Physicochemical Water Quality Influence on the Parasite Biodiversity in Juvenile Tilapia (Oreochromis spp.) Farmed at Valle Del Mezquital in the Central-Eastern Socioeconomic Region of Mexico
Abstract
:1. Introduction
2. Results
2.1. Frequency
2.2. Distribution and Biodiversity
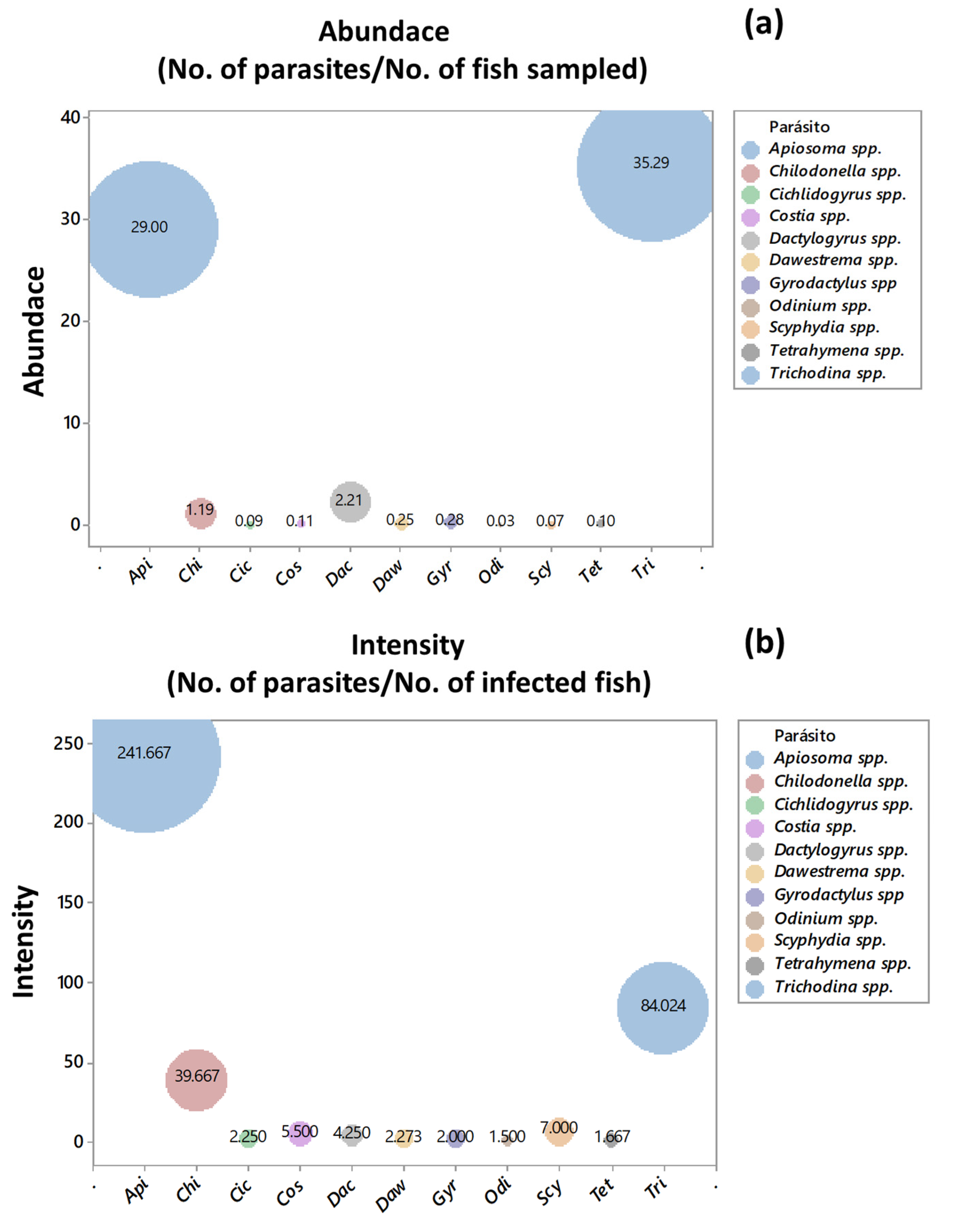
2.3. Ecological Indices of Abundance and Intensity
2.4. Water Quality
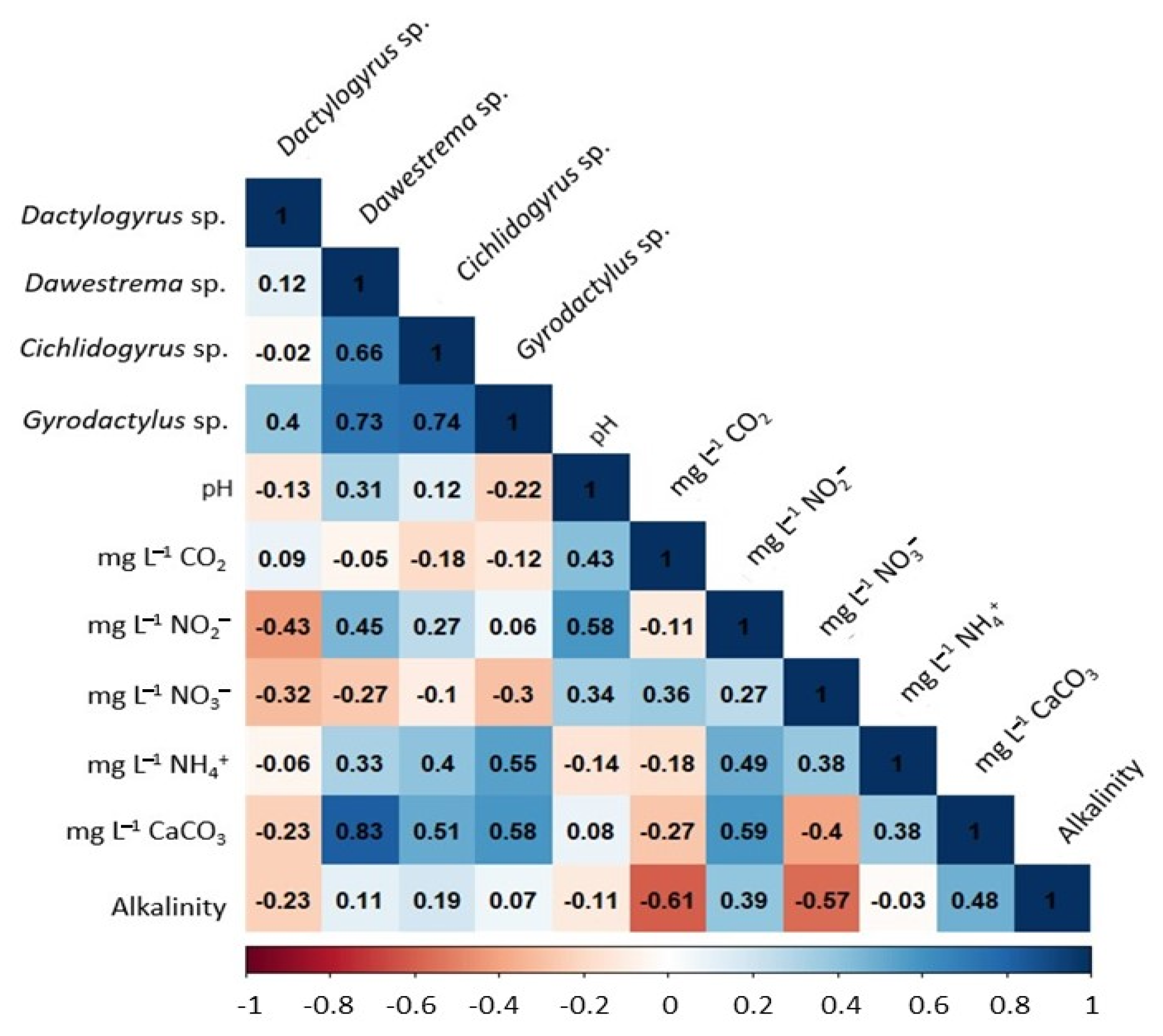
2.5. Influence of Water Quality on Parasite Biodiversity
3. Discussion
3.1. Frequency
3.2. Distribution and Biodiversity
3.3. Ecological Indices of Abundance and Intensity
3.4. Physicochemical Water Quality
3.5. Influence of Physicochemical Water Quality on Parasitoses
4. Materials and Methods
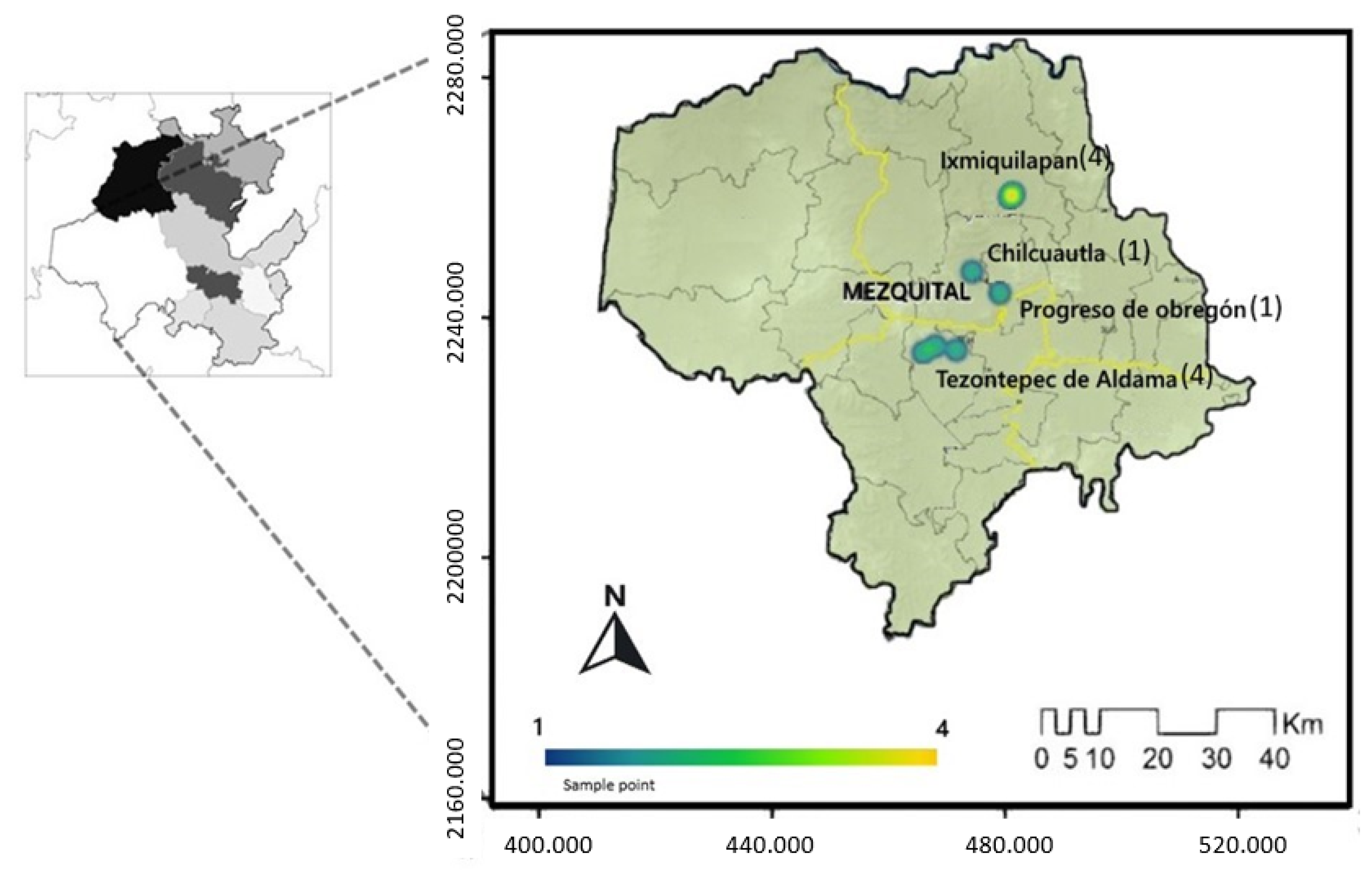
4.1. Study Area
4.2. Sampling and Parasitological Analysis
4.3. Determination of Physicochemical Water Quality Parameters
4.4. Analysis of the Influence of Physicochemical Water Quality on Parasitic Biodiversity
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Sayed, A.-F.M. Tilapia Culture, 1st ed.; Elsevier Inc.: Cairo, Egypt, 2020; pp. 21–31. [Google Scholar] [CrossRef]
- Gu, D.E.; Yu, F.D.; Yang, Y.X.; Xu, M.; Wei, H.; Luo, D.; Mu, X.D.; Hu, Y.C. Tilapia fisheries in Guangdong Province, China: Socio-economic benefits, and threats on native ecosystems and economics. Fish. Manag. Ecol. 2019, 26, 97–107. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Dawood, M.A.; Menanteau-Ledouble, S.; El-Matbouli, M. The nature and consequences of co-infections in tilapia: A review. J. Fish Dis. 2020, 43, 651–664. [Google Scholar] [CrossRef]
- Fajer-Ávila, E.J.; Medina-Guerrero, R.M.; Morales-Serna, F.N. Strategies for prevention and control of parasite diseases in cultured tilapia. Acta Agrícola y Pecu. 2017, 3, 25–31. [Google Scholar]
- Gjedrem, T.; Robinson, N.; Rye, M. The importance of selective breeding in aquaculture to meet future demands for animal protein: A review. Aquaculture 2012, 350–353, 117–129. [Google Scholar] [CrossRef]
- Vega, F.; Cortés, M.; Zuñiga, L.; Jaime, B.; Galindo, J.; Basto, M. Small-scale culture of tilapia (Oreochromis niloticus), ali-mentary alternative for rural and peri-urban families in Mexico? Redvelvet 2010, 11, 177–185. [Google Scholar]
- Mitiku, M.A.; Konecny, R.; Haile, A.L. Parasites of Nile tilapia (Oreochromis niloticus) from selected fish farms and Lake Koftuin central Ethiopia. Ethiop. Veter J. 2018, 22, 65. [Google Scholar] [CrossRef]
- Cascarano, M.C.; Stavrakidis-Zachou, O.; Mladineo, I.; Thompson, K.D.; Papandroulakis, N.; Katharios, P. Mediterranean Aquaculture in a Changing Climate: Temperature Effects on Pathogens and Diseases of Three Farmed Fish Species. Pathogens 2021, 10, 1205. [Google Scholar] [CrossRef]
- Wilson, J.R.; Saunders, R.J.; Hutson, K.S. Parasites of the invasive tilapia Oreochromis mossambicus: Evidence for co-introduction. Aquat Invasions 2019, 14, 332–349. [Google Scholar] [CrossRef]
- Rahman, M.; Shahjahan, M.; Ahmed, N. Tilapia Farming in Bangladesh: Adaptation to Climate Change. Sustainability 2021, 13, 7657. [Google Scholar] [CrossRef]
- Reid, G.; Gurney-Smith, H.; Marcogliese, D.; Knowler, D.; Benfey, T.; Garber, A.; Forster, I.; Chopin, T.; Brewer-Dalton, K.; Moccia, R.; et al. Climate change and aquaculture: Considering biological response and resources. Aquac. Environ. Interactions 2019, 11, 569–602. [Google Scholar] [CrossRef]
- Akoll, P.; Konecny, R.; Mwanja, W.W.; Nattabi, J.K.; Agoe, C.; Schiemer, F. Parasite fauna of farmed Nile tilapia (Oreochromis niloticus) and African catfish (Clarias gariepinus) in Uganda. Parasitol Res. 2011, 110, 315–323. [Google Scholar] [CrossRef]
- Cortés, D.A.; Dolz, G.; Romero-Zuñiga, J.R.; Rocha, A.E.J.; Alán, D.L. Centrocestus formosanus (Opisthorchiida: Heterophyidae) como causa de muerte de alevines de tilapia gris Oreochromis niloticus (Perciforme: Cichlidae) en el Pacífico seco de Costa Rica. Rev Biol Trop. 2009, 58, 1453–1465. [Google Scholar] [CrossRef]
- Manbe, M.Y.; Kabir, A.; Muaz, U.; Hussaini, K. Prevalence of protozoan parasites in some freshwater fishes of Dangana Lake Lapai, Niger State Nigeria. Int. J. Vet. Sci. Anim. Husb. 2020, 5, 13–16. Available online: https://www.researchgate.net/publication/341566173_International_Journal_of_Veterinary_Sciences_and_Animal_Husband-ry_2020_52_13-16_Prevalence_of_protozoan_parasites_in_some_freshwater_fishes_of_Dangana_Lake_Lapai_Niger_State_Nigeria (accessed on 6 May 2022).
- Mf, W.P.; R, L.N.; Rd, M.D.; Gb, R.M.; Montagner, D.; Tavares-Dias, M. Protozoan and metazoan parasites of Nile tilapia Oreochromis niloticus cultured in Brazil. Rev MVZ Cordoba. 2012, 17, 2812–2819. [Google Scholar] [CrossRef]
- Pinto, H.A.; Mati, V.L.T.; Melo, A.L. Metacercarial Infection of Wild Nile Tilapia (Oreochromis niloticus) from Brazil. Sci. World J. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Belmont, M.A.; Cantellano, E.; Ramirez-Mendoza, N. Multifunctional Wetlands. Environmental Contamination Remediation and Management, 1st ed.; Springer: Cham, Switzerland, 2018; pp. 241–252. [Google Scholar] [CrossRef]
- Sathish, S.; Chidambaram, P.; Uma, A. Prevalence of parasites in tilapia farms and their management practices in Tamil Nadu, India. J. Entomol. Zool. Stud. 2021, 9, 678–689. Available online: https://www.researchgate.net/publication/350631713_Prevalence_of_parasites_in_tilapia_farms_and_their_management_practices_in_Tamil_Nadu_India (accessed on 6 May 2022).
- Kolia, W.; Sunarto, S.; Widiyani, T. The infection of ectoparasitic protozoa on farmed Nile tilapia (Oreochromis niloticus) at three reservoirs in Central Java, Indonesia. Biodiversitas J. Biol. Divers. 2021, 22, 1975–1980. [Google Scholar] [CrossRef]
- Zago, A.C.; Franceschini, L.; Garcia, F.; Schalch, S.H.C.; Gozi, K.S.; Da Silva, R.J. Ectoparasites of Nile tilapia (Oreochromis niloticus) in cage farming in a hydroelectric reservoir in Brazil. J. Vet. Parasitol. Jaboticabal. 2014, 23, 171–178. [Google Scholar] [CrossRef]
- Salem, E.; Reda, A. A Review of some Ecto-and Endo Protozoan Parasites Infecting Sarotherodon Galilaeus and Tilapia Zillii from Damietta Branch of River Nile, Egypt. Egypt J. Am. Sci. 2011, 7, 1545–1603. [Google Scholar]
- Adamba, S.W.K.; Otachi, E.O.; Ong’Ondo, G.O. Parasite Communities of Oreochromis niloticus baringoensis (Trewavas, 1983) in Relation to Selected Water Quality Parameters in the Springs of Lorwai Swamp and Lake Baringo, Kenya. Acta Parasitol. 2020, 65, 441–451. [Google Scholar] [CrossRef]
- Otachi, E.O.; Szostakowska, B.; Jirsa, F.; Fellner-Frank, C. Parasite communities of the elongate tigerfish Hydrocynus forskahlii (Cuvier 1819) and redbelly tilapia Tilapia zillii (Gervais 1848) from Lake Turkana, Kenya: Influence of host sex and size. Acta Parasitol. 2014, 60, 9–20. [Google Scholar] [CrossRef]
- Georges, B.K.; Euphrasie, A.Y.; N’Doua, E.R.; Valentin, N. Structure of the gill monogenean parasites of Tylochromis jentinki (Teleostei: Haemulidae) from two sectors of Ebrié lagoon, Côte d’Ivoire. Int. J. Sci. Res. Publ. 2019, 9, 9055. [Google Scholar] [CrossRef]
- Marinho, R.; Tavares-Dias, M.; Dias-Grigório, M.; Neves, L.; Yoshioka, E.; Boijink, C.L.; Takemoto, R. Helminthes and protozoan of farmed pirarucu (Arapaima gigas) in eastern Amazon and host-parasite relationship. Arq. Bras. Med. Vet. e Zootec. 2013, 65, 1192–1202. [Google Scholar] [CrossRef]
- Morris, T.C.; van der Ploeg, J.; Awa, S.B.; van der Lingen, C.D.; Reed, C.C. Parasite community structure as a predictor of host population structure: An example using Callorhinchus capensis. Int. J. Parasitol. Parasites Wildl. 2019, 8, 248–255. [Google Scholar] [CrossRef]
- Villalba-Vasquez, P.J.; Violante-González, J.; Monks, S.; Marino-Romero, J.U.; Ibáñez, S.G.; Rojas-Herrera, A.A.; Flores-Garza, R.; Rosas-Guerrero, V. Temporal and spatial variations in the metazoan parasite communities of the Panama spadefish, Parapsettus panamensis (Pisces: Ephippidae), from the Pacific coast of Mexico. Invertebr. Biol. 2018, 137, 339–354. [Google Scholar] [CrossRef]
- Paredes-Trujillo, A.; Velázquez-Abunader, I.; Torres-Irineo, E.; Romero, D.; Vidal-Martínez, V.M. Geographical distribution of protozoan and metazoan parasites of farmed Nile tilapia Oreochromis niloticus (L.) (Perciformes: Cichlidae) in Yucatán, México. Parasites Vectors 2016, 9, 1–16. [Google Scholar] [CrossRef]
- Akoll, P.; Konecny, R.; Mwanja, W.W.; Schiemer, F. Risk assessment of parasitic helminths on cultured Nile tilapia (Oreochromis niloticus, L.). Aquaculture 2012, 356–357, 123–127. [Google Scholar] [CrossRef]
- Zhi, T.; Huang, C.; Sun, R.; Zheng, Y.; Chen, J.; Xu, X.; Brown, C.L.; Yang, T. Mucosal immune response of Nile tilapia Oreochromis niloticus during Gyrodactylus cichlidarum infection. Fish Shellfish Immunol. 2020, 106, 21–27. [Google Scholar] [CrossRef]
- Arguedas, C.D.; Ortega, S.C.; Martínez, C.S.; Astroza C, Á. Parasites of Nile Tilapia larvae Oreochromis niloticus (Pisces: Cichlidae) in concrete ponds in Guanacaste, Northern Costa Rica. UNED Res. J. 2017, 9, 1904. [Google Scholar] [CrossRef]
- Sharmin, F.; Rahman, M.; Shahjahan, M.; Chowdhury, P. Study of growth and productions of tilapia (Oreochromis niloticus) on different population densities in monoculture. Int. J. Agric. Res. Innov. Technol. 2020, 9, 76–83. [Google Scholar] [CrossRef]
- Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA). Manual de Buenas Prácticas de Pro-ducción Acuícola de Tilapia para la Inocuidad Alimentaria (Primera Eds), CDMX. 2008, pp. 59–61. Available online: https://www.gob.mx/cms/uploads/attachment/file/167794/7_Manual_Tilapia.pdf (accessed on 30 June 2022).
- Yusni, E.; Rambe, N. Identification of ectoparasites in Fry Tilapia (Oreochromis Niloticus) in Aquaculture Pond. IOP Conf. Series: Earth Environ. Sci. 2019, 260, 012110. [Google Scholar] [CrossRef]
- Cavalcante, D.D.H.; Caldini, N.N.; Da Silva, J.L.S.; Lima, F.R.D.S.; E Sá, M.V.D.C. Imbalances in the hardness/alkalinity ratio of water and Nile tilapia’s growth performance. Acta Sci. Technol. 2013, 36, 49–54. [Google Scholar] [CrossRef]
- Berman, J.J. Principles and Practice of Big Data. Preparing, Sharing, and Analyzing Complex Information, 2nd ed.; Elsevier Inc.: London, UK, 2018; pp. 231–257. [Google Scholar] [CrossRef]
- Ashmawy, K.; Hiekal, F.; AboAkadda, S.; Laban, N. The inter-relationship of water quality parameters and fish parasite occurrence. Alex. J. Veter Sci. 2018, 59, 97. [Google Scholar] [CrossRef]
- Setiawan, D.; Prayogo; Rahardja, B.S. Utilization of Nitrosomonas sp. and Nitrobacter sp. probiotic towards nitrite and nitrate level in nile tilapia (Oreochromis niloticus) using aquaponic system. IOP Conf. Series Earth Environ. Sci. 2021, 718, 012098. [Google Scholar] [CrossRef]
- Ojwala, R.A.; Otachi, E.O.; Kitaka, N.K. Effect of water quality on the parasite assemblages infecting Nile tilapia in selected fish farms in Nakuru County, Kenya. Parasitol Res. 2018, 117, 3459–3471. [Google Scholar] [CrossRef]
- Oliveira, M.S.B.; Adriano, E.A.; Tavares-Dias, M.; Corrêa, L.L. Community of Monogenea in populations of Cichla monoculus from two tributaries of the Amazon River in the Northern Brazil. Helminthologia 2019, 56, 1–10. [Google Scholar] [CrossRef]
- Modu, B.M.; Saiful, M.; Kartini, M.; Kassim, Z.; Hassan, M. Impact of Monogenean Parasite in Relation to Water quality Effects on the Structural changes in the Gills of Freshwater Cat Fish, Hemibagrus nemurus Valenciennes 1840. Curr. Res. J. Biol. Sci. 2012, 12, 1–6. [Google Scholar]
- Pérez-Serrano, D.; Cabirol, N.; Martínez-Cervantes, C.; Rojas-Oropeza, M. Mesquite management in the Mezquital Valley: A sustainability assessment based on the view point of the Hñähñú indigenous community. Environ. Sustain. Indic. 2021, 10, 100113. [Google Scholar] [CrossRef]
- Muthiah, M. Fisheries, 1st ed.; Allied Printers: Kaulagarh Road, Dehradun, India, 2014; p. 12. Available online: https://www.researchgate.net/publication/256423207_Transport_techniques_of_Fish_seeds_and_live_fishes_to_and_fro_watershed_ponds (accessed on 6 May 2022).
- Cifuentes, R.; Gonzalez, J.; Montoya, G.; Jara, A.; Ortiz, N.; Piedra, P.; Habit, E. Relación longitud-peso y factor de condición de los peces nativos del río San Pedro (cuenca del río Valdivia, Chile). Gayana 2012, 76, 86–100. [Google Scholar] [CrossRef]
- Scott, W.; Govett, P. Parasitology and necropsy of fish. Compend. Vet. 2009, 2, 1–7. Available online: https://www.researchgate.net/publication/24202851_Parasitology_and_necropsy_of_fish (accessed on 6 May 2022).
- Amaechi, C.E. Prevalence, intensity and abundance of endoparasites in Oreochromis niloticus and Tilapia zilli (Pisces: Cichlidae) from Asa Dam, Ilorin, Nigeria. UNED Res. J. 2015, 7, 67–70. [Google Scholar] [CrossRef]
- Justine, J.-L.; Briand, M.J.; Bray, R.A. A quick and simple method, usable in the field, for collecting parasites in suitable condition for both morphological and molecular studies. Parasitol Res. 2012, 111, 341–351. [Google Scholar] [CrossRef]
- Bautista-Hernández, C.E.; Pulido-Flores, G.; Violante-González, J.; Monks, S. Helminth parasites of Xiphophorus birchmanni (Pisces: Poeciliidae) from two localities of the Pánuco River drainage, Mexico. Rev. Mex. de Biodivers. 2019, 90, 1577. [Google Scholar] [CrossRef]
- Moreno, C. Métodos Para Medir la Biodiversidad, 1st ed.; MyT: Zaragoza, Spain, 2001; p. 87. Available online: https://www.researchgate.net/publication/304346666_Metodos_para_medir_la_biodiversidad (accessed on 6 May 2022).
- Wanja, D.W.; Mbuthia, P.G.; Waruiru, R.M.; Mwadime, J.M.; Bebora, L.C.; Nyaga, P.N.; Ngowi, H.A. Fish Husbandry Practices and Water Quality in Central Kenya: Potential Risk Factors for Fish Mortality and Infectious Diseases. Veter. Med. Int. 2020, 2020, 6839354. [Google Scholar] [CrossRef] [Green Version]

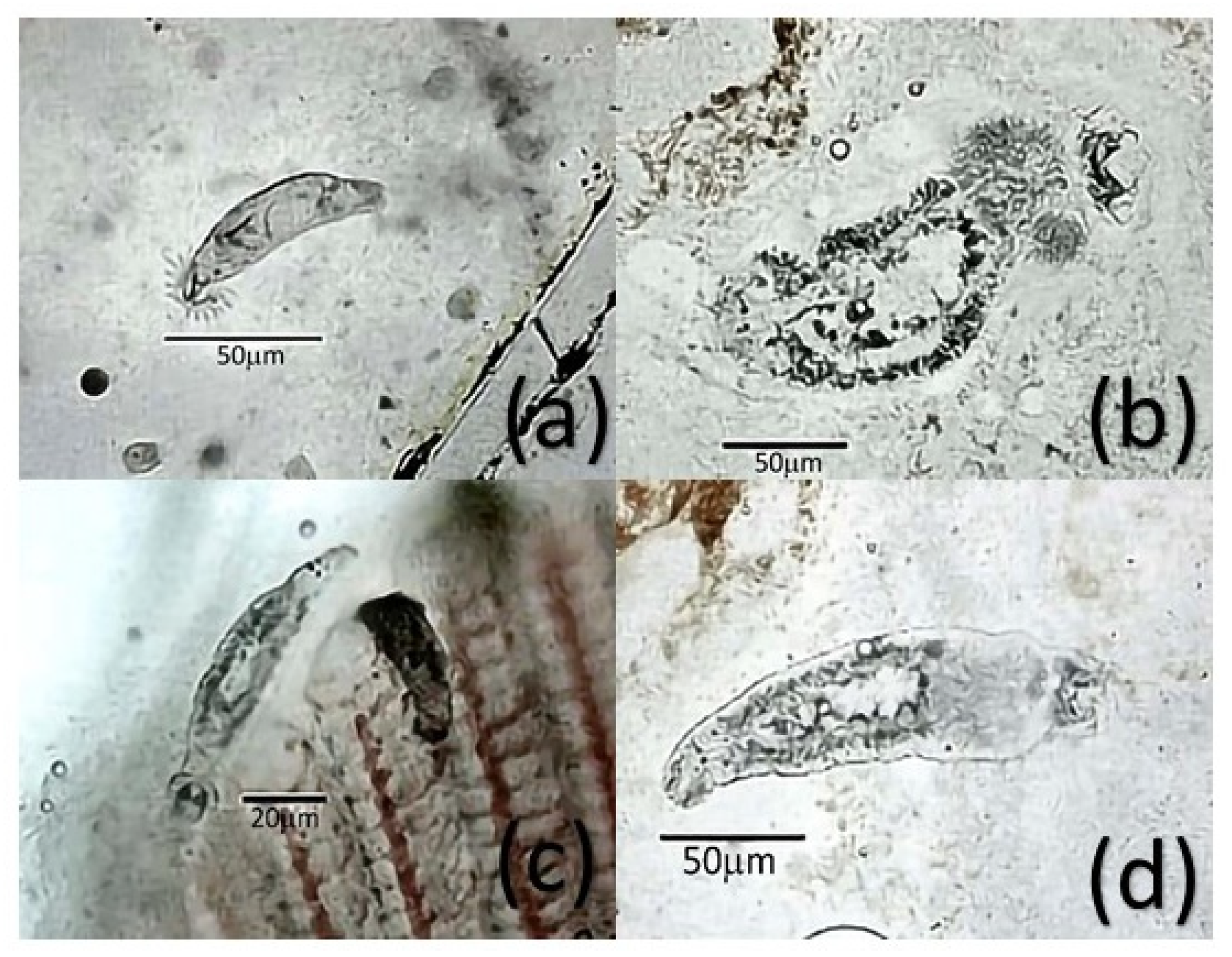

| Parasite | Sampling Point | Parasite Prevalence 1 (IC 95%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G1Ix | G2 Ix | G3Ix | G4Ch | G5Ix | G6Te | G7Te | G8Te | G9Te | G10Pr | ||
| Protozoa | |||||||||||
| Apiosoma spp. | - | - | - | - | - | - | - | 10 | 100 | 10 | 12% (9.90–14.10) |
| Chilodonella spp. | - | - | - | - | - | 10 | - | - | 20 | - | 3% (2.54–3.46) |
| Costia spp. | - | - | - | 10 | - | - | - | - | 10 | - | 2% (1.72–2.28) |
| Oodinium spp. | - | - | - | 10 | - | - | - | - | 10 | - | 2% (1.72–2.28) |
| Scyphydia spp. | - | - | - | 10 | - | - | - | - | - | - | 1% (0.79–1.21) |
| Tetrahymena spp. | - | - | 40 | - | - | - | - | - | 20 | - | 6% (5.09–6.91) |
| Trichodina spp. | 20 | 10 | 40 | 100 | 50 | 20 | 40 | 30 | 100 | 10 | 42% (39.76–44.24) |
| Monogeneans | |||||||||||
| Dactylogyrus spp. | 100 | 20 | 60 | 100 | 50 | 10 | - | 80 | 50 | 50 | 52% (49.65–54.35) |
| Dawestrema spp. | - | - | - | - | - | - | 10 | 50 | 50 | - | 11% (9.60–12.40) |
| Cichlidogyrus spp. | - | - | - | - | - | - | - | - | 40 | - | 4% (3.15–4.85) |
| Gyrodactylus spp. | 40 | - | 10 | - | - | - | - | 30 | 60 | - | 14% (12.54–15.46) |
| Total | 86% (84.26–87.74) | ||||||||||
| Sampling Point | Number of Parasitic Individuals | Richness of Parasitic Genera | Simpson Index | Berger–Parker Index |
|---|---|---|---|---|
| G1Ix | 36 | 3 | 0.60 | 0.53 (Dactylogyrus spp.) |
| G2Ix | 12 | 2 | 0.59 | 0.75 (Dactylogyrus spp.) |
| G3Ix | 38 | 4 | 0.53 | 0.55 (Dactylogyrus spp.) |
| G4Ch | 1954 | 5 | 0.07 | 0.95 (Trichodina spp.) |
| G5Ix | 306 | 2 | 0.05 | 0.97 (Trichodina spp.) |
| G6Te | 8 | 3 | 0.46 | 0.75 (Trichodina spp.) |
| G7Te | 77 | 2 | 0.02 | 0.98 (Trichodina spp.) |
| G8Te | 83 | 5 | 0.49 | 0.68 (Dactylogyrus spp.) |
| G9Te | 4325 | 10 | 0.46 | 0.66 (Apiosoma spp.) |
| G10Pr | 23 | 3 | 0.16 | 0.91 (Dactylogyrus spp.) |
| Totals | 6862 | 11 | 0.55 | 0.51 (Trichodina spp.) |
| Physicochemical Water Quality Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Sample | pH (p ≤ 0.001) | CO2 mg L−1 (p ≤ 0.001) | NO2−-N mg L−1 (p ≤ 0.001) | NO3--N mg L−1 (p = 0.001) | NH3-N mg L−1 (p ≤ 0.001) | CaCO3 mg L−1 (p ≤ 0.001) | Total Alkalinity (p ≤ 0.001) |
| G1Ix | 6.46 ± 0.05 e | 32 ± 1.73 c | 0.20 ± 3.4 × 10−17 i | 13.33 ± 5.77 b | 2.50 ± 0 a | 307 ± 9.16 de | 361 ± 4.58 cd |
| G2Ix | 6.90 ± 0.10 d | 79 ± 4.58 c | 0.20 ± 3.4 × 10−17 h | 20.00 ± 0 ab | 1.00 ± 0 cd | 273 ± 34.59 e | 317 ± 4.58 e |
| G3Ix | 7.03 ± 0.05 cd | 650 ± 17.32 a | 0.00 ± 0 j | 10.00 ± 0 b | 0.50 ± 0 d | 275 ± 18.33 e | 330 ± 3.00 de |
| G4Ch | 7.10 ± 0.10 bcd | 0 ± 0 c | 0.20 ± 3.4 × 10−17 g | 10.00 ± 0 b | 0.66 ± 0.28 d | 191 ± 6.92 f | 370 ± 17.32 cd |
| G5Ix | 7.46 ± 0.05 a | 590 ± 121.24 a | 1.00 ± 0 e | 33.33 ± 11.54 a | 2.16 ± 0.28 ab | 197 ± 4.58 f | 255 ± 30.44 f |
| G6Te | 6.96 ± 0.05 cd | 46 ± 3.46 c | 1.00 ± 0 d | 13.33 ± 5.77 b | 1.50 ± 0.50 bc | 379 ± 15.39 c | 507 ± 27.49 a |
| G7Te | 7.03 ± 0.05 cd | 41 ± 3.46 c | 1.00 ± 0 c | 16.66 ± 5.77 b | 2.00 ± 0 ab | 428 ± 4.58 b | 378 ± 3.00 bc |
| G8Te | 7.30 ± 0.10 ab | 290 ± 17.32 b | 1.00 ± 0 b | 10.00 ± 0 b | 1.50 ± 0 bc | 517 ± 9.16 a | 369 ± 6.00 cd |
| G9Te | 7.16 ± 0.05 bc | 60 ± 3.00 c | 1.00 ± 0 a | 13.33 ± 5.77 b | 2.33 ± 0.28 a | 511 ± 7.54 a | 416 ± 4.58 b |
| G10Pr | 7.26 ± 0.05 ab | 60 ± 3.00 c | 1.00 ± 0 f | 13.33 ± 5.77 b | 1.16 ± 0.28 cd | 345 ± 3.00 cd | 469 ± 4.58 a |
| ID | Municipality | Latitude | Longitude | No. of Fishes | Fulton Index (Weight-Length Ratio) | Fish Stage |
|---|---|---|---|---|---|---|
| G1Ix | Ixmiquilpan | 20.425037 | −99.16348 | 10 | 1.52 ± 0.42 | Offspring |
| G2Ix | Ixmiquilpan | 20.423151 | −99.165259 | 10 | 1.70 ± 0.16 | Juvenile |
| G3Ix | Ixmiquilpan | 20.424129 | −99.164666 | 10 | 1.74 ± 0.33 | Juvenile |
| G4Ch | Chilcuautla | 20.305693 | −99.228399 | 10 | 1.88 ± 0.28 | Juvenile |
| G5Ix | Ixmiquilpan | 20.418005 | −99.170325 | 10 | 1.62 ± 0.18 | Juvenile |
| G6Te | Tezontepec de Aldama | 20.186341 | −99.253102 | 10 | 1.86 ± 0.75 | Juvenile |
| G7Te | Tezontepec de Aldama | 20.192473 | −99.285839 | 10 | 2.27 ± 1.30 | Juvenile |
| G8Te | Tezontepec de Aldama | 20.182211 | −99.304982 | 10 | 2.82 ± 0.87 | Juvenile |
| G9Te | Tezontepec de Aldama | 20.18237 | −99.304968 | 10 | 2.51 ± 1.00 | Offspring |
| G10Pr | Progreso de Obregón | 20.273165 | −99.185246 | 10 | 2.46 ± 0.39 | Juvenile |
| Totals | 100 | 1.99 ± 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Pérez, V.-J.; Vega-Sánchez, V.; Fernández-Martínez, T.-E.; Zepeda-Velázquez, A.-P.; Reyes-Rodríguez, N.-E.; Ponce-Noguez, J.-B.; Peláez-Acero, A.; de-la-Rosa-Arana, J.-L.; Gómez-De-Anda, F.-R. Physicochemical Water Quality Influence on the Parasite Biodiversity in Juvenile Tilapia (Oreochromis spp.) Farmed at Valle Del Mezquital in the Central-Eastern Socioeconomic Region of Mexico. Pathogens 2022, 11, 1076. https://doi.org/10.3390/pathogens11101076
Acosta-Pérez V-J, Vega-Sánchez V, Fernández-Martínez T-E, Zepeda-Velázquez A-P, Reyes-Rodríguez N-E, Ponce-Noguez J-B, Peláez-Acero A, de-la-Rosa-Arana J-L, Gómez-De-Anda F-R. Physicochemical Water Quality Influence on the Parasite Biodiversity in Juvenile Tilapia (Oreochromis spp.) Farmed at Valle Del Mezquital in the Central-Eastern Socioeconomic Region of Mexico. Pathogens. 2022; 11(10):1076. https://doi.org/10.3390/pathogens11101076
Chicago/Turabian StyleAcosta-Pérez, Víctor-Johan, Vicente Vega-Sánchez, Tomás-Eduardo Fernández-Martínez, Andrea-Paloma Zepeda-Velázquez, Nydia-Edith Reyes-Rodríguez, Jesús-Benjamín Ponce-Noguez, Armando Peláez-Acero, Jorge-Luis de-la-Rosa-Arana, and Fabián-Ricardo Gómez-De-Anda. 2022. "Physicochemical Water Quality Influence on the Parasite Biodiversity in Juvenile Tilapia (Oreochromis spp.) Farmed at Valle Del Mezquital in the Central-Eastern Socioeconomic Region of Mexico" Pathogens 11, no. 10: 1076. https://doi.org/10.3390/pathogens11101076
APA StyleAcosta-Pérez, V. -J., Vega-Sánchez, V., Fernández-Martínez, T. -E., Zepeda-Velázquez, A. -P., Reyes-Rodríguez, N. -E., Ponce-Noguez, J. -B., Peláez-Acero, A., de-la-Rosa-Arana, J. -L., & Gómez-De-Anda, F. -R. (2022). Physicochemical Water Quality Influence on the Parasite Biodiversity in Juvenile Tilapia (Oreochromis spp.) Farmed at Valle Del Mezquital in the Central-Eastern Socioeconomic Region of Mexico. Pathogens, 11(10), 1076. https://doi.org/10.3390/pathogens11101076







