Evaluating the Role of Exogenously Applied Ascorbic Acid in Rescuing Soybean Plant Health in The Presence of Pathogen-Induced Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soybean Genotype and Macrophomina phaseolina Isolates
2.2. In Vitro Sensitivity of M. phaseolina to Ascorbic Acid
2.3. Soybean Stem Necrosis Assay
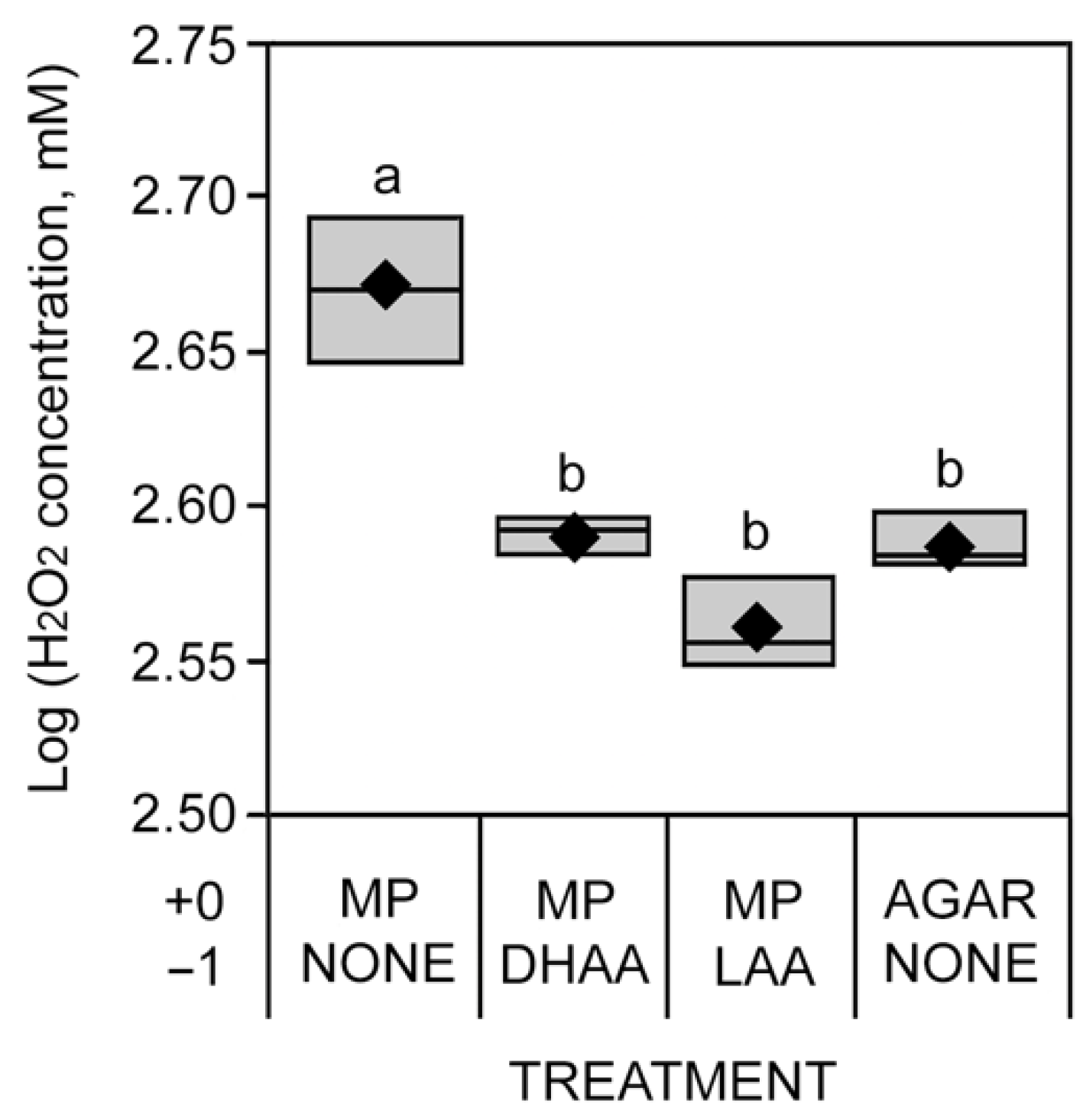
2.4. H2O2 Absorbance Assay
2.5. Statistics
3. Results
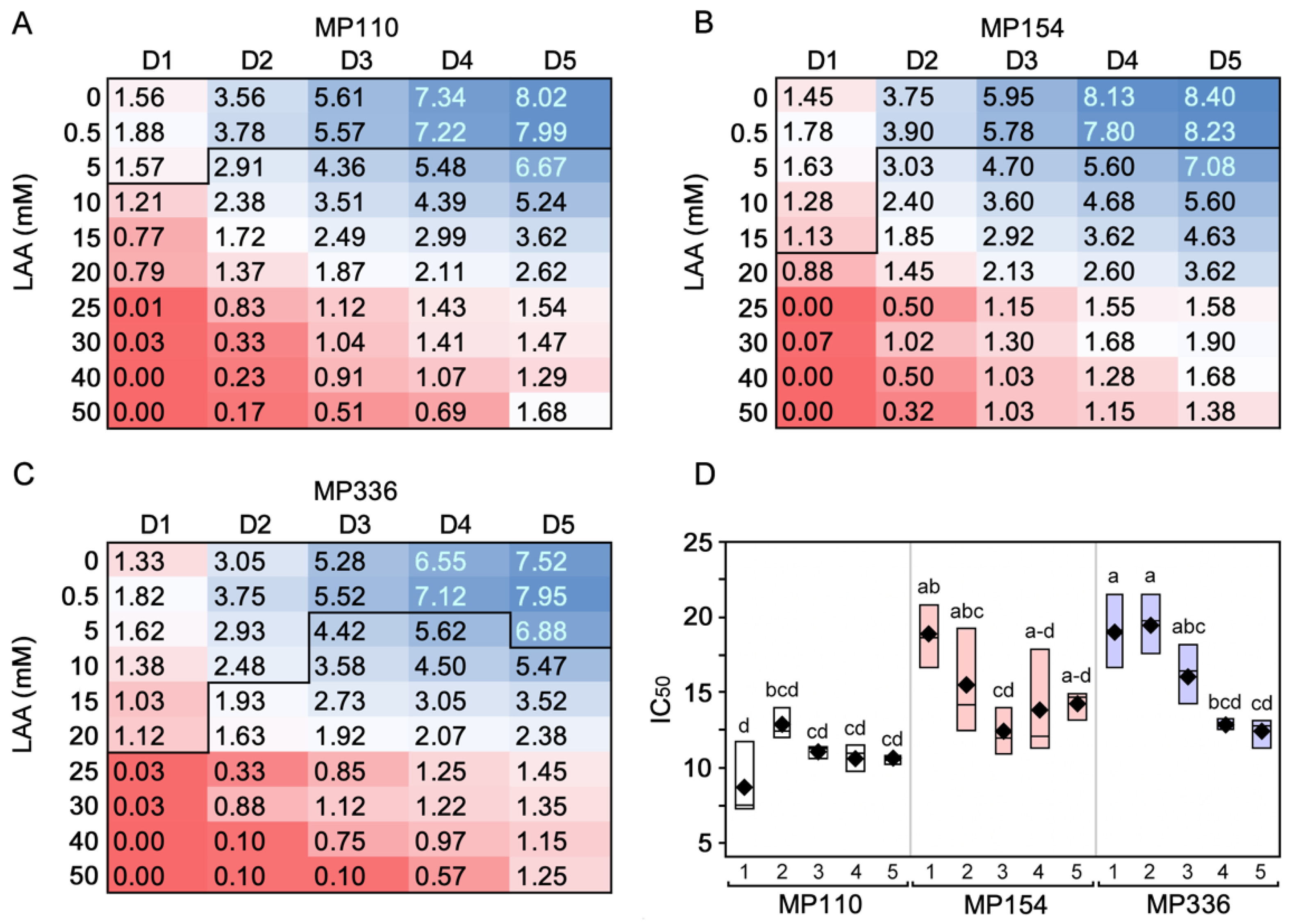
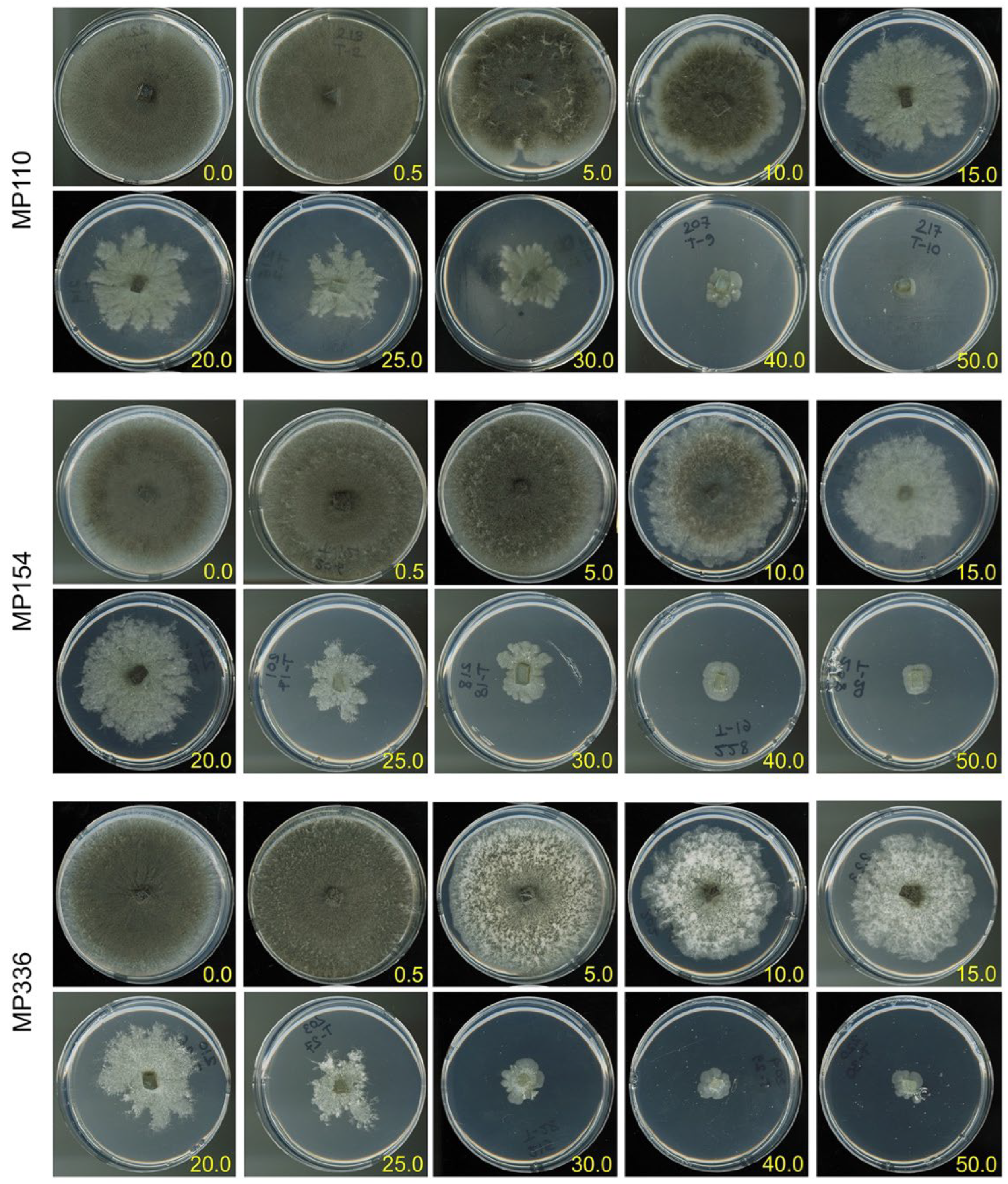
3.1. In Vitro Sensitivity Assay
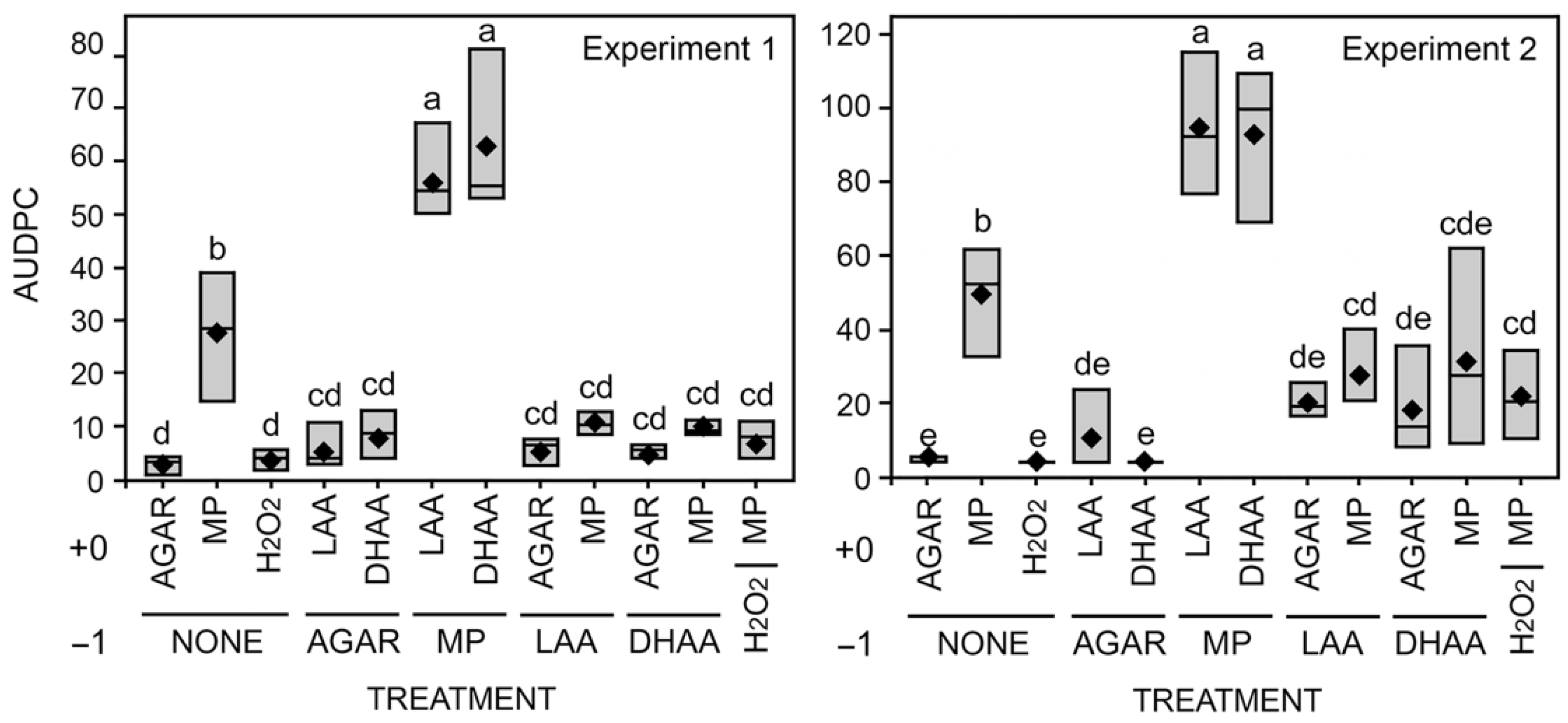
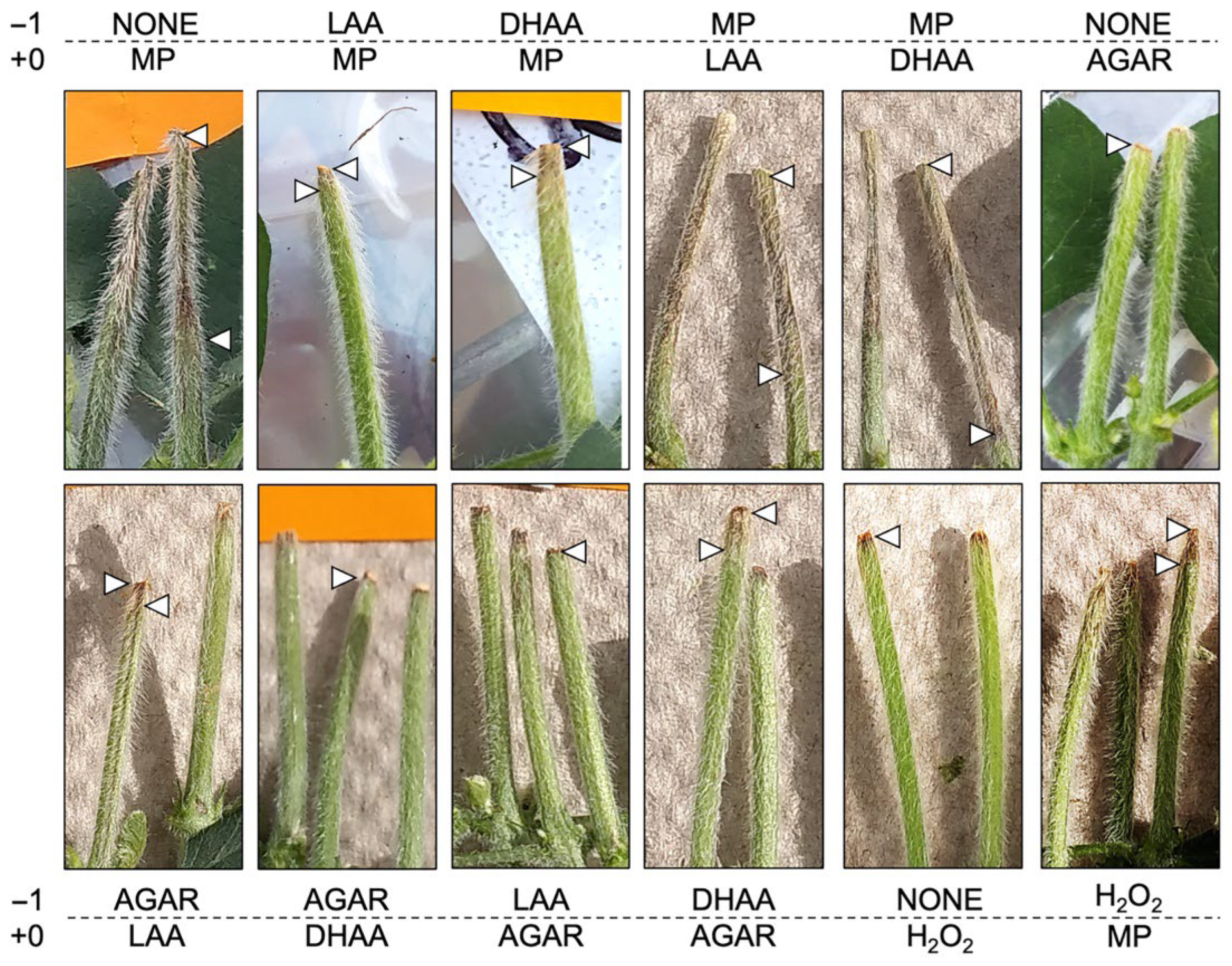
3.2. Cut-Stem Assay
3.3. H2O2 Absorbance Assay
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartman, G.L.; Rupe, J.C.; Sikora, E.J.; Domier, L.L.; Davis, J.A.; Steffey, K.L. Compendium of Soybean Diseases and Pests, 5th ed.; American Phytopathological Society: St. Paul, MN, USA, 2015; p. 201. [Google Scholar]
- Živanov, S.T.; Dedić, B.D.; Dimitrijević, A.; Duśanić, N.; Jocić, S.; Miklič, V.; Kovačević, B.; Miladinović, D. Analysis of genetic diversity among Macrophomina phaseolina (Tassi) Goid. Isolates from Euro-Asian countries. J. Plant Dis. Protect. 2019, 125, 556–573. [Google Scholar]
- Basandrai, A.K.; Pandey, A.K.; Somta, P.; Basandrai, D. Macrophomina phaseolina-host interface: Insights into an emerging dry root rot pathogen of mungbean and urdbean, and its mitigation strategies. Plant Pathol. 2021, 70, 1263–1265. [Google Scholar] [CrossRef]
- Rogers, L.W.; Koehler, A.M. Nondestructive sampling to monitor Macrophomina phaseolina root colonization in overwintering Stevia. Plant Health Progr. 2021, 22, 151–153. [Google Scholar] [CrossRef]
- Masi, M.; Sautua, F.; Zatout, R.; Castaldi, S.; Arrico, L.; Isticato, R.; Pescitelli, G.; Carmona, M.A.; Evidente, A. Phaseocyclopentenones A and B, phytotoxic penta-and tetrasubstituted cyclopentenones produced by Macrophomina phaseolina, the causal agent of charcoal rot of soybean in Argentina. J. Nat. Prod. 2021, 84, 459–465. [Google Scholar] [CrossRef]
- Bowers, G.R.; Russin, J.S.; Heatherly, L.G.; Hodges, H.F. Soybean disease management. In Soybean Production in the Midsouth, 1st ed.; Heatherly, L.G., Hodges, H.F., Eds.; CRC Press: New York, NY, USA, 1999; pp. 231–271. [Google Scholar]
- Islam, M.S.; Haque, M.S.; Islam, M.M.; Emdad, E.M.; Halim, A.; Hossen, Q.M.; Hossain, M.Z.; Ahmed, B.; Rahim, S.; Rahman, M.S.; et al. Tools to kill: Genome of one of the most destructive plant pathogenic fungi Macrophomina phaseolina. BMC Genom. 2012, 13, 493. [Google Scholar] [CrossRef]
- Twizeyimana, M.; Hill, C.B.; Pawlowski, M.; Paul, C.; Hartman, G.L. A cut-stem inoculation technique to evaluate soybean for resistance to Macrophomina phaseolina. Plant Dis. 2012, 96, 1210–1215. [Google Scholar] [CrossRef]
- Doubledee, M.D.; Rupe, J.C.; Rothrock, C.S.; Bajwa, S.G. Effect of root infection by Macrophomina phaseolina on stomatal conductance, canopy temperature and yield of soybean. Can. J. Plant Pathol. 2018, 40, 272–283. [Google Scholar] [CrossRef]
- Hemmati, P.; Zafari, D.; Mahmoodi, S.B.; Hashemi, M.; Gholamhoseini, M.; Dolatabadian, A.; Ataei, R. Histopathology of charcoal rot disease (Macrophomina phaseolina) in resistant and susceptible cultivars of soybean. Rhizosphere 2018, 7, 27–34. [Google Scholar] [CrossRef]
- Marquez, N.; Giachero, M.L.; Declerck, S.; Ducasse, D.A. Macrophomina phaseolina: General characteristics of pathogenicity and methods of control. Front. Plant Sci. 2021, 12, 634397. [Google Scholar] [CrossRef]
- Bandara, Y.M.A.Y.; Weerasooriya, D.K.; Liu, S.; Little, C.R. The necrotrophic fungus Macrophomina phaseolina promotes charcoal rot susceptibility in grain sorghum through induced host cell wall-degrading enzymes. Phytopathology 2018, 108, 948–956. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Ann. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Millet, Y.A.; Danna, C.H.; Clay, N.K.; Songnuan, W.; Simon, M.D.; Werck-Reichhart, D.; Ausubel, F.M. Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 2010, 22, 973–990. [Google Scholar] [CrossRef]
- Faris, J.D.; Zhang, Z.; Lu, H.; Lu, S.; Reddy, L.; Cloutier, S.; Fellers, J.P.; Meinhardt, S.W.; Rasmussen, J.B.; Xu, S.S.; et al. A unique wheat disease resistance-like gene governs effector-triggered susceptibility to necrotrophic pathogens. Proc. Natl. Acad. Sci. USA 2010, 107, 13544–13549. [Google Scholar] [CrossRef]
- Foley, R.C.; Kidd, B.N.; Hane, J.K.; Anderson, J.P.; Singh, K.B. Reactive oxygen species play a role in the infection of the necrotrophic fungi, Rhizoctonia solani in wheat. PLoS ONE 2016, 11, e0152548. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid—A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Sarkar, T.S.; Biswas, P.; Ghosh, S.K.; Ghosh, S. Nitric oxide production by necrotrophic pathogen Macrophomina phaseolina and the host plant in charcoal rot disease of jute: Complexity of the interplay between necrotrophy-host plant interactions. PLoS ONE 2014, 9, e107348. [Google Scholar] [CrossRef]
- Turkan, I. ROS and RNS: Key signaling molecules in plants. J. Exp. Bot. 2018, 69, 3313. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen peroxide: Its role in plant biology and crosstalk with signaling networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef] [Green Version]
- Habibi, G. Hydrogen peroxide (H2O2) generation, scavenging and signaling in plants. In Oxidative Damage to Plants: Antioxidant Networks and Signaling; Ahmad, P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 557–584. [Google Scholar]
- Nath, M.; Bhatt, D.; Prasad, R.; Gill, S.S.; Anjum, N.A.; Tuteja, N. Reactive oxygen species generation-scavenging and signaling during plant-arbuscular mycorrhizal and Piriformospora indica interaction under stress condition. Front. Plant Sci. 2016, 7, 1574. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Piasecki, C.; Chavarria, G.; Stewart, C.N.; Vargas, L. Defenses against ROS in crops and weeds: The effects of interference and herbicides. Int. J. Mol. Sci. 2019, 20, 1086. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.A.; Ahmed, H.U.; Todd, T.C.; Travers, S.E.; Zeller, K.; Leslie, J.F.; Garett, K.A. Relatedness of Macrophomina phaseolina isolates from tallgrass prairie, maize, soybean and sorghum. Mol. Ecol. 2010, 19, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.K.; Saxena, A.K.; Srivastava, A.K.; Arora, D.K. Identification and detection of Macrophomina phaseolina by using species-specific oligonucleotide primers and probe. Mycologia 2007, 99, 797–803. [Google Scholar] [CrossRef]
- AAT Bioquest, Inc. Quest GraphTM. IC50 Calculator. 2021. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 25 May 2022).
- Veljovic-Jovanovic, S.; Noctor, G.; Foyer, C.H. Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiol. Biochem. 2002, 40, 501–507. [Google Scholar] [CrossRef]
- Liu, Y.H.; Offler, C.E.; Ruan, Y.L. A simple, rapid, and reliable protocol to localize hydrogen peroxide in large plant organs by DAB-mediated tissue printing. Front. Plant Sci. 2014, 5, 745. [Google Scholar] [CrossRef]
- Cheeseman, J.M. Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 2006, 57, 2435–2444. [Google Scholar] [CrossRef]
- Krzywinski, M.; Altman, N. Visualizing samples with box plots. Nat. Meth. 2014, 11, 119–120. [Google Scholar] [CrossRef]
- Botanga, C.J.; Bethke, G.; Chen, Z.; Galillie, D.R.; Fiehn, O.; Glazebrook, J. Metabolite profiling of Arabidopsis inoculated with Alternaria brassicicola reveals that ascorbate reduces disease severity. Mol. Plant Micr. Interact. 2012, 25, 1628–1638. [Google Scholar] [CrossRef]
- Miguel, G.; Fontes, C.; Martins, D.; Neves, A.; Antunes, D. Effects of post-harvest treatment and storage time on the organic acid content in Assaria and Mollar pomegranate (Punica grantum L.) fruit. Ital. J. Food Sci. 2006, 18, 317–322. [Google Scholar]
- Khan, A.; Iqbal, I.; Shah, A.; Nawaz, H.; Ahmad, F.; Ibrahim, M. Alleviation of adverse effects of salt stress in brassica (Brassica campestris) by pre-sowing seed treatment with ascorbic acid. J. Agric. Environ. Sci. 2010, 7, 557–560. [Google Scholar]
- Naz, H.I.R.A.; Akram, N.A.; Ashraf, M. Impact of ascorbic acid on growth and some physiological attributes of cucumber (Cucumis sativus) plants under water-deficit conditions. Pak. J. Bot. 2016, 48, 877–883. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Ann. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, G.; Liu, Y.; Zhang, A.; Li, Y.; Liu, R.; Shi, L.; Jia, L.; Zhang, J. Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds. Journal Exp. Bot. 2012, 63, 1809–1822. [Google Scholar] [CrossRef]
- Khan, A.; Ashraf, M. Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ. Exp. Bot. 2008, 63, 224–231. [Google Scholar]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Ketsa, S. Cross reactivity between ascorbate peroxidase and phenol (guaiacol) peroxidase. Postharv. Biol. Technol. 2014, 95, 64–69. [Google Scholar] [CrossRef]
- Amin, B.; Mahleghah, G.; Mahmood, H.M.R.; Hossein, M. Evaluation of interaction effect of drought stress with ascorbate and salicylic acid on some of physiological and biochemical parameters in okra (Hibiscus esculentus L.). Res. J. Biol. Sci. 2009, 4, 380–387. [Google Scholar]
- Chowdhury, S.; Basu, A.; Kundu, S. Biotrophy-necrotrophy switch in pathogen evoke differential response in resistant and susceptible sesame involving multiple signaling pathways at different phases. Sci. Rep. 2017, 7, 17251. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska, D.; Zagdańska, B. ROS as signaling molecules and enzymes of plant response to unfavorable environmental conditions. In Oxidative Stress–Molecular Mechanisms and Biological Effects; In-Tech Publishers: Rijeka, Croatia, 2012; pp. 341–362. [Google Scholar]
- Nandini, Y.; Samir, S. Reactive oxygen species, oxidative stress and ROS scavenging system in plants. J. Chem. Pharm. Res. 2016, 8, 595–604. [Google Scholar]
- Maurino, V.G.; Flügge, U.-I. Experimental systems to assess the effects of reactive oxygen species in plant tissues. Plant Signal. Behav. 2008, 3, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Singh, L.P.; Gill, R.; Tuteja, N. Generation and scavenging of reactive oxygen species in plants under stress. In Improving Crop Resistance to Abiotic Stress; Tuteja, N., Gill, S.S., Tiburcio, A.F., Tuteja, R., Eds.; Wiley-Blackwell: New York, NY, USA, 2013; pp. 46–69. [Google Scholar]
- Chung, I.M.; Venkidasamy, B.; Upadhyaya, C.P.; Packiaraj, G.; Rajakumar, G.; Thiruvengadam, M. Alleviation of Phytophthora infestans mediated necrotic stress in the transgenic potato (Solanum tuberosum L.) with enhanced ascorbic acid accumulation. Plants 2019, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Govrin, E.M.; Levine, A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 2000, 10, 751–757. [Google Scholar] [CrossRef]
- Tsugane, K.; Kobayashi, K.; Niwa, Y.; Ohba, Y.; Wada, K.; Kobayashi, H. A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 1999, 11, 1195–1206. [Google Scholar] [CrossRef]
- El Hariri, D.M.; Sadak, M.S.; El-Bassiouny, H.M.S. Response of flax cultivars to ascorbic acid and α-tocophorol under salinity stress conditions. Int. J. Acad. Res. 2010, 2, 101–109. [Google Scholar]
- Shao, H.B.; Chu, L.Y.; Lu, Z.H.; Kang, C.M. Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int. J. Biol. Sci. 2008, 4, 8. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2021, 109, 373–379. [Google Scholar] [CrossRef]


| Pre-Treatment (Day −1) | Treatment(s) (Day +0) |
|---|---|
| No pre-treatment | Agar plug Inoculate w/MP 1 Hydrogen peroxide (H2O2) |
| Agar plug | L-ascorbic acid (LAA; reduced) Dehydroascorbic acid (DHAA; oxidized) |
| Inoculate w/MP | LAA DHAA |
| LAA | Agar plug Inoculate w/MP |
| DHAA | Agar plug Inoculate w/MP |
| H2O2 | Inoculate w/MP |
| Source | DF | D1 | D2 | D3 | D4 | D5 | |
|---|---|---|---|---|---|---|---|
| Model | 31 | F | 37.89 | 62.52 | 160.95 | 172.64 | 34.51 |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Replication | 2 | F | 1.16 | 0.96 | 1.59 | 3.58 | 0.21 |
| P | 0.3208 | 0.3891 | 0.2128 | 0.0341 | 0.8073 | ||
| Isolate (I) | 2 | F | 5.23 | 7.25 | 25.77 | 37.01 | 114.24 |
| P | 0.0082 | 0.0015 | <0.0001 | <0.0001 | <0.0001 | ||
| Concentration (C) | 9 | F | 120.26 | 210.35 | 542.98 | 580.33 | 114.24 |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| I x C | 18 | F | 4.42 | 1.59 | 2.66 | 2.64 | 1.30 |
| P | <0.0001 | 0.0933 | 0.0025 | 0.0027 | 0.2244 |
| Source | DF 1 | SS | MS | F | P |
|---|---|---|---|---|---|
| Model | 5 | 20,299.731 | 4059.946 | 17.00 | 0.0017 |
| Replication | 2 | 426.162 | 213.081 | 0.89 | 0.4580 |
| Treatment | 3 | 19,873.569 | 6624.523 | 27.73 | 0.0006 |
| Error | 6 | 1433.281 | 238.880 | ||
| Total | 11 | 21,733.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, A.; Little, C.R. Evaluating the Role of Exogenously Applied Ascorbic Acid in Rescuing Soybean Plant Health in The Presence of Pathogen-Induced Oxidative Stress. Pathogens 2022, 11, 1117. https://doi.org/10.3390/pathogens11101117
Noor A, Little CR. Evaluating the Role of Exogenously Applied Ascorbic Acid in Rescuing Soybean Plant Health in The Presence of Pathogen-Induced Oxidative Stress. Pathogens. 2022; 11(10):1117. https://doi.org/10.3390/pathogens11101117
Chicago/Turabian StyleNoor, Afsana, and Christopher R. Little. 2022. "Evaluating the Role of Exogenously Applied Ascorbic Acid in Rescuing Soybean Plant Health in The Presence of Pathogen-Induced Oxidative Stress" Pathogens 11, no. 10: 1117. https://doi.org/10.3390/pathogens11101117






