Fungal Rhinosinusitis Caused by a Curvularia sp. Infection in a Female Sumatran Orangutan: A Case Report
Abstract
:1. Introduction
2. Material, Methods, and Results
2.1. Case Description and Anesthesia
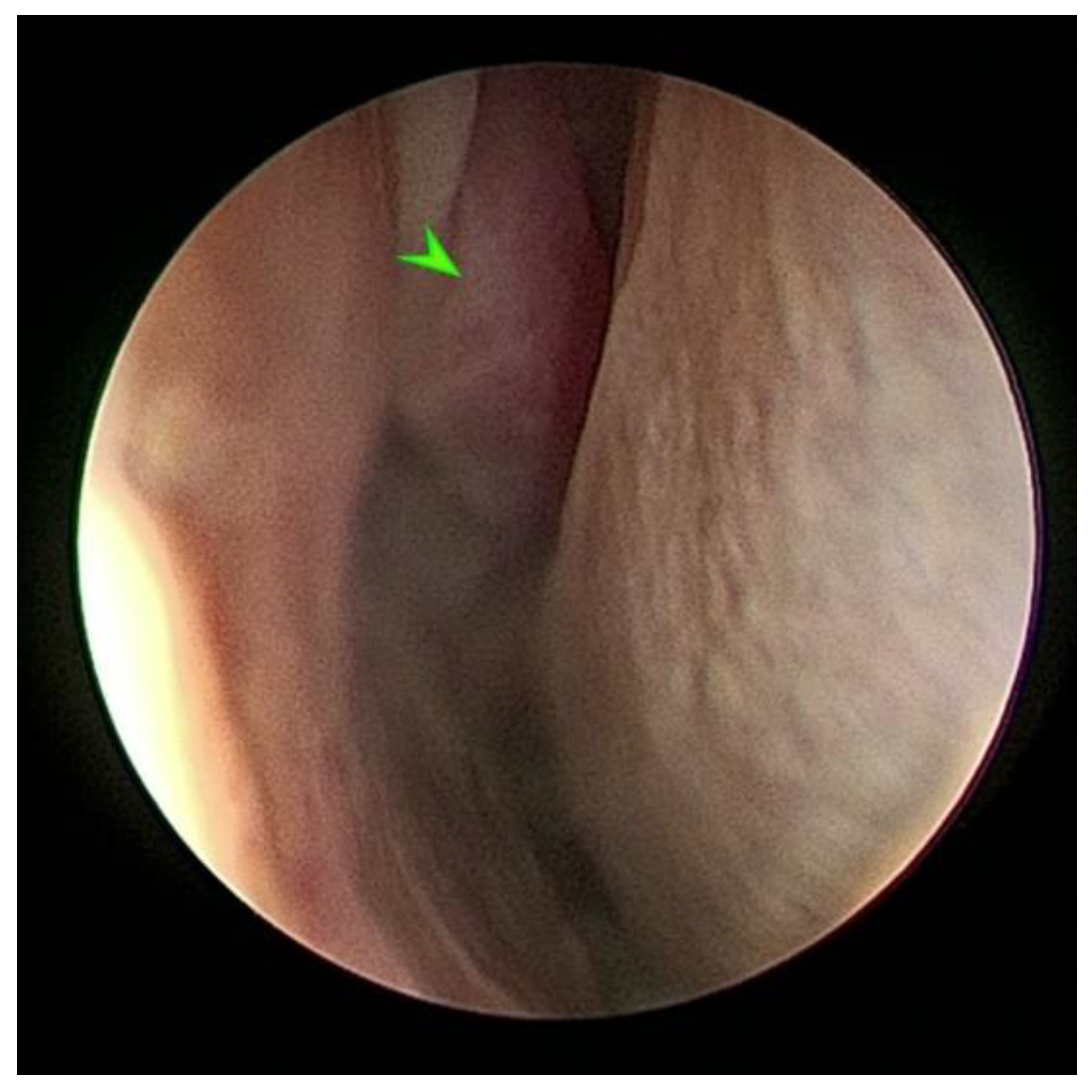
2.2. Endoscopic Sinus Surgery with Biopsy and Tissue Histopathology
2.3. Computerized Tomography Imaging
2.4. Peripheral Blood Lymphocytes and Serum Viral Antibody Analysis and Cryptococcus Antigen Detection
2.5. Endoscopic Sinus Surgery with Fungal Mass Excision, Fungal Identification and Medical Treatment
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemaitre, J.; Naninck, T.; Delache, B.; Creppy, J.; Huber, P.; Holzapfel, M.; Bouillier, C.; Contreras, V.; Martinon, F.; Kahlaoui, N.; et al. Non-human primate models of human respiratory infections. Mol. Immunol. 2021, 135, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Blaney, S.P. An allometric study of the frontal sinus in Gorilla, Pan and Pongo. Folia Primatol. 1986, 47, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Lowenstine, L.J.; Osborn, K.G. Respiratory System Diseases of Nonhuman Primates. Nonhum. Primates Biomed. Res. 2012, Volume 3, 413–481. [Google Scholar] [CrossRef]
- Hewitt, G.; MacLarnon, A.; Jones, K.E. The functions of laryngeal air sacs in primates: A new hypothesis. Folia Primatol. 2002, 73, 70–94. [Google Scholar] [CrossRef] [PubMed]
- Fox, M. Respiratory Disease in the Northe Ameraican Captive Orangutan Population; California State University: Fullerton, CA, USA, 2017. [Google Scholar]
- Zimmermann, N.; Pirovino, M.; Zingg, R.; Clauss, M.; Kaup, F.J.; Heistermann, M.; Hatt, J.M.; Steinmetz, H.W. Upper respiratory tract disease in captive orangutans (Pongo sp.): Prevalence in 20 European zoos and predisposing factors. J. Med. Primatol. 2011, 40, 365–375. [Google Scholar] [CrossRef]
- Strong, V.J.; Grindlay, D.; Redrobe, S.; Cobb, M.; White, K. A Systematic Review of the Literature Relating to Captive Great Ape Morbidity and Mortality. J. Zoo Wildl. Med. 2016, 47, 697–710. [Google Scholar] [CrossRef]
- Buitendijk, H.; Fagrouch, Z.; Niphuis, H.; Bogers, W.M.; Warren, K.S.; Verschoor, E.J. Retrospective serology study of respiratory virus infections in captive great apes. Viruses 2014, 6, 1442–1453. [Google Scholar] [CrossRef]
- Chandler, F.W.; McClure, H.M.; Campbell, W.G.; Watts, J.C. Pulmonary pneumocystosis in nonhuman primates. Arch. Pathol. Lab. Med. 1976, 100, 163–167. [Google Scholar]
- Furuta, T. Severe pulmonary pneumocystosis in simian acquired immunodeficiency syndrome induced by simian immunodeficiency virus. J. Eukaryot. Microbiol. 1997, 44, 52S. [Google Scholar] [CrossRef]
- Johnson, A.L.; Lewis, A.D. Two cases of cryptococcus gattii infection in the rhesus macaque (Macaca Mulatta). J. Med. Primatol. 2021, 50, 67–70. [Google Scholar] [CrossRef]
- Koistinen, K.; Mullaney, L.; Bell, T.; Zaki, S.; Nalca, A.; Frick, O.; Livingston, V.; Robinson, C.G.; Estep, J.S.; Batey, K.L.; et al. Coccidioidomycosis in Nonhuman Primates: Pathologic and Clinical Findings. Vet. Pathol. 2018, 55, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Wachtman, L.M.; Mansfield, K.G. Opportunistic infections in immunologically compromised nonhuman primates. ILAR J. 2008, 49, 191–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasikasin, V.; Nasomsong, W.; Srisuttiyakorn, C.; Mitthamsiri, W.; Oer-Areemitr, N.; Changpradub, D. Disseminated Phaeohyphomycosis Caused by Curvularia tuberculata in a Previously Healthy Man. Mycopathologia 2019, 184, 321–325. [Google Scholar] [CrossRef]
- Killingsworth, S.M.; Wetmore, S.J. Curvularia/Drechslera sinusitis. Laryngoscope 1990, 100, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Protocol, Hematoxylin and Eosin-Automated Method; Western Diagnostic Pathology: Jandakot, WA, Australia. Available online: https://www.wdp.com.au/ (accessed on 28 February 2022).
- Protocol, Methenamine Silver for Fungi and Pneumocystis carinii; Western Diagnostic Pathologyy: Jandakot, WA, Australia. Available online: https://www.wdp.com.au/guides-publications/ (accessed on 28 February 2021).
- Aronson, R.K.; Sriningsih, A.P.; Sulistyo, F.; Taylor-Cousar, J.L.; Aronson, S.A.; South, A.; Nutter, F.; Lung, N.P. Use of Computed Tomography (Ct) to Determine the Sensitivity of Clinical Signs as a Diagnostic Tool for Respiratory Disease in Bornean Orangutans (Pongo pygmaeus). J. Zoo Wildl. Med. 2021, 52, 470–478. [Google Scholar] [CrossRef] [PubMed]
- D’Orsogna, L.J.; Wright, M.P.; Krueger, R.G.; McKinnon, E.J.; Buffery, S.I.; Witt, C.S.; Staples, N.; Loh, R.; Cannell, P.K.; Christiansen, F.T.; et al. Allogeneic hematopoietic stem cell transplantation recipients have defects of both switched and igm memory B cells. Biol. Blood Marrow. Transplant 2009, 15, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.S.; Rosa-Bray, M.; Staats, J.; Zakroysky, P.; Chan, C.; Russo, M.A.; Dumbauld, C.; White, S.; Gierman, T.; Weinhold, K.J.; et al. Establishment of normative ranges of the healthy human immune system with comprehensive polychromatic flow cytometry profiling. PLoS ONE 2019, 14, e0225512. [Google Scholar] [CrossRef] [Green Version]
- McClure, H.M.; Keeling, M.E.; Guilloud, N.B. Hematologic and blood chemistry data for the gorilla (Gorilla gorilla). Folia Primatol. 1972, 18, 300–316. [Google Scholar] [CrossRef]
- Qiu, C.L.; Zhao, H.; Yang, G.B.; Liu, Q.; Shao, Y. Flow cytometric characterization of T lymphocyte subsets in the peripheral blood of Chinese rhesus macaques: Normal range, age- and sex-related differences. Vet. Immunol. Immunopathol. 2008, 124, 313–321. [Google Scholar] [CrossRef]
- Principles and Procedures for Detection of Fungi in Clinical Specimens-Direct Examination and Culture: Approved Guideline. M54-A; Clinical and Laboratory Sciences Institute: Hongkong, China, 2012. Available online: https://clsi.org/standards/products/microbiology/documents/m54/ (accessed on 12 February 2016).
- Pryce, T.M.; Palladino, S.; Kay, I.D.; Coombs, G.W. Rapid identification of fungi by sequencing the ITS1 and ITS2 regions using an automated capillary electrophoresis system. Med. Mycol. 2003, 41, 369–381. [Google Scholar] [CrossRef]
- Irinyi, L.; Serena, C.; Garcia-Hermoso, D.; Arabatzis, M.; Desnos-Ollivier, M.; Vu, D.; Cardinali, G.; Arthur, I.; Normand, A.C.; Giraldo, A.; et al. International Society of Human and Animal Mycology (ISHAM)-ITS reference DNA barcoding database--the quality controlled standard tool for routine identification of human and animal pathogenic fungi. Med. Mycol. 2015, 53, 313–337. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Ro, J.Y.; el-Naggar, A.K.; Sim, S.J.; Weber, R.S.; Ayala, A.G. Allergic fungal sinusitis: A clinicopathologic study of 16 cases. Hum. Pathol. 1996, 27, 793–799. [Google Scholar] [CrossRef]
- Travis, W.D.; Kwon-Chung, K.J.; Kleiner, D.E.; Geber, A.; Lawson, W.; Pass, H.I.; Henderson, D. Unusual aspects of allergic bronchopulmonary fungal disease: Report of two cases due to Curvularia organisms associated with allergic fungal sinusitis. Hum. Pathol. 1991, 22, 1240–1248. [Google Scholar] [CrossRef]
- Posteraro, B.; Scarano, E.; La Sorda, M.; Torelli, R.; De Corso, E.; Mule, A.; Paludetti, G.; Fadda, G.; Sanguinetti, M. Eosinophilic fungal rhinosinusitis due to the unusual pathogen Curvularia inaequalis. Mycoses 2010, 53, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Ter Beest, J.M.; Garner, M.M.; Love, D.M.; Bradway, D.S.; Daniels, J.B.; Surman, S.T.; Neiffer, D.L.; Ramer, J.C. Dermatitis and Rhinosinuitis Caused by Curvularia Species in a Chinese Goral (Naemorhedus griseus). J. Zoo Wildl. Med. 2020, 50, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Yew, S.M.; Chan, C.L.; Lee, K.W.; Na, S.L.; Tan, R.; Hoh, C.C.; Yee, W.Y.; Ngeow, Y.F.; Ng, K.P. A five-year survey of dematiaceous fungi in a tropical hospital reveals potential opportunistic species. PLoS ONE 2014, 9, e104352. [Google Scholar] [CrossRef]
- Steinmetz, H.W.; Zimmermann, N. Computed tomography for the diagnosis of sinusitis and air sacculitis in orangutans. In M. Fowler’s Zoo and Wild Animal Medicine Current Therapy, 7th ed.; Fowler, M.E., Miller, R.E.F., Eds.; Elsevier: St. Louis, MO, USA, 2011; Volume 7, pp. 420–430. [Google Scholar]
- Lapid, R.; Eshar, D. Fatal Herpes simplex virus 1 (HSV-1) infection in a group of zoo-kept white-faced saki monkeys (Pithecia pithecia) in Israel. Isr. J. Vet. Med. 2017, 72, 51–55. [Google Scholar]
- Unwin, S.; Chatterton, J.; Chantrey, J. Management of severe respiratory tract disease caused by human respiratory syncytial virus and Streptococcus pneumoniae in captive chimpanzees (Pan troglodytes). J. Zoo Wildl. Med. 2013, 44, 105–115. [Google Scholar] [CrossRef]
- Kumar, S.; Fox, B.; Owston, M.; Hubbard, G.B.; Dick, E.J., Jr. Pathology of spontaneous air sacculitis in 37 baboons and seven chimpanzees and a brief review of the literature. J. Med. Primatol. 2012, 41, 266–277. [Google Scholar] [CrossRef] [Green Version]
- Lawson, B.; Garriga, R.; Galdikas, B.M. Airsacculitis in fourteen juvenile southern Bornean orangutans (Pongo pygmaeus wurmbii). J. Med. Primatol. 2006, 35, 149–154. [Google Scholar] [CrossRef]
- Zimmermann, N.; Zingg, R.; Makara, M.; Hatt, J.-M.; Steinmetz, H.W. Computertomographic evaluation of the upper respiratory tract in orangutans. (Pongo pygmaeus, Pongo abelii). In Proceedings of the International Conference on Diseases of Zoo, Barcelona, Spain, 13–16 May 2015; pp. 102–103. [Google Scholar]
- Furuta, T.; Fujita, M.; Mukai, R.; Sakakibara, I.; Sata, T.; Miki, K.; Hayami, M.; Kojima, S.; Yoshikawa, Y. Severe pulmonary pneumocystosis in simian acquired immunodeficiency syndrome induced by simian immunodeficiency virus: Its characterization by the polymerase-chain-reaction method and failure of experimental transmission to immunodeficient animals. Parasitol. Res. 1993, 79, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Keita, M.B.; Hamad, I.; Bittar, F. Looking in apes as a source of human pathogens. Microb Pathog 2014, 77, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Cambre, R.C.; Edwards, J.E.; Wilson, H.L.; Todd, J.K.; Strain, J.D.; Handee, R.W.; Jaskunas, J.M.; Chang, J.H.T. Maxillary and Ethmoid Sinusitis with Orbital and Intracranial Extension in an Infant Orangutan (Pongo pygmaeus). J. Zoo Wildl. Med. 1995, 26, 144–151. [Google Scholar]
- Madrid, H.; da Cunha, K.C.; Gene, J.; Dijksterhuis, J.; Cano, J.; Sutton, D.A.; Guarro, J.; Crous, P.W. Novel Curvularia species from clinical specimens. Persoonia 2014, 33, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiss, N.; Homa, M.; Manikandan, P.; Mythili, A.; Krizsán, K.; Revathi, R.; Varga, M.; Papp, T.; Vágvölgyi, C.; Kredics, L.; et al. New Species of the Genus Curvularia: C. tamilnaduensis and C. coimbatorensis from Fungal Keratitis Cases in South India. Pathogens 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Barnes, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef] [Green Version]
- Priyadharsini, P.; Muthukumar, T. The root endophytic fungus Curvularia geniculata from Parthenium hysterophorus roots improves plant growth through phosphate solubilization and phytohormone production. Fungal Ecology 2017, 27, 69–77. [Google Scholar] [CrossRef]
- Index Fungorium. Available online: http:www.indexfungorgum.org/ (accessed on 1 June 2022).
- Tan, Y.P.; Crou, P.W.S.; Shivas, R.G. Cryptic species of Curvularia in the culture collection of the Queensland Plant Pathology Herbarium. MycoKeys 2018, 35, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Nakada, M.; Tanaka, C.; Tsunewaki, K.; Tsuda, M. RFLP analysis for species separation in the genera Bipolaris and Curvularia. Mycoscience 1994, 35, 271–278. [Google Scholar] [CrossRef]
- Hyde, K.D.; Nilsson, R.H.; Alias, S.A.; Ariyawansa, H.A.; Blair, J.E.; Cai, L.; de Cock, A.W.A.M.; Dissanayake, A.J.; Glockling, S.L.; Goonasekara, I.D.; et al. One stop shop: Backbones trees for important phytopathogenic genera: I (2014). Fungal Divers. 2014, 67, 21–125. [Google Scholar] [CrossRef] [Green Version]
- Balla, A.; Pierson, J.; Hugh, J.; Wojewoda, C.; Gibson, P.; Greene, L. Disseminated cutaneous Curvularia infection in an immunocompromised host; diagnostic challenges and experience with voriconazole. J. Cutan. Pathol. 2016, 43, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Graf, E.; Green, A.M. Invasive Curvularia Infection in Pediatric Patients with Hematologic Malignancy Identified by Fungal Sequencing. J. Pediatric Infect. Dis. Soc. 2019, 8, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Al-Odaini, N.; Wei, J.Y.; Zheng, Y.Q.; Zheng, D.Y.; Khader, J.A.; Cao, C.W. A Special Tinea Nigra Caused by Curvularia lunata: Case Report and Literature Review. Mycopathologia 2022, 187, 291–298. [Google Scholar] [CrossRef]
- Ghosh, A.; Kaur, H.; Gupta, A.; Singh, S.; Rudramurthy, S.M.; Gupta, S.; Chakrabarti, A. Emerging Dematiaceous and Hyaline Fungi Causing Keratitis in a Tertiary Care Centre from North India. Cornea 2020, 39, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.P.; Joseph, J.; Pathengay, A.; Pappuru, R.R.; Das, T. Clinical presentations, diagnosis, and management outcomes of Curvularia endophthalmitis and a review of literature. Retina 2020, 40, 370–375. [Google Scholar] [CrossRef]
- Pimentel, J.D.; Mahadevan, K.; Woodgyer, A.; Sigler, L.; Gibas, C.; Harris, O.C.; Lupino, M.; Athan, E. Peritonitis due to Curvularia inaequalis in an elderly patient undergoing peritoneal dialysis and a review of six cases of peritonitis associated with other Curvularia spp. J. Clin. Microbiol. 2005, 43, 4288–4292. [Google Scholar] [CrossRef] [Green Version]
- Carter, E.; Boudreaux, C. Fatal cerebral phaeohyphomycosis due to Curvularia lunata in an immunocompetent patient. J. Clin. Microbiol. 2004, 42, 5419–5423. [Google Scholar] [CrossRef] [Green Version]
- Skovrlj, B.; Haghighi, M.; Smethurst, M.E.; Caridi, J.; Bederson, J.B. Curvularia abscess of the brainstem. World Neurosurg. 2014, 82, 241.e9–241.e13. [Google Scholar] [CrossRef]
- Lake, F.R.; Froudist, J.H.; McAleer, R.; Gillon, R.L.; Tribe, A.E.; Thompson, P.J. Allergic bronchopulmonary fungal disease caused by Bipolaris and Curvularia. Aust. N. Z. J. Med. 1991, 21, 871–874. [Google Scholar] [CrossRef]
- Thekkedath, E.; Burden, Z.; Steinberg, S.; Cury, J. Curvularia Pneumonia Presenting as a Mass-Like Lesion. Cureus 2022, 14, 1–3. [Google Scholar] [CrossRef]
- Kawabata, T.; Suga, H.; Takeuchi, K.; Nagata, Y.; Sakakibara, M.; Ushida, K.; Ozone, C.; Enomoto, A.; Kawamoto, I.; Itagaki, I.; et al. A new primate model of hypophyseal dysfunction. Sci. Rep. 2021, 11, 10729. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E.; Baik, F.M.; Liddell, R.M.; Ayars, A.G.; Branch, K.R.; Pottinger, P.S.; Hillel, A.D.; Helmick, K.; Collins, D. Gorilla endoscopic sinus surgery: A life-saving collaboration between human and veterinary medicine. Int. Forum. Allergy Rhinol. 2018, 8, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Chai, N. Endoscopy and Endosurgery in Nonhuman Primates. Vet. Clin. North Am. Exot. Anim. Pract. 2015, 18, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Lawson, W.; Ho, Y. Open Frontal Sinus Surgery: A Lost Art. Otolaryngol. Clin. North. Am. 2016, 49, 1067–1089. [Google Scholar] [CrossRef]
- Noon, E.; Hopkins, C. Review article: Outcomes in endoscopic sinus surgery. BMC Ear Nose Throat Disord. 2016, 16, 9. [Google Scholar] [CrossRef]


| Lymphocyte Populations Female Sumatran Orangutan | Reference Range Human [20] | Reference Range Orangutan [21] | Reference Range Rhesus Macaques [22] |
|---|---|---|---|
| Total Lymphocytes: 92 | ND | 13.0–85.0 | 16.9–89.8 |
| Total T cells: 69 | 66.0–88.1 | ND | 16.9–89.8 |
| Total B cells: 21 | 4.7–22.5 | ND | ND |
| CD4 T cells: 39 | 36.8–68.3 | ND | 20.3–70.2 |
| CD8 T cells: 24 | 10.1–37.6 | ND | 0.2–5.9 |
| NK T cells: 2 | 2.5–19.4 | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uwiera, R.R.E.; Vijayasekaran, S.; Wallace, A.M.; Reese, D.J.; Walsh, A.L.; Uwiera, T.C.; Vaughan-Higgins, R.; Vitali, S.D. Fungal Rhinosinusitis Caused by a Curvularia sp. Infection in a Female Sumatran Orangutan: A Case Report. Pathogens 2022, 11, 1166. https://doi.org/10.3390/pathogens11101166
Uwiera RRE, Vijayasekaran S, Wallace AM, Reese DJ, Walsh AL, Uwiera TC, Vaughan-Higgins R, Vitali SD. Fungal Rhinosinusitis Caused by a Curvularia sp. Infection in a Female Sumatran Orangutan: A Case Report. Pathogens. 2022; 11(10):1166. https://doi.org/10.3390/pathogens11101166
Chicago/Turabian StyleUwiera, Richard R. E., Shyan Vijayasekaran, Alisa M. Wallace, David J. Reese, Audra L. Walsh, Trina C. Uwiera, Rebecca Vaughan-Higgins, and Simone D. Vitali. 2022. "Fungal Rhinosinusitis Caused by a Curvularia sp. Infection in a Female Sumatran Orangutan: A Case Report" Pathogens 11, no. 10: 1166. https://doi.org/10.3390/pathogens11101166
APA StyleUwiera, R. R. E., Vijayasekaran, S., Wallace, A. M., Reese, D. J., Walsh, A. L., Uwiera, T. C., Vaughan-Higgins, R., & Vitali, S. D. (2022). Fungal Rhinosinusitis Caused by a Curvularia sp. Infection in a Female Sumatran Orangutan: A Case Report. Pathogens, 11(10), 1166. https://doi.org/10.3390/pathogens11101166






