Treponema denticola Induces Neuronal Apoptosis by Promoting Amyloid-β Accumulation in Mice
Abstract
:1. Introduction
2. Results
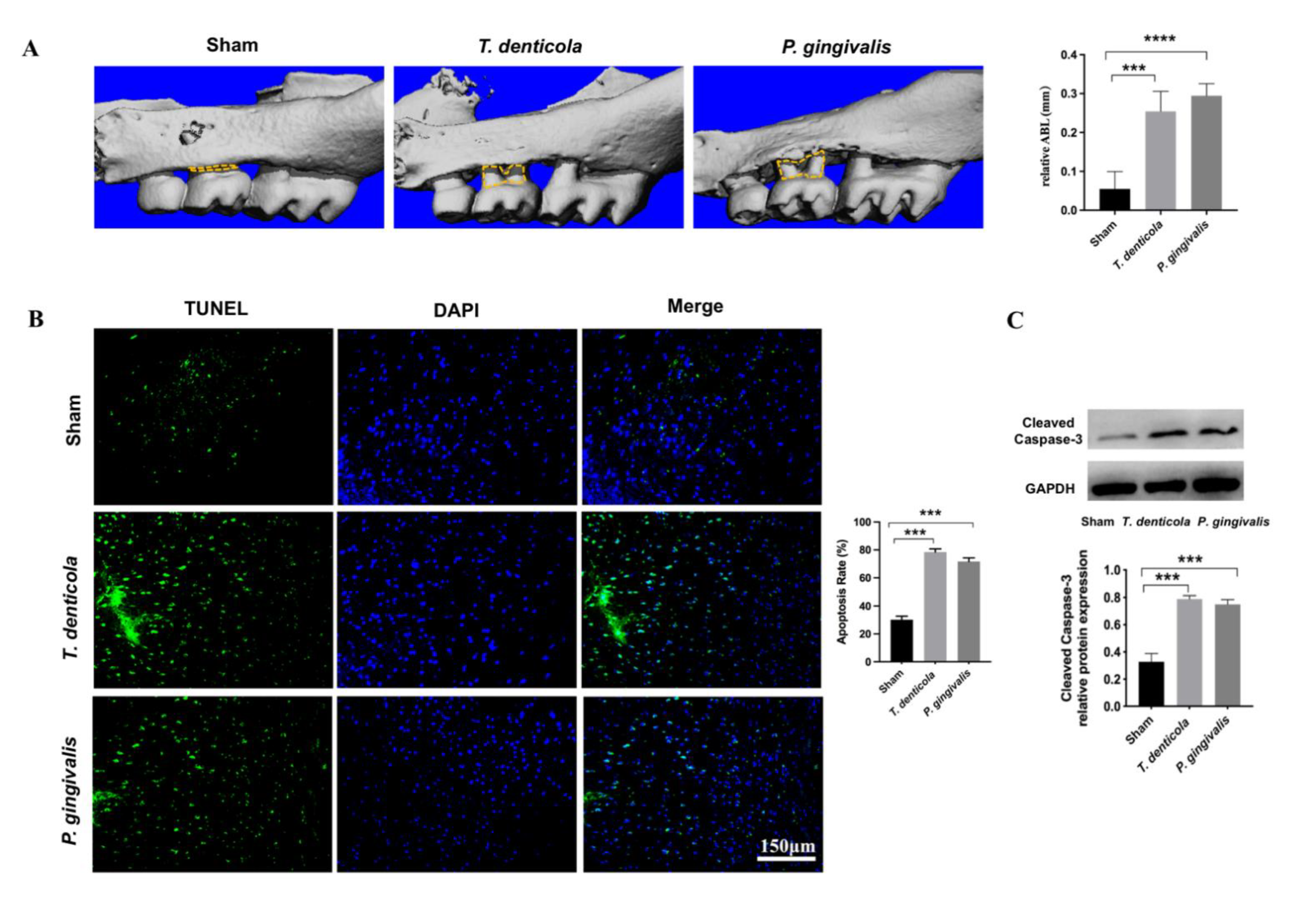
2.1. T. denticola Induced Alveolar Bone Resorption and Neuronal Apoptosis in the Mouse Hippocampi
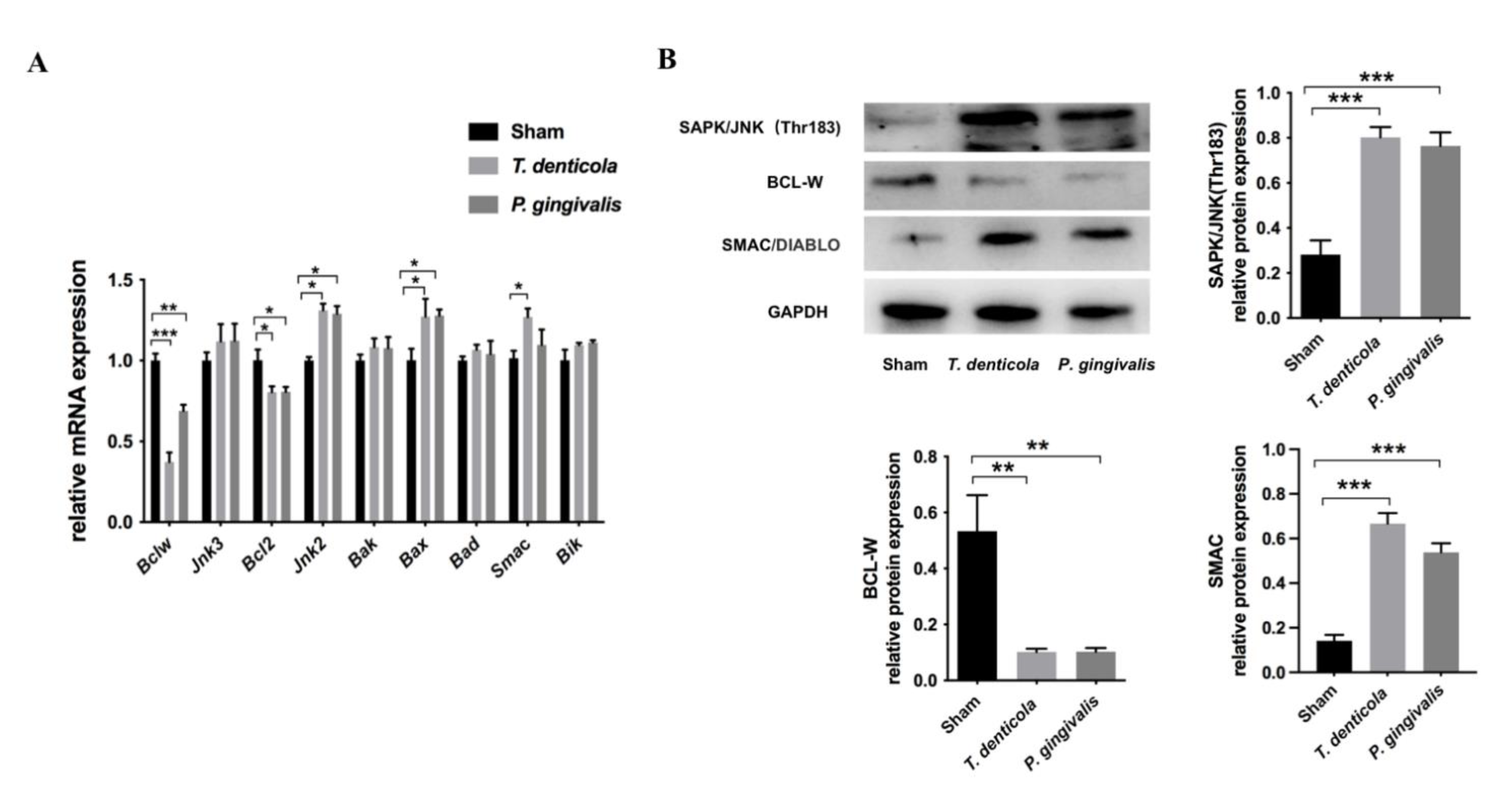
2.2. T. denticola Oral Infection Regulated the Expressions of Apoptosis-Associated Genes and Proteins
2.3. Amyloid-β1–42 Mediated T. denticola Infection-Induced Neuronal Apoptosis
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Mouse Treatment
4.3. PCR
4.4. Measurement of Alveolar Bone Loss
4.5. Cell Culture and Treatment
4.6. TUNEL Staining
4.7. Western Blotting
4.8. qRT-PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hardy, J. A hundred years of Alzheimer’s disease research. Neuron 2006, 52, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collaborators, G.B.D.D. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar]
- Hodson, R. Alzheimer’s disease. Nature 2018, 559, S1. [Google Scholar] [CrossRef] [Green Version]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Kaushal, V.; Dye, R.; Pakavathkumar, P.; Foveau, B.; Flores, J.; Hyman, B.; Ghetti, B.; Koller, B.H.; LeBlanc, A.B. Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ 2015, 22, 1676–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol 2000 2017, 75, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ren, J.; Yu, H.; Yu, W.; Zhou, Y. Porphyromonas gingivalis, a periodontitis causing bacterium, induces memory impairment and age-dependent neuroinflammation in mice. Immun. Ageing 2018, 15, 6. [Google Scholar] [CrossRef] [Green Version]
- Ide, M.; Harris, M.; Stevens, A.; Sussams, R.; Hopkins, V.; Culliford, D.; Fuller, J.; Ibbett, P.; Raybould, R.; Thomas, R.; et al. Periodontitis and Cognitive Decline in Alzheimer’s Disease. PLoS ONE 2016, 11, e0151081. [Google Scholar] [CrossRef] [Green Version]
- Kamer, A.R.; Craig, R.G.; Dasanayake, A.P.; Brys, M.; Glodzik-Sobanska, L.; de Leon, M.J. Inflammation and Alzheimer’s disease: Possible role of periodontal diseases. Alzheimers Dement. 2008, 4, 242–250. [Google Scholar] [CrossRef]
- Kamer, A.R.; Craig, R.G.; Niederman, R.; Fortea, J.; de Leon, M.J. Periodontal disease as a possible cause for Alzheimer’s disease. Periodontol 2000 2020, 83, 242–271. [Google Scholar] [CrossRef]
- Iwasaki, M.; Kimura, Y.; Ogawa, H.; Yamaga, T.; Ansai, T.; Wada, T.; Sakamoto, R.; Ishimoto, Y.; Fujisawa, M.; Okumiya, K.; et al. Periodontitis, periodontal inflammation, and mild cognitive impairment: A 5-year cohort study. J. Periodontal Res. 2019, 54, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kamer, A.R.; Pirraglia, E.; Tsui, W.; Rusinek, H.; Vallabhajosula, S.; Mosconi, L.; Yi, L.; McHugh, P.; Craig, R.G.; Svetcov, S.; et al. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol. Aging 2015, 36, 627–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marcyzk, A.; Konradi, A.; Nguyn, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilievski, V.; Zuchowska, P.K.; Green, S.J.; Toth, P.T.; Ragozzino, M.E.; Le, K.; Aljewari, H.W.; O’Brien-Simpson, N.; Reynolds, E.C.; Watanabe, K. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS ONE 2018, 13, e0204941. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Liang, D.; Cheng, M.; Su, X.; Liu, R.; Zhang, Y.; Wu, H. Effects of Porphyromonas gingivalis and Its Underlying Mechanisms on Alzheimer-Like Tau Hyperphosphorylation in Sprague-Dawley Rats. J. Mol. Neurosci. 2021, 71, 89–100. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; . Guo, J.; Sun, J.; Sun, Q. Salvianolic Acid B improves cognitive impairment by inhibiting neuroinflammation and decreasing Abeta level in Porphyromonas gingivalis-infected mice. Aging 2020, 12, 10117–10128. [Google Scholar] [CrossRef]
- Miklossy, J. Alzheimer’s disease—A spirochetosis? Neuroreport 1993, 4, 841–848. [Google Scholar] [CrossRef]
- Miklossy, J. Alzheimer’s disease—A neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J. Neuroinflamm. 2011, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Riviere, G.R.; Riviere, K.H.; Smith, K.S. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol. Immunol. 2002, 17, 113–118. [Google Scholar] [CrossRef]
- Su, X.; Tang, Z.; Lu, Z.; Liu, Y.; He, W.; Jiang, J.; Zhang, Y.; Wu, H. Oral Treponema denticola Infection Induces Aβ1–40 and Aβ1–42 Accumulation in the Hippocampus of C57BL/6 Mice. J. Mol. Neurosci. 2021, 71, 1506–1514. [Google Scholar] [CrossRef]
- Tang, Z.; Cheng, X.; Su, X.; Wu, L.; Cai, Q.; Wu, H. Treponema denticola Induces Alzheimer-Like Tau Hyperphosphorylation by Activating Hippocampal Neuroinflammation in Mice. J. Dent. Res. 2022, 101, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.B.; Bonni, A. Cell cycle regulation of neuronal apoptosis in development and disease. Prog. Neurobiol. 2004, 72, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.M., Jr. Possible role of neuronal apoptosis in Alzheimer’s disease. Neurobiol. Aging 1994, 15, S187–S189. [Google Scholar] [CrossRef]
- Gosztyla, M.L.; Brothers, H.M.; Robinson, S.R. Alzheimer’s Amyloid-beta is an Antimicrobial Peptide: A Review of the Evidence. J. Alzheimers Dis. 2018, 62, 1495–1506. [Google Scholar] [CrossRef] [Green Version]
- Wirths, O.; Zampar, S. Emerging roles of N- and C-terminally truncated Aβ species in Alzheimer’s disease. Expert Opin. Ther. Targets 2019, 23, 991–1004. [Google Scholar] [CrossRef]
- Liu, T.; Wang, F.; LePochat, P.; Woo, J.-A.A.; Bukhari, M.Z.; Hong, K.W.; Trotter, C.; Kang, D.E. Cofilin-mediated Neuronal Apoptosis via p53 Translocation and PLD1 Regulation. Sci. Rep. 2017, 7, 11532. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-K.; Ma, P.-L.; Ji, S.-Y.; Zhao, X.-L.; Tan, J.-X.; Sun, X.-J.; Huang, F.-D. Age-dependent alterations in the presynaptic active zone in a Drosophila model of Alzheimer’s disease. Neurobiol. Dis. 2013, 51, 161–167. [Google Scholar] [CrossRef]
- Arbor, S.C.; LaFontaine, M.; Cumbay, M. Amyloid-beta Alzheimer targets—protein processing, lipid rafts, and amyloid-beta pores. Yale J. Biol. Med. 2016, 89, 5–21. [Google Scholar]
- Lesné, S.; Koh, M.T.; Kotilinek, L.; Kayed, R.; Glabe, C.G.; Yang, A.; Gallagher, M.; Ashe, K.H. A specific amyloid-β protein assembly in the brain impairs memory. Nature 2006, 440, 352–357. [Google Scholar] [CrossRef]
- Noguchi, H.; Moore, J.W. A Demonstration of Treponema Pallidum in the Brain in Cases of General Paralysis. J. Exp. Med. 1913, 17, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Gatz, M.; Mortimer, J.A.; Fratiglioni, L.; Johansson, B.; Berg, S.; Reynolds, C.A.; Pedersen, N.L. Potentially modifiable risk factors for dementia in identical twins. Alzheimers Dement. 2006, 2, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S. Interleukin 1 genetics, inflammatory mechanisms, and nutrigenetic opportunities to modulate diseases of aging. Am. J. Clin. Nutr. 2006, 83, 475S–483S. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.B. A Novel Approach to the Treatment and Prevention of Alzheimer’s Disease Based on the Pathology and Microbiology. J. Alzheimers Dis. 2021, 84, 61–67. [Google Scholar] [CrossRef]
- Su, J.H.; Anderson, A.J.; Cummings, B.J.; Cotman, C.W. Immunohistochemical evidence for apoptosis in Alzheimer’s disease. Neuroreport 1994, 5, 2529–2533. [Google Scholar] [CrossRef]
- Lucassen, P.J.; Chung, W.C.; Kamphorst, W.; Swaab, D.F. DNA damage distribution in the human brain as shown by in situ end labeling; area-specific differences in aging and Alzheimer disease in the absence of apoptotic morphology. J. Neuropathol. Exp. Neurol. 1997, 56, 887–900. [Google Scholar] [CrossRef] [Green Version]
- Obulesu, M.; Lakshmi, M.J. Apoptosis in Alzheimer’s disease: An understanding of the physiology, pathology and therapeutic avenues. Neurochem. Res. 2014, 39, 2301–2312. [Google Scholar] [CrossRef]
- Hugon, J.; Terro, F.; Esclaire, F.; Yardin, C. Markers of apoptosis and models of programmed cell death in Alzheimer’s disease. J. Neural. Transm. Suppl. 2000, 59, 125–131. [Google Scholar] [PubMed]
- Masliah, E.; Mallory, M.; Alford, M.; Tanaka, S.; Hansen, L.A. Caspase dependent DNA fragmentation might be associated with excitotoxicity in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1998, 57, 1041–1052. [Google Scholar] [CrossRef]
- Volkmann, N.; Marassi, F.M.; Newmeyer, D.D.; Hanein, D. The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ 2014, 21, 206–215. [Google Scholar] [CrossRef]
- Zaitoun, I.S.; Wintheiser, C.M.; Jamali, N.; Wang, S.; Suscha, A.; Darjatmoko, S.R.; Schlek, K.; Hanna, B.A.; Lindner, V.; Sheibani, N.; et al. Bcl-2 Expression in Pericytes and Astrocytes Impacts Vascular Development and Homeostasis. Sci. Rep. 2019, 9, 9700. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Wang, Y.; Ogawa, O.; Lee, H.; Raina, A.K.; Siedlek, S.L.; Harris, P.L.R.; Fujioka, H.; Shimohama, S.; Tabaton, M.; et al. Neuroprotective properties of Bcl-w in Alzheimer disease. J. Neurochem. 2004, 89, 1233–1240. [Google Scholar] [CrossRef]
- Hartman, M.L.; Czyz, M. BCL-w: Apoptotic and non-apoptotic role in health and disease. Cell Death Dis 2020, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Repici, M.; Centeno, C.; Tomasi, S.; Forloni, G.; Bonny, C.; Vercelli, A.; Borsello, T. Time-course of c-Jun N-terminal kinase activation after cerebral ischemia and effect of D-JNKI1 on c-Jun and caspase-3 activation. Neuroscience 2007, 150, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Spigolon, G.; Veronesi, C.; Bonny, C.; Vercelli, A. c-Jun N-terminal kinase signaling pathway in excitotoxic cell death following kainic acid-induced status epilepticus. Eur. J. Neurosci. 2010, 31, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Dibling, B. The true face of JNK activation in apoptosis. Aging Cell 2002, 1, 112–116. [Google Scholar] [CrossRef]
- Yao, M.; Nguyen, T.V.; Pike, C.J. Beta-amyloid-induced neuronal apoptosis involves c-Jun N-terminal kinase-dependent downregulation of Bcl-w. J. Neurosci. 2005, 25, 1149–1158. [Google Scholar] [CrossRef] [Green Version]
- Marques, C.A.; Keil, U.; Bonert, A.; Steiner, B.; Haass, C.; Muller, W.E.; Eckert, A. Neurotoxic mechanisms caused by the Alzheimer’s disease-linked Swedish amyloid precursor protein mutation: Oxidative stress, caspases, and the JNK pathway. J. Biol. Chem. 2003, 278, 28294–28302. [Google Scholar] [CrossRef] [Green Version]
- Sahara, N.; Murayama, M.; Lee, B.; Park, J.-M.; Lagalwar, S.; Binder, L.I.; Takashima, A. Active c-jun N-terminal kinase induces caspase cleavage of tau and additional phosphorylation by GSK-3beta is required for tau aggregation. Eur. J. Neurosci. 2008, 27, 2897–2906. [Google Scholar] [CrossRef]
- Brenner, D.; Mak, T.W. Mitochondrial cell death effectors. Curr. Opin. Cell Biol. 2009, 21, 871–877. [Google Scholar] [CrossRef]
- Adrain, C.; Creagh, E.M.; Martin, S.J. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 2001, 20, 6627–6636. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.L.; Olsson, T.; Johansson, I.M.; Brännström, T.; Wester, P. Dynamic changes of the anti- and pro-apoptotic proteins Bcl-w, Bcl-2, and Bax with Smac/Diablo mitochondrial release after photothrombotic ring stroke in rats. Eur. J. Neurosci. 2004, 20, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T.; Bieger, S.C.; Bruhl, B.; Tienari, P.J.; Ida, N.; Allsop, D.; Roberts, G.W.; Masters, C.L.; Dotti, C.G.; Unsicker, K.; et al. Distinct sites of intracellular production for Alzheimer’s disease A beta40/42 amyloid peptides. Nat. Med. 1997, 3, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Bathini, P.; Foucras, S.; Dupanloup, I.; Imeri, H.; Perna, A.; Berreux, J.-L.; Doucey, M.-A.; Annoni, J.-M.; Alberi, L.A. Classifying dementia progression using microbial profiling of saliva. Alzheimers Dement. 2020, 12, e12000. [Google Scholar] [CrossRef] [PubMed]
- Tzanoulinou, S.; Brandi, R.; Arisi, I.; D’Onofrio, M.; Urfer, S.M.; Sandi, C.; Constam, D.; Capsoni, S. Pathogen-free husbandry conditions alleviate behavioral deficits and neurodegeneration in AD10 anti-NGF mice. J. Alzheimers Dis. 2014, 38, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Loeb, M.B.; Molloy, D.W.; Smieja, M.; Standish, T.; Goldsmith, C.H.; Mahony, J.; Smith, S.; Borrie, M.; Decoteau, E.; Davidson, W.; et al. A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer’s disease. J. Am. Geriatr. Soc. 2004, 52, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Sun, C.; Zheng, M.; Liu, S.; Shi, R. Amentoflavone suppresses amyloid beta1-42 neurotoxicity in Alzheimer’s disease through the inhibition of pyroptosis. Life Sci. 2019, 239, 117043. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Vecino, B.; Fernández-Novoa, L.; Álvarez, A.; Cacabelos, R. Neuroprotective role of S12024 against neurodegeneration in the rat dentate gyrus. Eur Neuropsychopharmacol 1998, 8, 203–208. [Google Scholar] [CrossRef]
- Viola, K.L.; Klein, W.L. Amyloid beta oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015, 129, 183–206. [Google Scholar] [CrossRef]
- Xu, S.; Zhong, M.; Zhang, L.; Wang, Y.; Zhou, Z.; Hao, Y.; Zhang, W.; Yang, X.; Wei, A.; Pei, L.; et al. Overexpression of Tfam protects mitochondria against beta-amyloid-induced oxidative damage in SH-SY5Y cells. FEBS J. 2009, 276, 3800–3809. [Google Scholar] [CrossRef]
- Wang, X.T.; Pei, D.S.; Xu, J.; Guan, Q.-H.; Sun, Y.-F.; Liu, X.-M.; Zhang, G.-Y. Opposing effects of Bad phosphorylation at two distinct sites by Akt1 and JNK1/2 on ischemic brain injury. Cell Signal 2007, 19, 1844–1856. [Google Scholar] [CrossRef]
- Longpre, F.; Garneau, P.; Christen, Y.; Ramassamy, C. Protection by EGb 761 against beta-amyloid-induced neurotoxicity: Involvement of NF-kappaB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free Radic. Biol. Med. 2006, 41, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Nagano, K.; Sugiura, S.; Hagiwara, M.; Tanigawa, N.; Abiko, Y.; Yoshimura, F.; Furuichi, Y.; Matsushita, K. E-selectin mediates Porphyromonas gingivalis adherence to human endothelial cells. Infect. Immun. 2012, 80, 2570–2576. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cheng, L.; Liu, D.; Wang, J.; Zhang, X.; Shu, R.; Liang, J. Role of p38 mitogen-activated protein kinase pathway in Porphyromonas gingivalis lipopolysaccharide-induced VCAM-1 expression in human aortic endothelial cells. J. Periodontol. 2012, 83, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Liu, Y.; Huang, W.; Qing, H.; Kadowaki, T.; Kashiwazaki, H.; Ni, J.; Wu, Z. Receptor for advanced glycation end products up-regulation in cerebral endothelial cells mediates cerebrovascular-related amyloid beta accumulation after Porphyromonas gingivalis infection. J. Neurochem. 2021, 158, 724–736. [Google Scholar] [CrossRef]
- Kouki, M.A.; Pritchard, A.B.; Alder, J.E.; Crean, S. Do Periodontal Pathogens or Associated Virulence Factors Have a Deleterious Effect on the Blood-Brain Barrier, Contributing to Alzheimer’s Disease? J. Alzheimers Dis. 2022, 85, 957–973. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Rivera, M.F.; Chukkapalli, S.S.; Velsko, I.M.; Lee, J.-Y.; Bhattacharyya, I.; Dolce, C.; Toro, E.J.; Holliday, S.; Kesavalu, L. Bis-enoxacin blocks rat alveolar bone resorption from experimental periodontitis. PLoS ONE 2014, 9, e92119. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, S.; Sharma, R.; Gupta, S.; Cakar, Z.; de Geyter, C.; Agarwal, A. Inter- and intra-laboratory standardization of TUNEL assay for assessment of sperm DNA fragmentation. Andrology 2017, 5, 477–485. [Google Scholar] [CrossRef] [Green Version]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]



| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Amplicon Size | Genebank Accession Number |
|---|---|---|---|---|
| Bcl-2 | GAGAGCGTCAACAGGGAGATG | CCAGCCTCCGTTATCCTGGA | 108 bp | AH001858 |
| Bcl-w | ACTGAACAGGGTTTTGTGACTT | CCAGTTATTCCCCTTAGCAAGGT | 105 bp | U59746 |
| Bax | CCGGCGAATTGGAGATGAACT | CCAGCCCATGATGGTTCTGAT | 229 bp | AB029557 |
| Bak | CAGCTTGCTCTCATCGGAGAT | GGTGAAGAGTTCGTAGGCATTC | 108 bp | Y13231 |
| Bad | TGAGCCGAGTGAGCAGGAA | GCCTCCATGATGACTGTTGGT | 154 bp | NM_001285453 |
| Bik | ACGTGGACCTCATGGAGTG | TGTGTATAGCAATCCCAGGCA | 129 bp | NM_007546 |
| Smac | TCTTGGCTAACTCTAAGAAACGC | TGCTTCGTTACTGAGAGACTGA | 140 bp | NM_023232 |
| Jnk2 | AGAACCAAACGCACGCAAAG | GCTGAATGCAGATGCTTGATG | 250 bp | AB005664 |
| Jnk3 | CCATGTCTGTGTTCTTTCTCACG | TTGGTTCCAACTGTGAAGAGTC | 118 bp | AB005665 |
| Gapdh | TGGCCTTCCGTGTTCCTAC | GAGTTGCTGTTGAAGTCGCA | 178 bp | AY618199 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Su, X.; Tang, Z.; Jian, L.; Zhu, H.; Cheng, X.; Wu, H. Treponema denticola Induces Neuronal Apoptosis by Promoting Amyloid-β Accumulation in Mice. Pathogens 2022, 11, 1150. https://doi.org/10.3390/pathogens11101150
Wu L, Su X, Tang Z, Jian L, Zhu H, Cheng X, Wu H. Treponema denticola Induces Neuronal Apoptosis by Promoting Amyloid-β Accumulation in Mice. Pathogens. 2022; 11(10):1150. https://doi.org/10.3390/pathogens11101150
Chicago/Turabian StyleWu, Linrui, Xinyi Su, Zhiqun Tang, Lixiang Jian, He Zhu, Xingqun Cheng, and Hongkun Wu. 2022. "Treponema denticola Induces Neuronal Apoptosis by Promoting Amyloid-β Accumulation in Mice" Pathogens 11, no. 10: 1150. https://doi.org/10.3390/pathogens11101150
APA StyleWu, L., Su, X., Tang, Z., Jian, L., Zhu, H., Cheng, X., & Wu, H. (2022). Treponema denticola Induces Neuronal Apoptosis by Promoting Amyloid-β Accumulation in Mice. Pathogens, 11(10), 1150. https://doi.org/10.3390/pathogens11101150






