Parasites Circulating in Wild Synanthropic Capybaras (Hydrochoerus hydrochaeris): A One Health Approach
Abstract
:1. Introduction
2. Results
2.1. Fecal Macroscopical Examination
2.2. Microscopical, Coproantigen, and Molecular Parasite Identification
3. Discussion
4. Materials and Methods
4.1. Study Areas and Sample Collection
4.2. Parasitological and Immunological Analysis
4.3. Molecular Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, D.E.; Reeder, D.M. Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; Wilson, D.E., Reeder, D.M., Eds.; Johns Hopkins University Press: Baltimore, MA, USA, 2005; ISBN 0-8018-8221-4. [Google Scholar]
- Alderton, D. Rodents of the World; Facts On File: Blandford, UK, 1999; ISBN 0713727896. [Google Scholar]
- Vucetich, M.G.; Deschamps, C.M.; Pérez, M.E. Paleontology, Evolution and Systematics of Capybara. In Capybara: Biology, Use and Conservation of an Exceptional Neotropical Species; Springer: New York, NY, USA, 2012; pp. 39–59. ISBN 9781461440000. [Google Scholar]
- Schai-Braun, S.; Hackländer, K. Lagomorphs and Rodents I. Family Leporidae. In Handbook of the Mammals of the World; Wilson, D.E., Mittermeier, R.A., Eds.; Lynx Edicions: Barcelona, Spain, 2016; Volume 6, pp. 145–147. ISBN 978-84-941892-3-4. [Google Scholar]
- Timm, R.M. Capybaras: Behavior, Ecology, and Management. J. Mamm. Evol. 2010, 17, 217–219. [Google Scholar] [CrossRef]
- Nogueira-Filho, S.L.G.; da Cunha Nogueira, S.S. Capybara meat: An extraordinary resource for food security in South America. Meat Sci. 2018, 145, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Ramírez, J.; Prado, V.; Solheim, H. Life cycle assessment and socioeconomic evaluation of the illicit crop substitution policy in Colombia. J. Ind. Ecol. 2019, 23, 1237–1252. [Google Scholar] [CrossRef]
- Unda, M.; Etter, A. Conservation opportunities of the land restitution program areas in the Colombian post-conflict period. Sustainability 2019, 11, 2048. [Google Scholar] [CrossRef] [Green Version]
- Goyes, D.R.; Sollund, R. Contesting and contextualising CITES: Wildlife trafficking in Colombia and Brazil. Int. J. Crime Justice Soc. Democr. 2016, 5, 87–102. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Yates, J.A.; Jorgenson, J.P. Dipetalonema (Alafilaria) hydrochoerus subgen. et sp. n. (Nematoda: Filarioidea) from Colombian Capybaras. J. Parasitol. 1983, 69, 606. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, M.L.; Morales, G.A.; Orihel, T.C. Cruorifilaria tuberocauda gen. et sp. n. (Nematoda: Filarioidea) from the Capybara, Hydrochoerus hydrochaeris in Colombia. J. Parasitol. 1976, 62, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.A.; Guzmàn, V.H.; Angel, D. Vascular damage caused by Cruorifilaria tuberocauda in the Capybara (Hydrochoerus hydrochaeris). J. Wildl. Dis. 1978, 14, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.F.; Reis, J.L.; Barbosa, C.H.G.; Gardiner, C.H.; de Sant’Ana, F.J.F. Aspectos anatomopatológicos do parasitismo por nematódeos da superfamília filarioidea em capivaras (Hydrochoerus hydrochaeris) de vida livre no Centro-Oeste brasileiro. Pesqui. Vet. Bras. 2015, 35, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.A.; Catto, J.B. Helminth parasites of capybaras (Hydrochaeris hydrochaeris) on sub-region of Nhecolândia, Pantanal, Mato Grosso do Sul. Rev. Bras. Biol. 1994, 54, 39–48. [Google Scholar]
- Souza, G.T.R.; Ribeiro, T.S.; Antonucci, A.M.; Ueda, B.H.; Carniel, M.K.; Karling, L.C.; Eiras, J.C.; Takemoto, R.M.; Pavanelli, G.C. Endoparasite Fauna of Wild Capybaras (Hydrochoerus hydrochaeris) (Linnaeus, 1766) from the Upper Paraná River Floodplain, Brazil. Aquat. Mamm. 2015, 41, 213–221. [Google Scholar] [CrossRef]
- Sinkoc, A.L.; Brum, J.G.W.; Muller, G. Gastrintestinal helminths of capybara (Hydrochoerus hydrochaeris, linnaeus, 1766) in cattle breeding farm in the area of the ecological reserve of taim, rio grande. Braz. Arch. Biol. Technol. 2009, 52, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Salas, V.; Herrera, E.A. Intestinal helminths of capybaras, Hydrochoerus hydrochaeris, from Venezuela. Memórias do Instituto Oswaldo Cruz 2004, 99, 563–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiacchio, R.G.-D.; Prioste, F.E.S.; Vanstreels, R.E.T.; Knöbl, T.; Kolber, M.; Miyashiro, S.I.; Matushima, E.R. Health evaluation and survey of zoonotic pathogens in free-ranging capybaras (Hydrochoerus hydrochaeris). J. Wildl. Dis. 2014, 50, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.C.; Zalles, L.M.; Patrick, M.J.; Dailey, M. Intestinal helminths of capybara (Hydrochaeris hydrochaeris) from Bolivia. J. Helminthol. Soc. Washingt. 1995, 62, 87–88. [Google Scholar]
- Bonuti, M.R.; do Nascimento, A.A.; Mapelli, E.B.; Arantes, I.G. Helmintos gastrintestinais de capivaras (Hydrochoerus hydrochaeris) na sub-região de Paiaguás, Pantanal do Mato Grosso do Sul, Brasil. Semina Ciências Agrárias 2002, 23, 57. [Google Scholar] [CrossRef] [Green Version]
- Corriale, M.J.; Milano, A.M.F.; Herrera, E.A. Prevalence of gastrointestinal parasites in a natural population of capybaras, Hydrochoerus hydrochaeris, in Esteros del Iberá (Argentina). Rev. Ibero Latinoam. Parasitol. 2011, 70, 189–196. [Google Scholar]
- Sprenger, L.K.; Yoshitani, U.Y.; Buzatti, A.; Molento, M.B. Occurrence of gastrointestinal parasites in wild animals in state of Paraná, Brazil. Anais Acad. Bras. Ciências 2018, 90, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Del Rosario Robles, M.; Eberhardt, M.A.T.; Bain, O.; Beldomenico, P.M. Redescription of Echinocoleus hydrochoeri (Travassos, 1916) (Nematoda: Trichuridae) from Hydrochoeris hydrochaeris Linnaeus, 1766 (Rodentia: Caviidae) from Argentina. J. Parasitol. 2013, 99, 624–633. [Google Scholar] [CrossRef]
- Eberhardt, A.T.; del Rosario Robles, M.; Monje, L.D.; Beldomenico, P.M.; Callejón, R. A new Trichuris species (Nematoda: Trichuridae) from capybaras: Morphological-molecular characterization and phylogenetic relationships. Acta Trop. 2019, 190, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.O. A new nematode, Habronema clarki, n. sp. (Spiruridae), from Hydrochoerus isthmius Goldman. Proc. Helm. Soc. Wash. 1937, 4, 64–65. [Google Scholar]
- Labruna, M.B.; Costa, F.B.; Port-Carvalho, M.; Oliveira, A.S.; Souza, S.L.P.; Castro, M.B. Lethal Fascioliasis in Capybaras (Hydrochoerus hydrochaeris) in Brazil. J. Parasitol. 2018, 104, 173–176. [Google Scholar] [CrossRef]
- Alvarez, J.D.; Moriena, R.A.; Ortiz, M.I.; Racioppi, O. Hallazgo de Fasciola hepatica (Trematoda: Digenea) en un carpincho (Hydrochaeris hydrochaeris) de la Provincia de Corrientes, Argentina. Rev. Vet. 2009, 20, 132–134. [Google Scholar] [CrossRef]
- Assis, J.C.A.; Lopez-Hernández, D.; Pulido-Murillo, E.A.; Melo, A.L.; Pinto, H.A. A morphological, molecular and life cycle study of the capybara parasite Hippocrepis hippocrepis (Trematoda: Notocotylidae). PLoS ONE 2019, 14, e0221662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, R.M.; dos Santos, L.C.; Tortelly, R.; Menezes, R.C.; de Moraes, W.; Juvenal, J.C.; Gomes, D.C. Pathology and first report of natural infections of the eye trematode Philophthalmus lachrymosus Braun, 1902 (Digenea, Philophthalmidae) in a non-human mammalian host. Memórias Inst. Oswaldo Cruz 2005, 100, 579–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meireles, M.V.; Soares, R.M.; Bonello, F.; Gennari, S.M. Natural infection with zoonotic subtype of Cryptosporidium parvum in Capybara (Hydrochoerus hydrochaeris) from Brazil. Vet. Parasitol. 2007, 147, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Durán, A.; Blanco Palma, L.C.; Peña Flórez, R. Main gastrointestinal protozoa in wild capybara (Hydrochoerus hydrochaeris) in a village in the municipality of Arauca, Colombia. Zootec. Trop. 2015, 33, 261–268. [Google Scholar]
- Fagundes Gurgel, A.C.; Dos Santos Sartori, A.; Pacheco De Araújo, F.A. Eimeriosis in capybaras (Hydrochaeris hydrochaeris) in the state of Rio Grande do Sul, Brazil. Parasitol. Latinoam. 2007, 62, 76–78. [Google Scholar] [CrossRef]
- Casas, M.C.; Duszynski, D.W.; Zalles, L.M. Three New Eimerians in Capybara (Hydrochaeris hydrochaeris) Populations from Eastern Bolivia and Southern Venezuela. J. Parasitol. 1995, 81, 247. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, K.; Chávez, A. Trypanosoma evansi Isolated from Capybara (Hydrochaeris hydrochaeris). Memórias Inst. Oswaldo Cruz 2001, 96, 945–946. [Google Scholar] [CrossRef] [Green Version]
- Eberhardt, A.T.; Monje, L.D.; Zurvera, D.A.; Beldomenico, P.M. Detection of Trypanosoma evansi infection in wild capybaras from Argentina using smear microscopy and real-time PCR assays. Vet. Parasitol. 2014, 202, 226–233. [Google Scholar] [CrossRef]
- Herrera, E.A.; Castro, Y. Trypanosoma evansi (Kinetoplastida: Trypanosomatidae) in capybaras (Hydrochoerus hydrochaeris, Rodentia: Hydrochoeridae): Prevalence, effect and sexual selection. Rev. Biol. Trop. 2017, 65, 229. [Google Scholar] [CrossRef] [Green Version]
- Filgueiras, A.; da Silva Barros, J.H.; Xavier, S.C.C.; de Souza, S.F.; dos Santos Medeiros, L.; Ribeiro, V.M.F.; Jansen, A.M.; Roque, A.L.R. Natural Trypanosoma (Trypanozoon) evansi (Steel, 1885) infection among mammals from Brazilian Amazon. Acta Trop. 2019, 190, 92–98. [Google Scholar] [CrossRef]
- De Abreu, J.A.P.; Krawczak, F.D.S.; Nunes, F.P.; Labruna, M.B.; de Pena, H.F. Anticorpos anti-Toxoplasma gondii e anti-Neospora caninum em capivaras (Hydrochoerus hydrochaeris) do Município de Itu, São Paulo. Rev. Bras. Parasitol. Vet. 2016, 25, 116–118. [Google Scholar] [CrossRef] [Green Version]
- Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission; Childs, J.E.; Mackenzie, J.S.; Richt, J.A. (Eds.) Current Topics in Microbiology and Immunology; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2007; Volume 315, ISBN 978-3-540-70961-9. [Google Scholar]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E.J. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Luz, H.R.; Costa, F.B.; Benatti, H.R.; Ramos, V.N.; De A Serpa, M.C.; Martins, T.F.; Acosta, I.C.L.; Ramirez, D.G.; Muñoz-Leal, S.; Ramirez-Hernandez, A.; et al. Epidemiology of capybara-associated Brazilian spotted fever. PLoS Negl. Trop. Dis. 2019, 13, e1365. [Google Scholar] [CrossRef]
- Ma, J.; Khan, M.S.; Tkach, V.V.; Muhammad, N.; Zhang, D.; Zhu, X.-Q. Characterization of the complete mitochondrial genome of Plagiorchis maculosus (Digenea, Plagiorchiidae), Representative of a taxonomically complex digenean family. Parasitol. Int. 2019, 71, 99–105. [Google Scholar] [CrossRef]
- McMullen, D.B. An Experimental Infection of Plagiorchis muris in Man. J. Parasitol. 1937, 23, 113. [Google Scholar] [CrossRef]
- Hong, S.J.; Woo, H.C.; Chai, J.Y. A human case of Plagiorchis muris (Tanabe, 1922: Digenea) infection in the Republic of Korea: Freshwater fish as a possible source of infection. J. Parasitol. 1996, 82, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Youn, H. Review of zoonotic parasites in medical and veterinary fields in the Republic of Korea. Korean J. Parasitol. 2009, 47, S133. [Google Scholar] [CrossRef]
- Chai, J.Y.; Lee, S.H. Food-borne intestinal trematode infections in the Republic of Korea. Parasitol. Int. 2002, 51, 129–154. [Google Scholar] [CrossRef]
- Asada, J.; Otagaki, H.; Morita, M.; Takeuchi, T.; Sakai, Y.; Konoshi, T.; Okahashi, K. A case report on the human infection with Plagiorchis muris Tanabe, 1922 in Japan. J. Parasitol. 1962, 11, 512–516. [Google Scholar]
- Chaisiri, K.; Siribat, P.; Ribas, A.; Morand, S. Potentially Zoonotic Helminthiases of Murid Rodents from the Indo-Chinese Peninsula: Impact of Habitat and the Risk of Human Infection. Vector Borne Zoonotic Dis. 2015, 15, 73–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabiee, M.H.; Mahmoudi, A.; Siahsarvie, R.; Kryštufek, B.; Mostafavi, E. Rodent-borne diseases and their public health importance in Iran. PLoS Negl. Trop. Dis. 2018, 12, e0006256. [Google Scholar]
- Franssen, F.; Swart, A.; van Knapen, F.; van der Giessen, J. Helminth parasites in black rats (Rattus rattus) and brown rats (Rattus norvegicus) from different environments in the Netherlands. Infect. Ecol. Epidemiol. 2016, 6, 31413. [Google Scholar] [CrossRef] [Green Version]
- Catalano, S.; Nadler, S.A.; Fall, C.B.; Marsh, K.J.; Léger, E.; Sène, M.; Priestnall, S.L.; Wood, C.L.; Diouf, N.D.; Bâ, K.; et al. Plagiorchis sp. in small mammals of Senegal and the potential emergence of a zoonotic trematodiasis. Int. J. Parasitol. Parasites Wildl. 2019, 8, 164–170. [Google Scholar] [CrossRef]
- Rogan, M.T.; Craig, P.S.; Hide, G.; Heath, S.; Pickles, A.; Storey, D.M. The occurrence of the trematode Plagiorchis muris in the wood mouse Apodemus sylvaticus in North Yorkshire, UK. J. Helminthol. 2007, 81, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.-M.; Chai, J.-Y. Infection status with helminthes in feral cats purchased from a market in Busan, Republic of Korea. Korean J. Parasitol. 2005, 43, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, H. A contribution to the study of the life cycle of digenetic trematodes. A study of a new species Lepoderma muris n. sp. Okayama Igakkai Zasshi 1922, 385, 47–58. [Google Scholar]
- Hong, S.J. Surface ultrastructure of Plagiorchis muris growth and developmental stages in rats, the final host. Parasitol. Res. 2009, 105, 1077–1083. [Google Scholar] [CrossRef]
- Kurpiers, L.A.; Schulte-Herbrüggen, B.; Ejotre, I.; Reeder, D.A.M. Bushmeat and emerging infectious diseases: Lessons from Africa. In Problematic Wildlife: A Cross-Disciplinary Approach; Springer International Publishing: Cham, Switzerland, 2015; pp. 507–551. ISBN 9783319222462. [Google Scholar]
- Carrero, J.C.; Reyes-López, M.; Serrano-Luna, J.; Shibayama, M.; Unzueta, J.; León-Sicairos, N.; de la Garza, M. Intestinal amoebiasis: 160 years of its first detection and still remains as a health problem in developing countries. Int. J. Med. Microbiol. 2020, 310, 151358. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.L.; Ramirez-Avila, L. Current world status of Balantidium coli. Clin. Microbiol. Rev. 2008, 21, 626–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.; Ijaz, M.; Ayyub, R.M.; Ghaffar, A.; Ghauri, H.N.; Aziz, M.U.; Ali, S.; Altaf, M.; Awais, M.; Naveed, M.; et al. Balantidium coli in domestic animals: An emerging protozoan pathogen of zoonotic significance. Acta Trop. 2020, 203, 105298. [Google Scholar] [CrossRef] [PubMed]
- Krolewiecki, A.; Nutman, T.B. Strongyloidiasis: A Neglected Tropical Disease. Infect. Dis. Clin. N. Am. 2019, 33, 135–151. [Google Scholar] [CrossRef]
- De Oliveira Avelar, I.; Silva, A.P.C.; Gardiner, C.; de Lima Santos, R.; dos Santos Lima, W.; Ecco, R. Pathological and parasitological characterization of infection by trematodes (Paramphistomatidae) in the large intestine of capybaras. Rev. Bras. Parasitol. Vet. 2015, 24, 345–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Chang, H.; Wang, Y.; Huang, C.; Han, S.; He, H. Molecular Characterization of Cryptosporidium spp. in Brandt’s Vole in China. Front. Vet. Sci. 2020, 7, 300. [Google Scholar] [CrossRef] [PubMed]
- Perz, J.F.; Le Blancq, S.M. Cryptosporidium parvum Infection Involving Novel Genotypes in Wildlife from Lower New York State. Appl. Environ. Microbiol. 2001, 67, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- García-Livia, K.; Martín-Alonso, A.; Foronda, P. Diversity of Cryptosporidium spp. in wild rodents from the Canary Islands, Spain. Parasit. Vectors 2020, 13, 445. [Google Scholar] [CrossRef] [PubMed]
- Stenger, B.L.S.; Clark, M.E.; Kváč, M.; Khan, E.; Giddings, C.W.; Dyer, N.W.; Schultz, J.L.; McEvoy, J.M. Highly divergent 18S rRNA gene paralogs in a Cryptosporidium genotype from eastern chipmunks (Tamias striatus). Infect. Genet. Evol. 2015, 32, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, C.; Zhang, L.; Wang, R.; Jian, F.; Zhang, S.; Ning, C.; Wang, H.; Feng, C.; Wang, X.; Ren, X.; et al. Cryptosporidium spp. in Wild, Laboratory, and Pet Rodents in China: Prevalence and Molecular Characterization. Appl. Environ. Microbiol. 2009, 75, 7692–7699. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, P.E.; Wade, S.E.; Schaaf, S.L.; Stern, D.A.; Nadareski, C.A.; Mohammed, H.O. Prevalence of Cryptosporidium species in wildlife populations within a watershed landscape in southeastern New York State. Vet. Parasitol. 2007, 147, 176–184. [Google Scholar] [CrossRef]
- Zahedi, A.; Ryan, U. Cryptosporidium—An update with an emphasis on foodborne and waterborne transmission. Res. Vet. Sci. 2020, 132, 500–512. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future köppen-geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [Green Version]
- Uribe, M.; Payán, E.; Brabec, J.; Vélez, J.; Taubert, A.; Chaparro-Gutiérrez, J.J.; Hermosilla, C. Intestinal Parasites of Neotropical Wild Jaguars, Pumas, Ocelots, and Jaguarundis in Colombia: Old Friends Brought Back from Oblivion and New Insights. Pathogens 2021, 10, 822. [Google Scholar] [CrossRef]
- Sikes, R.S.; Gannon, W.L. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2016, 92, 235–253. [Google Scholar] [CrossRef]
- Yang, J.; Scholten, T. A Fixative for Intestinal Parasites Permitting the Use of Concentration and Permanent Staining Procedures. Am. J. Clin. Pathol. 1977, 67, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Dennis, W.R.; Stone, W.M.; Swanson, L.E. A new laboratory and field diagnostic test for fluke ova in feces. J. Am. Vet. Med. Assoc. 1954, 124, 47–50. [Google Scholar] [PubMed]
- Heine, J. A simple technic for the demonstration of cryptosporidia in feces. Zentralbl. Veterinarmed. B 1982, 29, 324–327. [Google Scholar] [CrossRef]
- Vieira, F.M.; de Lima, S.D.S.; Bessa, E.C.D.A. Morphology and biometry of eggs and larvae of Strongyloides sp. Grassi, 1879 (Rhabditoidea: Strongyloididae), a gastrointestinal parasite of Hydrochaeris hydrochaeris (Linnaeus, 1766) (Rodentia: Hydrochaeridae), in the municipality of Juiz de Fora, Minas. Rev. Bras. Parasitol. Vet. 2006, 15, 7–12. [Google Scholar]
- Cedrola, F.; Fregulia, P.; D’agosto, M.; Júnio Pedroso Dias, R. Intestinal ciliates of Brazilian Capybara (Hydrochoerus hydrochaeris L.). Acta Protozologica 2018, 57, 61–67. [Google Scholar] [CrossRef]
- Haverkost, T.R.; Gardner, S.L. A redescription of three species of Monoecocestus (Cestoda: Anoplocephalidae) including Monoecocestus threlkeldi based on new material. J. Parasitol. 2009, 95, 695–701. [Google Scholar] [CrossRef] [Green Version]
- Tkach, V.V.; Littlewood, D.T.J.; Olson, P.D.; Kinsella, J.M.; Swiderski, Z. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst. Parasitol. 2003, 56, 1–15. [Google Scholar] [CrossRef]
- Lee, S.-U.; Huh, S.; Sohn, W.-M. Molecular phylogenic location of the Plagiorchis muris (Digenea, Plagiorchiidae) based on sequences of partial 28S D1 rDNA and mitochondrial cytochrome C oxidase subunit I. Korean J. Parasitol. 2004, 42, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783. [Google Scholar] [CrossRef]
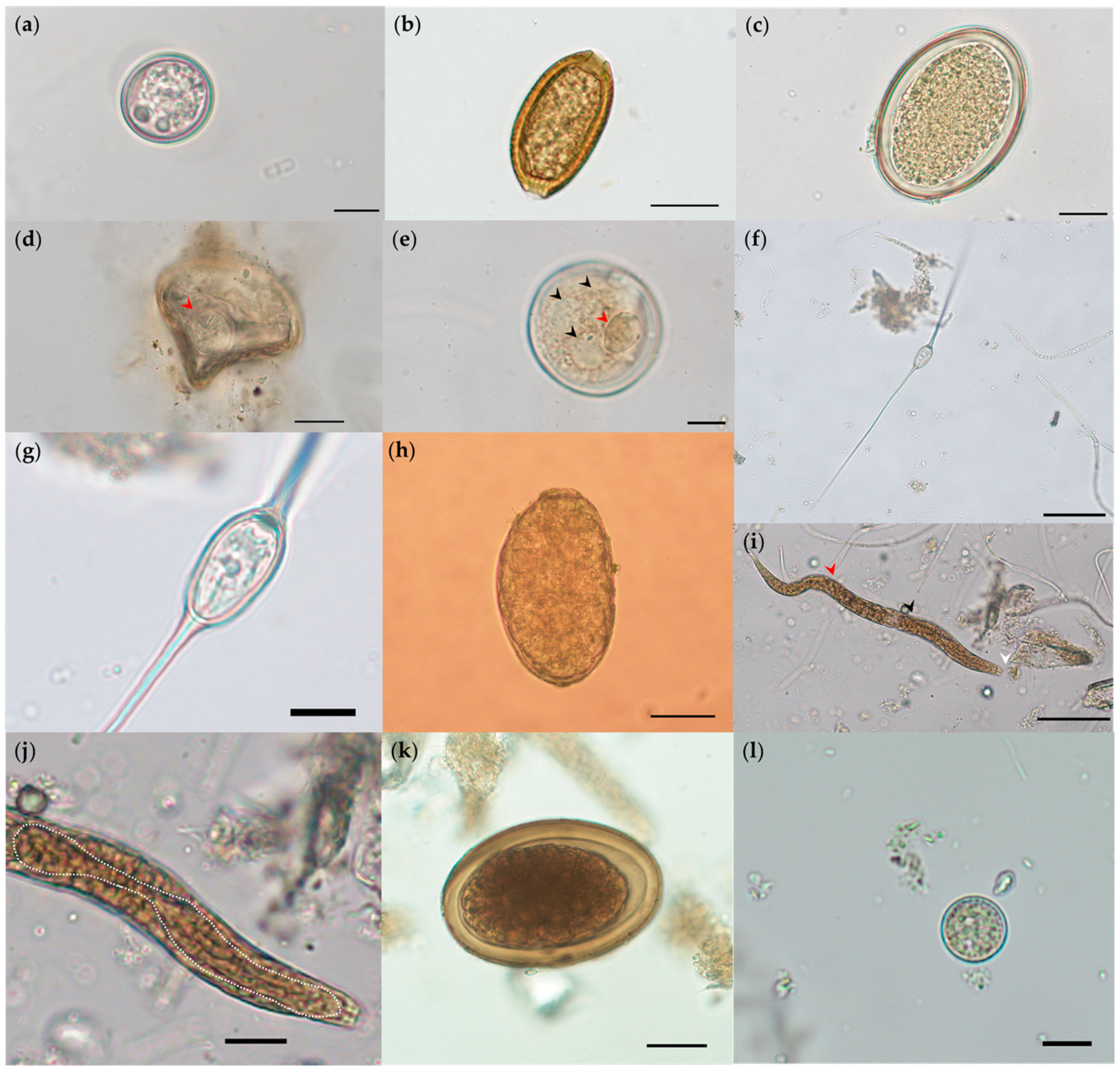


| Parasite | Localization a | Tissue | Feces | Blood | Literature |
|---|---|---|---|---|---|
| Metazoa | |||||
| Nematoda | |||||
| Dipetalonema hydrochoerus | C | x | [11] | ||
| Cruorifilaria tuberocauda | C, Br | x | [12,13,14,15] | ||
| Protozoophaga obesa‡ | A, Br, Bo, C, V | x | [15,16,17,18,19,20,21,22] | ||
| Strongyloides‡ | A, Br, C | x | [16,17,19,22] | ||
| Strongyloides chapini | Br | x | [15,21] | ||
| Capillaria spp. | Br | x | [23] | ||
| Echinocoleus hydrochoerib, ‡ | A, Br, C | x | [15,17,21,22,24] | ||
| Viannella spp. | Br | x | [19] | ||
| Vianella hydrochoeri | Br, Bo, V | x | [15,17,18,20,21] | ||
| Hydrochoerisnema anomalobursata | Br | x | [17,21] | ||
| Trichuris spp. | Br | x | [17] | ||
| Trichuris cutillasae n. sp. | A | x | [25] | ||
| Trichostrongylus axei | Br | x | [15,21] | ||
| Habronema clarki | Br, Pa | x | [20,26] | ||
| Yatesia hydrochoerus | Br | x | [15] | ||
| Fam: Trichostrongyloidea ‡ | A, C | x | [22] | ||
| Ord: Ascaridida | A | x | [22] | ||
| Cestoda | |||||
| Monoecocestus‡ | C | Present study | |||
| Monoecocestus hagmanni | Br, Bo, V | x | [18,20,21] | ||
| Monoecocestus hydrochoeri | A, Br, Bo | x | [15,16,17,20,21,22] | ||
| Monoecocestus jacobi | Br | x | [17] | ||
| Monoecocestus macrobursatus | Br, Bo | [16,20,21] | |||
| Fam: Anoplocephalidae | Br | x | [16] | ||
| Trematoda | |||||
| Fasciola hepatica | A, Br | x | [23,27,28] | ||
| Hippocrepis fuelleborni | Br | x | [16] | ||
| Hippocrepis hippocrepis‡ | Br, C, V | x | [15,16,17,18,21,29] | ||
| Hydrochoeristrema cabrali | Br | x | [17] | ||
| Neocotyle neocotyle | Br | x | [21] | ||
| Nudacotyle tertius | Br | x | [15,21] | ||
| Nudacotyle valdevaginatus | Br | x | [21] | ||
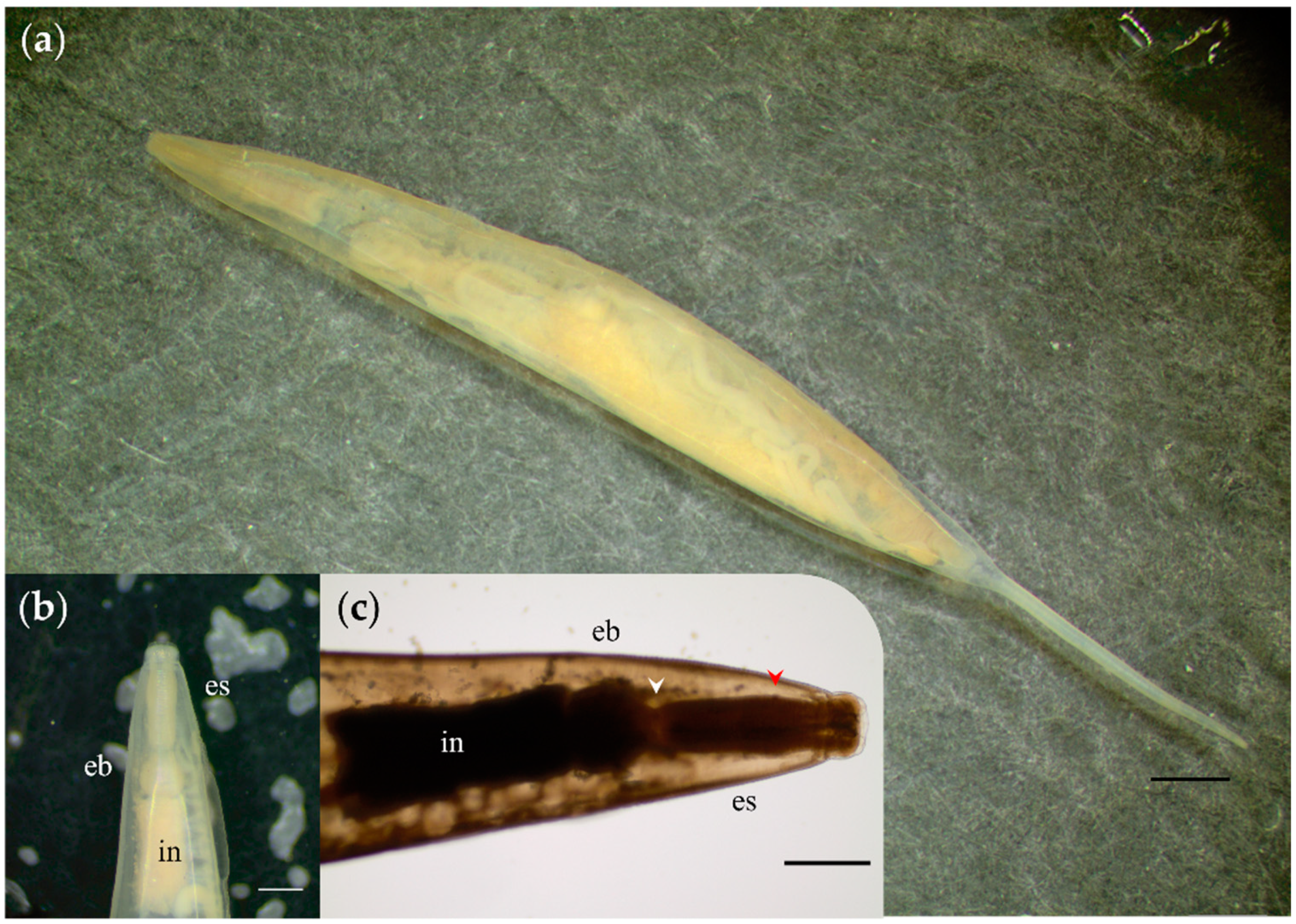
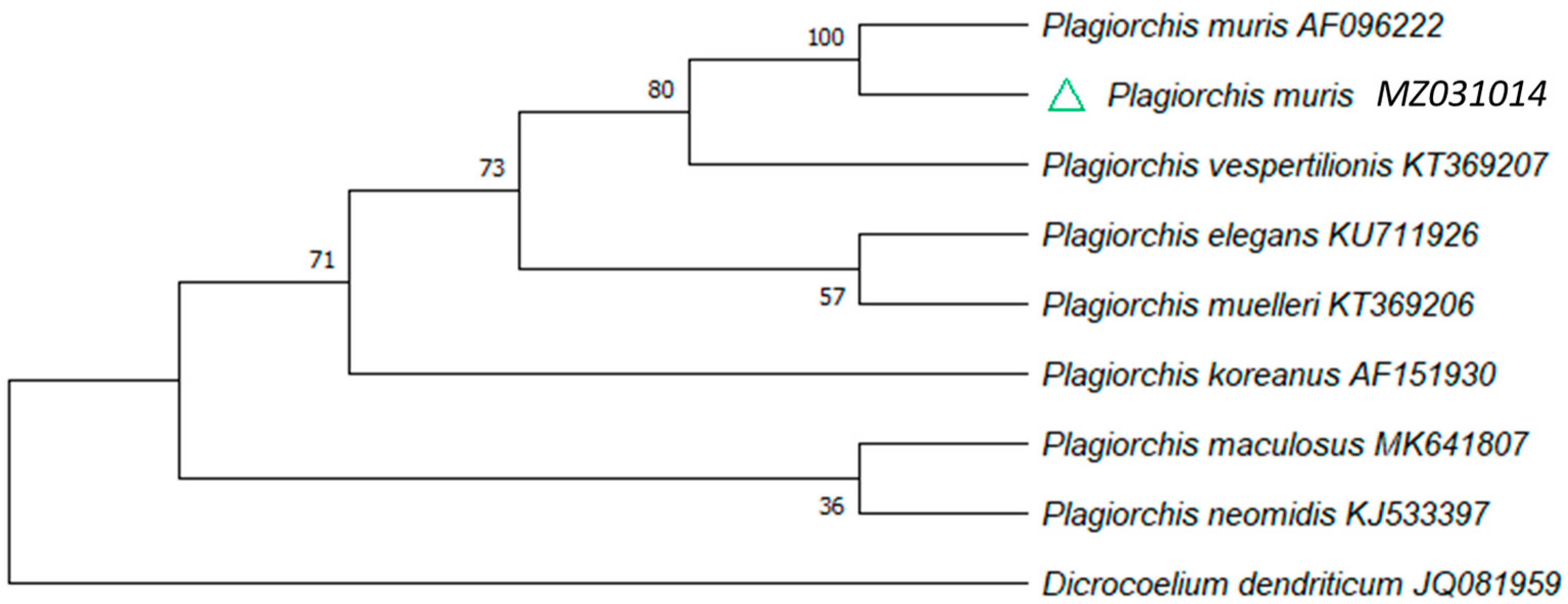
| Plagiorchis muris‡ | C | x | Present study | ||
| Philophthalmus lachrymosus | Br | x | x | [16,30] | |
| Taxorchis schistocotyle‡ | A, Br, C, V | x | [15,16,18,21,22] | ||
| Protozoa | |||||
| Neobalantidium coli‡ | A, C | x | [22] | ||
| Blastocystis sp. | A | x | [22] | ||
| Cryptosporidium‡/C. parvum | B | x | [31] | ||
| Entamoeba‡ | C | x | Present study | ||
| Eimeria sp./spp. | A, C | x | [22,32] | ||
| Eimeria araside | Br | x | [33] | ||
| Eimeria boliviensis | Bo, Br, V | x | [33,34] | ||
| Eimeria ichiloensis | Bo, Br, V | x | [33,34] | ||
| Eimeria trinidadensis‡ | Bo, Br, C, V | x | [33,34] | ||
| Trypanosoma evansi | A, Br, Pe, V | x | [35,36,37,38] | ||
| Toxoplasma gondii | Br | x | [39] | ||
| Giardia spp. | C | x | [32] | ||
| Sarcocystis spp. | C | x | [32] | ||
| Fam: Cycloposthiidae ‡ | C | x | Present study |
| Phylum | Parasite | Stage a | Technique b | Bocas del Arauca n = 15 | Cinaruco n = 8 | La Maporita n = 23 | Total (%) |
|---|---|---|---|---|---|---|---|
| Apicomplexa | Cryptosporidium | O | coproELISA | 2 | 6 | 8 | 34.8 (16/46) |
| Eimeria trinidadensis | O | SAF | 2 | 2 | 6 | 21.7 (10/46) | |
| Amoebozoa | Entamoeba | C | SAF | 4 | 2 | 2 | 17.4 (8/46) |
| Ciliophora | Neobalantidium coli | SAF | 1 | 1 | 4.3 (2/46) | ||
| Cycloposthiidae | C | SAF | 6 | 4 | 21.7 (10/46) | ||
| Platyhelminthes | |||||||
| Nematoda | Ascarididae | E | SAF | 3 | 2 | 8 | 28.3 (13/46) |
| Echinocoleus hydrochoeri | E | CF/SAF | 7 | 7 | 13 | 58.7 (27/46) | |
| Protozoophaga obesa | E/L/A | SS/CF/SAF | 3 | 1 | 4 | 17.4 (8/46) | |
| Strongyloides-like | L | SAF | 7 | 2 | 10 | 41.3 (19/46) | |
| Cestoda | Monoecocestus | E | CF/SAF | 1 | 2 | 6.5 (3/46) | |
| Trematoda | Hippocrepis hippocrepis | E/A | SF/SS/SAF | 4 | 1 | 3 | 17.4 (8/46) |
| Plagiorchis muris | A | Sequencing | 1 | 2.2 (1/46) | |||
| Taxorchis schistocotyle | E | SS/SAF | 6 | 6 | 8 | 43.5 (20/46) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uribe, M.; Hermosilla, C.; Rodríguez-Durán, A.; Vélez, J.; López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Cortés-Vecino, J.A. Parasites Circulating in Wild Synanthropic Capybaras (Hydrochoerus hydrochaeris): A One Health Approach. Pathogens 2021, 10, 1152. https://doi.org/10.3390/pathogens10091152
Uribe M, Hermosilla C, Rodríguez-Durán A, Vélez J, López-Osorio S, Chaparro-Gutiérrez JJ, Cortés-Vecino JA. Parasites Circulating in Wild Synanthropic Capybaras (Hydrochoerus hydrochaeris): A One Health Approach. Pathogens. 2021; 10(9):1152. https://doi.org/10.3390/pathogens10091152
Chicago/Turabian StyleUribe, Manuel, Carlos Hermosilla, Arlex Rodríguez-Durán, Juan Vélez, Sara López-Osorio, Jenny J. Chaparro-Gutiérrez, and Jesús A. Cortés-Vecino. 2021. "Parasites Circulating in Wild Synanthropic Capybaras (Hydrochoerus hydrochaeris): A One Health Approach" Pathogens 10, no. 9: 1152. https://doi.org/10.3390/pathogens10091152
APA StyleUribe, M., Hermosilla, C., Rodríguez-Durán, A., Vélez, J., López-Osorio, S., Chaparro-Gutiérrez, J. J., & Cortés-Vecino, J. A. (2021). Parasites Circulating in Wild Synanthropic Capybaras (Hydrochoerus hydrochaeris): A One Health Approach. Pathogens, 10(9), 1152. https://doi.org/10.3390/pathogens10091152











