] [PubMed]
- Punnath, K.; Dayanand, K.K.; Midya, V.; Chandrashekar, V.N.; Achur, R.N.; Kakkilaya, S.B.; Ghosh, S.K.; Kumari, S.N.; Gowda, D.C. Acquired antibody responses against merozoite surface protein-119 antigen during Plasmodium falciparum and P.vivax infections in South Indian city of Mangaluru. J. Parasit. Dis. 2021, 45, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Feleke, S.M.; Brhane, B.G.; Mamo, H.; Assefa, A.; Woyessa, A.; Ogawa, G.M.; Cama, V. Sero-Identification of the aetiologies of human malaria exposure (Plasmodium spp.) in the Limu Kossa District of Jimma Zone, South Western Ethiopia. Malar. J. 2019, 18. [Google Scholar] [CrossRef] [Green Version]
- Muerhoff, A.S.; Birkenmeyer, L.G.; Coffey, R.; Dille, B.J.; Barnwell, J.W.; Collins, W.E.; Sullivan, J.S.; Dawson, G.J.; Desai, S.M. Detection of Plasmodium falciparum, P. vivax, P. ovale, and P. malariae merozoite surface protein 1-p19 antibodies in human malaria patients and experimentally infected nonhuman primates. Clin. Vaccine. Immunol. 2010, 17, 1631–1638. [Google Scholar] [CrossRef] [Green Version]
- Cowan, G.J.M.; Bockau, U.; Eleni-Muus, J.; Aldag, I.; Samuel, K.; Creasey, A.M.; Hartmann, M.W.W.; Cavanagh, D.R. A Novel Malaria vaccine candidate antigen expressed in Tetrahymena thermophila. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Yuen, D.; Leung, W.-H.; Cheung, R.; Hashimoto, C.; Ng, S.F.; Ho, W.; Hui, G. Antigenicity and immunogenicity of the N-Terminal 33-KDa processing fragment of the Plasmodium falciparum merozoite surface protein 1, MSP1: Implications for vaccine development. Vaccine 2007, 25, 490–499. [Google Scholar] [CrossRef]
- Cassiano, G.C.; Furini, A.A.C.; Capobianco, M.P.; Storti-Melo, L.M.; Almeida, M.E.; Barbosa, D.R.L.; Póvoa, M.M.; Nogueira, P.A.; Machado, R.L.D. Immunogenetic markers associated with a naturally acquired humoral immune response against an n-terminal antigen of Plasmodium vivax merozoite surface protein 1 (PvMSP-1). Malar. J. 2016, 15. [Google Scholar] [CrossRef] [Green Version]
- Storti-Melo, L.M.; Souza-Neiras, W.C.; Cassiano, G.C.; Taveira, L.C.; Cordeiro, A.J.; Couto, V.S.C.A.; Póvoa, M.M.; Cunha, M.G.; Echeverry, D.M.; Rossit, A.R.B.; et al. Evaluation of the naturally acquired antibody immune response to the Pv200L N-Terminal fragment of Plasmodium vivax merozoite surface protein-1 in four areas of the Amazon Region of Brazil. Am. J. Trop. Med. Hyg. 2011, 84, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Priest, J.W.; Plucinski, M.M.; Huber, C.S.; Rogier, E.; Mao, B.; Gregory, C.J.; Candrinho, B.; Colborn, J.; Barnwell, J.W. Specificity of the IgG antibody response to Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale MSP119 subunit proteins in multiplexed serologic assays. Malar. J. 2018, 17, 417. [Google Scholar] [CrossRef] [Green Version]
- Elizardez, Y.B.; Fotoran, W.L.; Junior, A.J.G.; Curado, I.; Junior, N.K.; Monteiro, E.F.; Romero Neto, I.; Wunderlich, G.; Kirchgatter, K. Recombinant proteins of Plasmodium malariae merozoite surface protein 1 (PmMSP1): Testing immunogenicity in the BALB/c model and potential use as diagnostic tool. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Carlos, B.C.; Rona, L.D.P.; Christophides, G.K.; Souza-Neto, J.A. A Comprehensive Analysis of malaria transmission in Brazil. Pathog. Glob. Health 2019, 113, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Nunes, M.D.; Malafronte, R.D.S.; Luz, B.D.A.; Souza, E.A.D.; Martins, L.C.; Rodrigues, S.G.; Chiang, J.O.; Vasconcelos, P.F.D.C.; Muniz, P.T.; Ferreira, M.U. The Acre Project: The epidemiology of malaria and arthropod-borne virus infections in a rural Amazonian population. Cad. Saúde Pública 2006, 22, 1325–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buery, J.C.; de Alencar, F.E.C.; Duarte, A.M.R.D.C.; Loss, A.C.; Vicente, C.R.; Ferreira, L.M.; Fux, B.; Medeiros, M.M.; Cravo, P.; Arez, A.P.; et al. Atlantic Forest Malaria: A review of more than 20 years of epidemiological investigation. Microorganisms 2021, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.M.; Fotoran, W.L.; dalla Martha, R.C.; Katsuragawa, T.H.; Pereira da Silva, L.H.; Wunderlich, G. Natural antibody response to Plasmodium falciparum merozoite antigens MSP5, MSP9 and EBA175 is associated to clinical protection in the Brazilian Amazon. BMC Infect. Dis. 2013, 13, 608. [Google Scholar] [CrossRef]
- Curado, I.; dos Santos Malafronte, R.; de Castro Duarte, A.M.R.; Kirchgatter, K.; Branquinho, M.S.; Bianchi Galati, E.A. Malaria epidemiology in low-endemicity areas of the Atlantic Forest in the Vale Do Ribeira, São Paulo, Brazil. Acta Tropica. 2006, 100, 54–62. [Google Scholar] [CrossRef]
- Bousema, T.; Okell, L.; Felger, I.; Drakeley, C. Asymptomatic malaria infections: Detectability, transmissibility and public health relevance. Nat. Rev. Microbiol. 2014, 12, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, P.A.; Piovesan Alves, F.; Fernandez-Becerra, C.; Pein, O.; Rodrigues Santos, N.; Pereira da Silva, L.H.; Plessman Camargo, E.; del Portillo, H.A. A reduced risk of infection with Plasmodium vivax and clinical protection against malaria are associated with antibodies against the N Terminus but not the C Terminus of merozoite surface protein 1. Infect. Immun. 2006, 74, 2726–2733. [Google Scholar] [CrossRef] [Green Version]
- Wipasa, J.; Suphavilai, C.; Okell, L.C.; Cook, J.; Corran, P.H.; Thaikla, K.; Liewsaree, W.; Riley, E.M.; Hafalla, J.C.R. Long-Lived antibody and B cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 2010, 6, e1000770. [Google Scholar] [CrossRef]
- de Castro Duarte, A.M.R.; Fernandes, L.N.; Silva, F.S.; Sicchi, I.L.; Mucci, L.F.; Curado, I.; Fernandes, A.; Medeiros-Sousa, A.R.; Ceretti-Junior, W.; Marrelli, M.T.; et al. Complexity of malaria transmission dynamics in the Brazilian Atlantic Forest. Curr. Res. Parasitol. Vector Borne Dis. 2021, 1, 100032. [Google Scholar] [CrossRef]
- Multini, L.C.; Marrelli, M.T.; Beier, J.C.; Wilke, A.B.B. Increasing complexity threatens the elimination of Extra-Amazonian malaria in Brazil. Trends Parasitol. 2019, 35, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Miguel, R.B.; Albuquerque, H.G.; Sanchez, M.C.A.; Coura, J.R.; Santos, S.D.S.; Silva, S.D.; Moreira, C.J.D.C.; Suárez-Mutis, M.C. Asymptomatic Plasmodium infection in a residual malaria transmission area in the Atlantic Forest region: Implications for elimination. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180537. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.V.; Alves, J.R.S.; Lima, B.A.S.; Paula, R.B.; Costa, H.L.; Torres, L.M.; Sousa, T.N.; Soares, I.S.; Sanchez, B.A.M.; Fontes, C.J.F.; et al. Blood-stage Plasmodium vivax antibody dynamics in a low transmission setting: A nine year follow-up study in the Amazon Region. PLoS ONE 2018, 13, e0207244. [Google Scholar] [CrossRef] [PubMed]
- Pratt-Riccio, L.R.; De Souza Perce-Da-Silva, D.; Da Costa Lima-Junior, J.; Pratt Riccio, E.K.; Ribeiro-Alves, M.; Santos, F.; Arruda, M.; Camus, D.; Druilhe, P.; Oliveira-Ferreira, J.; et al. Synthetic antigens derived from Plasmodium falciparum sporozoite, liver, and blood stages: Naturally acquired immune response and human leukocyte antigen associations in individuals living in a Brazilian endemic area. Am. J. Trop. Med. Hyg. 2017, 97, 1581–1592. [Google Scholar] [CrossRef] [Green Version]
- Aschar, M.; Levi, J.E.; Farinas, M.L.R.N.; Montebello, S.C.; Mendrone-Junior, A.; Di Santi, S.M. The hidden Plasmodium malariae in blood donors: A risk coming from areas of low transmission of malaria. Rev. Inst. Med. trop. S. Paulo 2020, 62, e100. [Google Scholar] [CrossRef]
- World Health Organization. Malaria Rapid Diagnostic Test. Performance-Results of WHO Product Testing of Malaria RDTs: Round 1 (2008); World Health Organization: Geneva, Switzerland, 2009; ISBN 978 92 4 159807 1. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, E.F.; Fernandez-Becerra, C.; Curado, I.; Wunderlich, G.; Hiyane, M.I.; Kirchgatter, K. Antibody Profile Comparison against MSP1 Antigens of Multiple Plasmodium Species in Human Serum Samples from Two Different Brazilian Populations Using a Multiplex Serological Assay. Pathogens 2021, 10, 1138. https://doi.org/10.3390/pathogens10091138
Monteiro EF, Fernandez-Becerra C, Curado I, Wunderlich G, Hiyane MI, Kirchgatter K. Antibody Profile Comparison against MSP1 Antigens of Multiple Plasmodium Species in Human Serum Samples from Two Different Brazilian Populations Using a Multiplex Serological Assay. Pathogens. 2021; 10(9):1138. https://doi.org/10.3390/pathogens10091138
Chicago/Turabian StyleMonteiro, Eliana Ferreira, Carmen Fernandez-Becerra, Izilda Curado, Gerhard Wunderlich, Meire Ioshie Hiyane, and Karin Kirchgatter. 2021. "Antibody Profile Comparison against MSP1 Antigens of Multiple Plasmodium Species in Human Serum Samples from Two Different Brazilian Populations Using a Multiplex Serological Assay" Pathogens 10, no. 9: 1138. https://doi.org/10.3390/pathogens10091138






