Abstract
Thelaziosis caused by Thelazia callipaeda is an emerging disease in Europe. Only two reports of naturally infected lagomorphs have been published so far. The aim of this study was to evaluate the status of the Romanian populations of European brown hares, Lepus europaeus as reservoir hosts for T. callipaeda. Between November 2019 and November 2021, the eyes of 326 L. europaeus carcasses were examined for the presence of ocular parasites. Nematodes were stored in plastic vials with physiological saline, followed by morphological and molecular identification. QGis 3.20 and EpiInfoTM 7 were used for mapping and statistical analysis. Four (1.23%) hares harbored T. callipaeda infection, with a total of 84 nematodes collected (mean intensity 21 nematodes/host), with 45 males, 39 females (two sexually immature, seven with only eggs, and 30 with eggs and larvae). One specimen from each host was successfully sequenced resulting in a 100% similarity with several other sequences of T. callipaeda haplotype 1. Statistical analysis revealed no significant results. The current study represents a first report of T. callipaeda in the European brown hare in Romania, and the second in Europe, also reiterating the role of lagomorphs as reservoir hosts for this zoonotic ocular nematode.
1. Introduction
Thelaziosis caused by Thelazia callipaeda (Spirurida, Thelaziidae) is a rapidly emerging zoonosis reported across most of Europe and Asia [1]. Domestic and wild carnivores are considered the primary vertebrate hosts of T. callipaeda [2]. Still, occasionally, adult nematodes were reported from other mammals such as other carnivores, lagomorphs, wild boars, and humans [3,4,5,6,7].
Infections in both domestic and wild carnivores are commonly reported across the distribution range of this nematode [1]. Human ocular infections follow an emerging trend in most countries where T. callipaeda had been reported in the main reservoir hosts [6,7,8]. These findings not only underline the zoonotic potential of this nematode but also highlight the clinical implications of the disease. Symptoms range from mild to severe conjunctivitis [9], further complicated by bacterial or fungal infections, which may lead to corneal ulcers [7].
In Romania, the disease was first diagnosed in 2014 [10], in a domestic dog from the western part of the country. Subsequent surveillance documented the spread across most of Romania’s territory, in a wide variety of hosts: domestic dogs [11,12,13], domestic cats [13], jackals, wolves, wildcats [14], foxes [15], and mustelids [16]. However, despite its wide distribution in animals, no human cases have been documented in Romania, so far. Although lagomorphs (hares, rabbits) are known as suitable hosts for T. callipaeda [3,4], there are no studies or reports of these hosts in Romania. Hence, we aimed to investigate the presence of T. callipaeda in hares, Lepus europaeus collected in various regions of Romania, and to evaluate their reservoir role.
2. Results
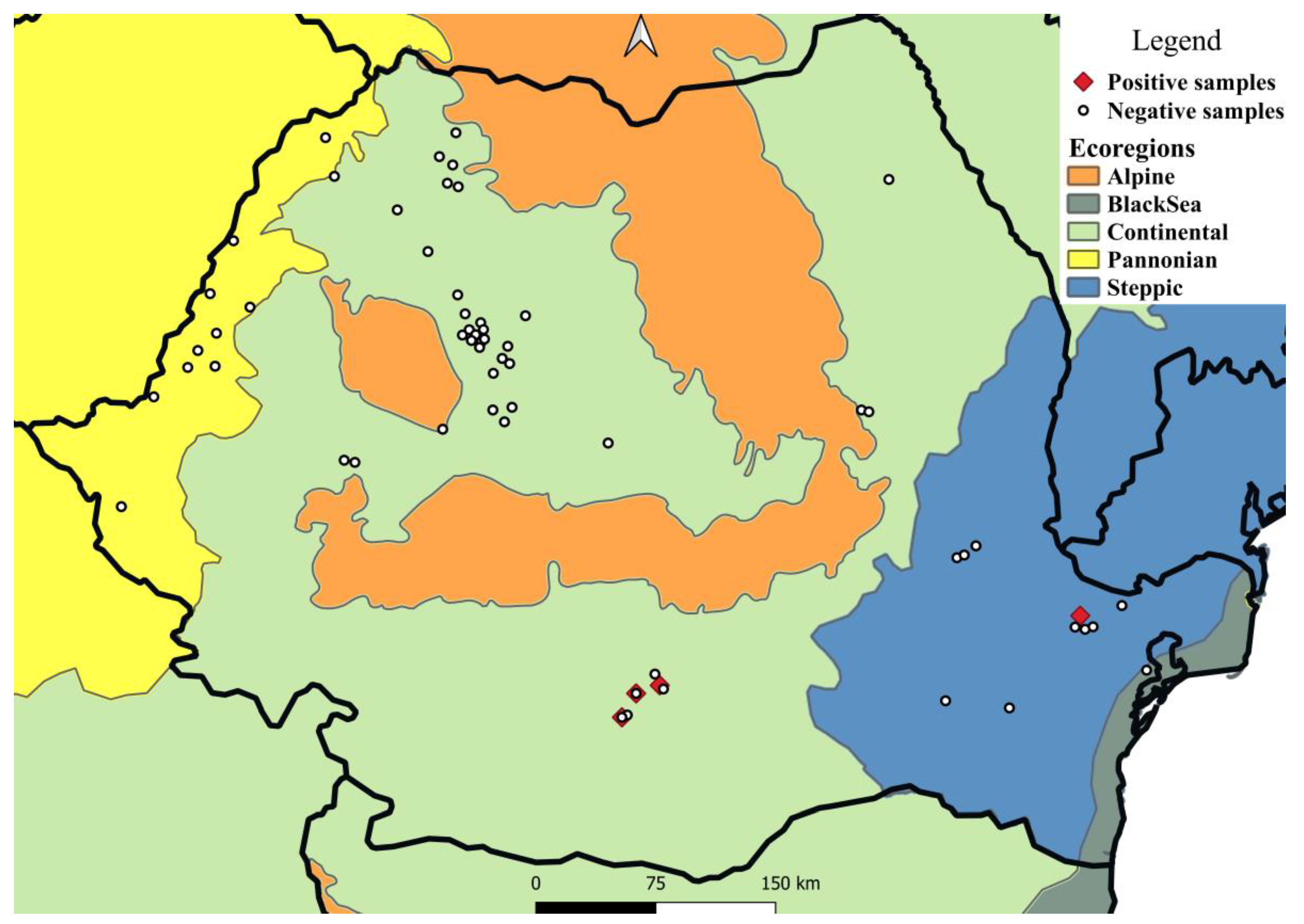
Four of the 326 European brown hares examined were positive for ocular nematodes (1.23%) (Table 1, Figure 1 and Figure 2). A total of 84 nematodes were collected. All nematodes were morphologically identified as T. callipaeda. The intensity varied between 1 and 70 nematodes/hare, with a mean intensity of 21 (Table 2). Seven (18.9%) of the 37 mature females presented non-blastomerized eggs and blastomerized eggs, whereas 30 (81.1%) also presented larvated eggs as well as larvae inside the uterus.

Table 1.
Sampled European brown hares according to the sex, altitude, and ecoregion.
Figure 1.
Sampling areas and distribution of T. callipaeda in European brown hares from Romania, by ecoregion.
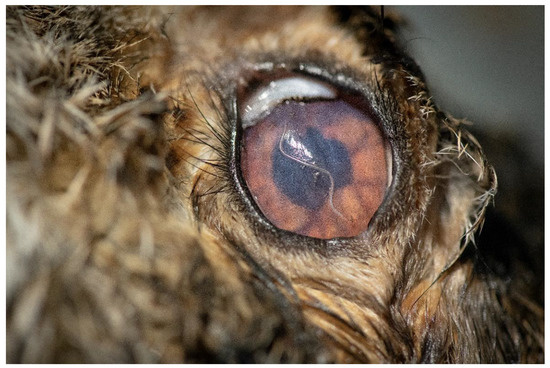
Figure 2.
L. europaeus with a specimen of T. callipaeda present in its left eye.

Table 2.
Thelazia callipaeda infection intensity and population structure in European brown hares from Romania.
All four specimens selected for molecular analysis were successfully sequenced, showing a 100% similarity with several sequences of T. callipaeda haplotype 1 (GenBank: MK546436- MK546439, MF578281; MG913802; AP017700; OM470911).
Statistical analysis, using Pearson’s chi-squared test, correlating sex (p = 0.6563), ecoregion (p = 0.1171), altitude intervals (p = 0.1721) to infection status revealed no significant results, the p values exceeding the 0.05 benchmark.
3. Discussion
The current study serves as the first report of T. callipaeda in European brown hares in Romania and the second in Europe [3]. Moreover, the presence of larvae in the adult females of T. callipaeda is a strong indicator that hares are definitive (final) and also reservoir hosts, being able to transmit the infection in natural conditions. However, due to the low prevalence, we cannot suggest that hares are significant reservoir hosts in Romania, mainly as the area is known as hypernedmic for T. callipaeda in red foxes [15]. For instance, in Italy, the prevalence in hares was significantly higher (3/13, 23.1%) [3]. The higher prevalence in foxes could be linked to their crepuscular activity, which fits the one of Phortica variegata [17], whereas European brown hares are predominantly crepuscular and nocturnal, with a peak in the late afternoon, during the mating season, in spring [18,19]. Additionally, two wild European rabbits, Oryctolagus cuniculus from Portugal, also harbored T. callipaeda infection [4], highlighting the susceptibility of European lagomorphs to infection with this nematode.
Lagomorphs were only reported as natural hosts for T. callipaeda in Europe and Russia [3,4,20]. An experimental study, in Russia, concluded that the estimated life span of sexually mature forms of T. callipaeda in laboratory rabbits, O. cuniculus is of around six months [21], which, under natural conditions, could overlap with the activity period of the vectors. Moreover, the shallow burrowing behavior of brown hares during their inactive periods might leave them exposed to the activity of P. variegata [22].
The potential susceptibility of European brown hares as feeding hosts for Phortica variegata was suggested by Otranto et al. [3]. Interestingly, landscape diversity for the European hare has seen a shift towards woodlands, brushes, unimproved grasslands, and field margins [23], potentially making them more susceptible both to predators [24] and diseases. This follows the trends of the decline of farmland biodiversity, which can be attributed to the intensification of the agricultural industry in the late 20th century in Europe, according to several studies on agri-environment schemes [25,26,27].
Because of the scarcity of hare carcass availability outside of the hunting season, which takes place between November and January, the current study is mainly limited to mature and immature adult stages of T. callipaeda. Subsequently, any larval stages present during the peak infestation season March–June [28] will have either matured or died, affecting both the overall prevalence rate as well as the intensity within the host.
Therefore, there is an increasing need to determine the complexity of the sylvatic cycle and the diversity of reservoir hosts in relation to their ecology to better understand and implement preventive measures for limiting this zoonotic disease. This latter aspect has become a key point over the past ten years, as more human cases have emerged worldwide [6,7,8].
4. Materials and Methods
Between November 2019 and November 2021, 326 carcasses of European hares, Lepus europaeus, were examined as part of a broader survey of their parasites (unpublished data). Of these, six were collected outside of the November–February period, whereas another eight had an unknown collection date (Table 1). The study area comprised 17 counties covering four ecoregions (Figure 1). The carcasses originated from legally hunted individuals or roadkills. The following information was recorded for each animal: date and location of collection, and sex.
As part of the necropsy procedure, the eyes of each carcass were examined under a stereo zoom microscope, with the lateral and medial canthus dissected to uncover the entire globe. Upon detection, nematodes were placed in a vial with physiological saline (0.9%), followed by a morphological examination.
Morphological identification of the ocular nematodes was performed using the keys provided by [20,29], during which each nematode’s sex and developmental stages were recorded. Intact specimens underwent detailed morphometric analysis following preservation in 4% formalin solution. All measurements were done using an Olympus microscope (Olympus BX61) and dedicated software.
One randomly selected nematode from each brown hare was stored in 70% ethanol and used for molecular characterization. DNA extraction was performed individually from each nematode using the ISOLATE II Genomic DNA Kit (Bioline Meridian Bioscience, Luckenwalde, Germany), according to the manufacturer’s instructions, and stored at −20 °C until further use. The samples were processed by PCR amplification of a 670-bp gene region the cytochrome c oxidase subunit 1 (cox 1), using a C1000™ Thermal Cycler (Bio-Rad, London, UK) and the NTF/NTR primer pair, as previously described [30]. Sequencing was performed by Macrogen Europe (Amsterdam, The Netherlands), while the assembled chromatograms and consensus sequences were translated and edited using the Geneious 4.8.5 software (Biomatter Ltd., Auckland, New Zealand). Lastly, using the Basic Local Alignment Search Tool (BLAST), the consensus sequences were compared with the available data in the GenBank® database.
Mapping was performed using the free open source QGis Geographic Information System (version 3.20 Odense, QGis Development Team, 2021), including ecoregions as a layer. Statistical analysis was performed using the EpiInfoTM 7 software (CDC, Atlanta, GA, USA, 2021), recording and calculating values for the frequency, prevalence, as well as 95% confidence interval of infestation, according to several parameters (Table 1).
5. Conclusions
The current study represents the first report of the presence of T. callipaeda in European brown hares in Romania, while also emphasizing the role as a reservoir host of lagomorphs for the aforementioned nematode.
Author Contributions
Conceptualization, V.-D.C., C.M.G. and A.D.M.; methodology, V.-D.C. and A.M.I.; validation, A.D.M., A.M.I. and C.M.G.; formal analysis, V.-D.C., C.D.C. and A.M.I.; investigation, V.-D.C., K.A.H. and C.M.G.; resources, C.M.G. and A.D.M.; data curation, A.D.M. and C.M.G.; writing—original draft preparation, V.-D.C. and A.D.M.; writing—review and editing, V.-D.C., A.D.M. and C.M.G.; visualization, V.-D.C.; supervision, C.M.G.; project administration, C.M.G. and A.D.M.; funding acquisition, A.D.M. and C.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the Internal Grant “Soluții” 24843/05.11.2021.
Institutional Review Board Statement
The study was conducted in compliance with the national animal welfare regulations and approved by the Bioethics Commission of the University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca (345/12.09.2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated or analyzed during this study is included in this published article.
Acknowledgments
The work of A.M.I. was conducted under the framework of grant number TE49/2022, by UEFISCDI Romania.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- do Vale, D.; Lopes, A.P.; Fontes, M.d.C.; Silvestre, M.; Cardoso, L.; Coelho, A.C. Systematic review on infection and disease caused by Thelazia callipaeda in Europe: 2001–2020. Parasite 2020, 11, 98. [Google Scholar]
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission, 2nd ed.; CABI Publishing: Guilford, UK, 2000; p. 672. [Google Scholar]
- Otranto, D.; Dantas-Torres, F.; Mallia, E.; DiGeronimo, P.M.; Brianti, E.; Testini, G.; Traversa, D.; Lia, R.P. Thelazia callipaeda (Spirurida, Thelaziidae) in wild animals: Report of new host and ecological implications. Vet. Parasitol. 2009, 166, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Gama, A.; Pires, I.; Canado, M.; Coutinho, T.; Lopes, A.P.; Latrofa, M.S.; Cardoso, L.; Dantas-Torres, F.; Ontranto, D. First report of Thelazia callipaeda infection in wild European rabbits (Oryctolagus cuniculus) in Portugal. Parasites Vectors 2016, 9, 236. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Wei, J.; Wen, Y.; He, N.; Tang, L.; Lin, D.; Lin, J. A first report of Thelazia callipaeda infection in Phortica okadai and wildlife in national nature reserves in China. Parasites Vectors 2021, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Morgado, A.C.T.; do Vale, B.; Ribeiro, P.; Coutinho, T.; Santos-Silva, S.; de Sousa Moreira, A.; Rodrigues, F.T.; Coelho, A.C.; Lopes, A.P.; Mesquita, J.R.; et al. First report of human Thelazia callipaeda infection in Portugal. Acta Trop. 2022, 231, 106436. [Google Scholar] [CrossRef]
- Wei, X.; Liu, B.; Li, Y.; Wang, K.; Gao, L.; Yang, Y. A human corneal ulcer caused by Thelazia callipaeda in Southwest China: Case report. Parasitol. Res. 2020, 119, 3531–3534. [Google Scholar] [CrossRef]
- Dolff, S.; Kehrmann, J.; Eisermann, P.; Dalbah, S.; Tappe, D.; Rating, P. Case Report: Thelazia callipaeda Eye Infection: The First Human Case in Germany. Am. J. Trop. Med. Hyg. 2020, 102, 350–351. [Google Scholar] [CrossRef]
- Kregel-Weber, M.K.; Delling, C.; Dyachenko, V.; Luttgenau, H. Okulare Thelaziose bei einem Hund in Deutschland–Ein autochthoner Fall? Tierärztliche Prax. Ausg. K Kleintiere/Heimtiere 2020, 49, 55–59. [Google Scholar] [CrossRef]
- Mihalca, A.D.; D’Amico, G.; Scurtu, I.; Chirilă, R.; Matei, I.A.; Ionică, A.M. Further spreading of canine oriental eyeworm in Europe: First report of Thelazia callipaeda in Romania. Parasites Vectors 2015, 8, 48. [Google Scholar] [CrossRef]
- Ioniță, M.; Mitrea, I.L.; Ionică, A.M.; Morariu, S.; Mihalca, A.D. New cases of Thelazia callipaeda haplotype 1 in dogs suggest a wider disribution in Romania. Vector-Borne Zoonotic Dis. 2016, 16, 172–175. [Google Scholar] [CrossRef]
- Tudor, P.; Bădicu, A.; Mateescu, R.; Tudor, N.; Mateescu, C.; Ionaşcu, I. First report of canine ocular thelaziosis in the Muntenia Region, Romania. Parasitol. Res. 2016, 115, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Dumitrache, M.O.; Györke, A.; Mircean, M.; Benea, M.; Mircean, V. Ocular thelaziosis due Thelazia callipaeda (Spirurida: Thelaziidae) in Romania: First report in domestic cat and new geographical records of canine cases. Parasitol. Res. 2018, 117, 4037–4042. [Google Scholar] [CrossRef] [PubMed]
- Mihalca, A.D.; Ionică, A.M.; D’Amico, G.; Daskalaki, A.A.; Deak, G.; Matei, I.A.; Șimonca, V.; Iordache, D.; Modrý, D.; Gherman, C.M. Thelazia callipaeda in wild carnivores from Romania: New host and geographical records. Parasites Vectors 2016, 9, 350. [Google Scholar] [CrossRef]
- Ionică, A.M.; Deak, G.; Matei, I.A.; D′Amico, G.; Cotuţiu, V.D.; Gherman, C.M.; Mihalca, A.D. Thelazia callipaeda, an Endemic Parasite of Red Foxes (Vulpes vulpes) in Western Romania. J. Wildl. Dis. 2018, 54, 829–833. [Google Scholar] [CrossRef]
- Ionică, A.M.; Deak, G.; D’Amico, G.; Stan, G.F.; Chișamera, G.B.; Constantinescu, I.C.; Adam, C.; Lefkaditis, M.; Gherman, C.M.; Mihalca, A.D. Thelazia callipaeda in mustelids from Romania with the European badger, Meles meles, as a new host for this parasite. Parasites Vectors 2019, 12, 370. [Google Scholar] [CrossRef]
- Otranto, D.; Cantacessi, C.; Dantas-Torres, F.; Brianti, E.; Pfeffer, M.; Genchi, C.; Guberti, V.; Capelli, G.; Deplazes, P. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: Helminths and arthropods. Vet. Parasitol. 2015, 213, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Tottewitz, F. Erste Ergebnisse zur Lebensraumnutzung un Aktitätsperiodik des Feldhasen (Lepus europaeus) in großflächig landwirtschaftlich genutzen Gebieten. Beiträge Zur Jagd Wildforschung 1993, 18, 135–139. [Google Scholar]
- Pèpin, D.; Cargnelluti, B. Individual variations of daily activity patterns in radiotracked European hares during winter. Acta Theriol. 1994, 39, 399–409. [Google Scholar] [CrossRef]
- Skrjabin, K.I.; Soboley, A.A.; Ivanshkin, V.M. Spirurata of animals and man and the diseases caused by them Part 4: Thelazioidea. In Essential of Nematodology; Skrjabin, K.I., Ed.; Academy of Sciences of the USSR: Moscow, Russia, 1967; Volume XVI, pp. 24–32. [Google Scholar]
- Kozlov, D.P. The life cycle of the nematode Thelazia callipaeda parasite in the eye of man and carnivores. Dokl. Akad. Nauk SSSR 1961, 142, 732–733. (In Russian) [Google Scholar]
- Trocchi, V.; Riga, F. I Lagomorfi in Italia. Linee guida per la conservazione e la Gestione. Minist. Politiche Agric. For. Ist. Naz. Fauna Selvatica Doc. Tec. 2005, 25, 128. [Google Scholar]
- Petrovan, S.O.; Ward, A.; Wheeler, P.M. Habitat selection guiding agri-environment schemes for a farmland specialist, the brown hare. Anim. Conserv. 2012, 16, 344–352. [Google Scholar] [CrossRef]
- Schmidt, N.M.; Asferg, T.; Forchhammer, M.C. Long-term patterns in European brown hare population dynamics in Denmark: Effects of agriculture, predation and climate. BMC Ecol. 2004, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Stoate, C.; Boatman, N.; Borralho, R.; Carvalhob, C.R.; Snoo, G.; Eden, P. Ecological impacts of arable intensification in Europe. J. Environ. Manag. 2001, 63, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.A.; Sutherland, W.J. Post-war changes in arable farming and biodiversity in Great Britain. J. Appl. Ecol. 2002, 39, 157–176. [Google Scholar] [CrossRef]
- Hackländer, K.; Klansek, E.; Steineck, T.; Ruf, T. Reproduction and juvenile mortality in European hare (Lepus europaeus) populations in intensive agricultural landscapes. Mamm. Biol. 2003, 68, 29–30. [Google Scholar]
- Otranto, D.; Lia, R.P.; Buono, V.; Traversa, D.; Giangaspero, A. Biology of Thelazia callipaeda (Spirurida, Thelaziidae) eyeworms in naturally infected definitive hosts. Parasitology 2004, 129, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Lia, R.P.; Traversa, D.; Giannetto, S. Thelazia callipaeda (Spirurida, Thelaziidae) of carnivores and humans: Morphological study by light and scanning electron microscopy. Parassitologia 2003, 45, 125–133. [Google Scholar]
- Casiraghi, M.; Anderson, T.; Bandi, C.; Bazzocchi, C.; Genchi, C. A phylogenetic analysis of filarial nematodes: Comparison with the phylogeny of Wolbachia endosymbionts. Parasitology 2001, 122, 93–103. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).