Activation of Human Platelets by Staphylococcus aureus Secreted Protease Staphopain A
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacterial Strains Used
2.3. Ammonium Sulphate Precipitation of S. aureus Supernatant
2.4. Extraction and Purification of Staphopain A
2.5. Preparation of Platelet Rich Plasma
2.6. Human Washed Platelet Preparation
2.7. Light Transmission Aggregometry
2.8. Flow Cytometry
2.9. In Vitro Thrombus Formation
2.10. Statistical Analysis
3. Results
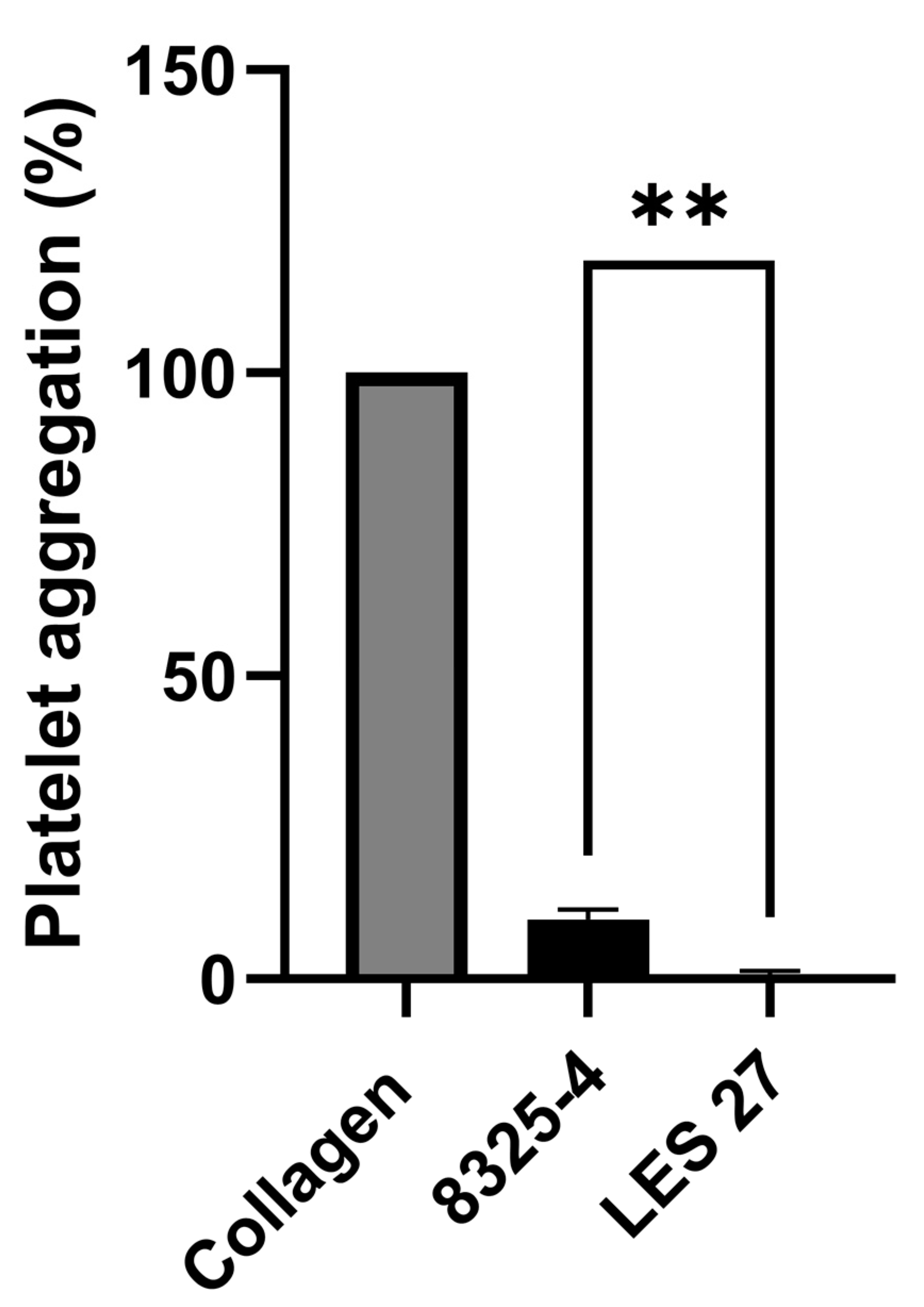
3.1. Staphopain A Causes Platelet Activation
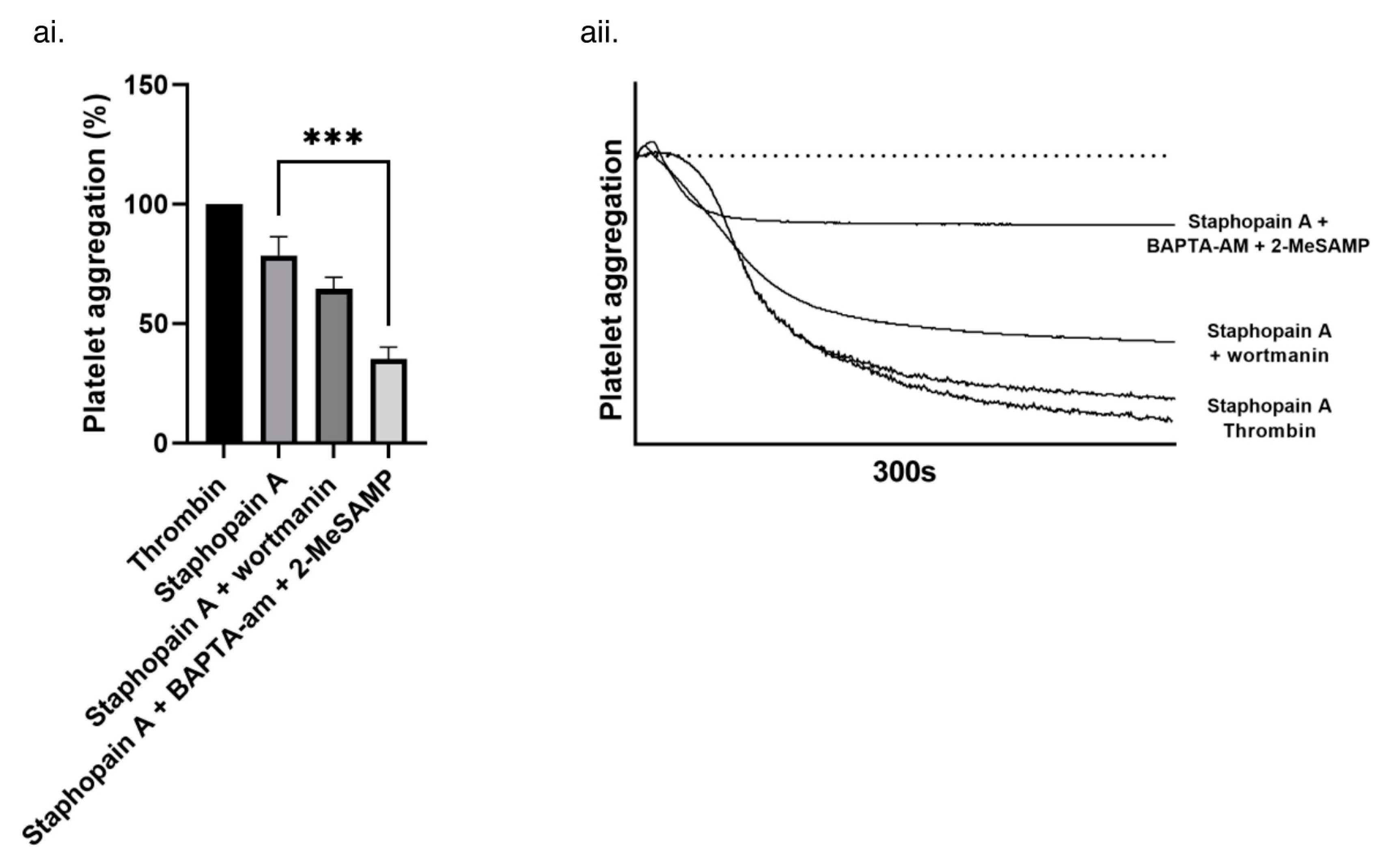
3.2. Staphopain A Protease Activity Is Required for Platelet Activation and Intracellular Signaling
3.3. Staphopain A Potentiates Thrombus Formation under Physiological Flow Conditions
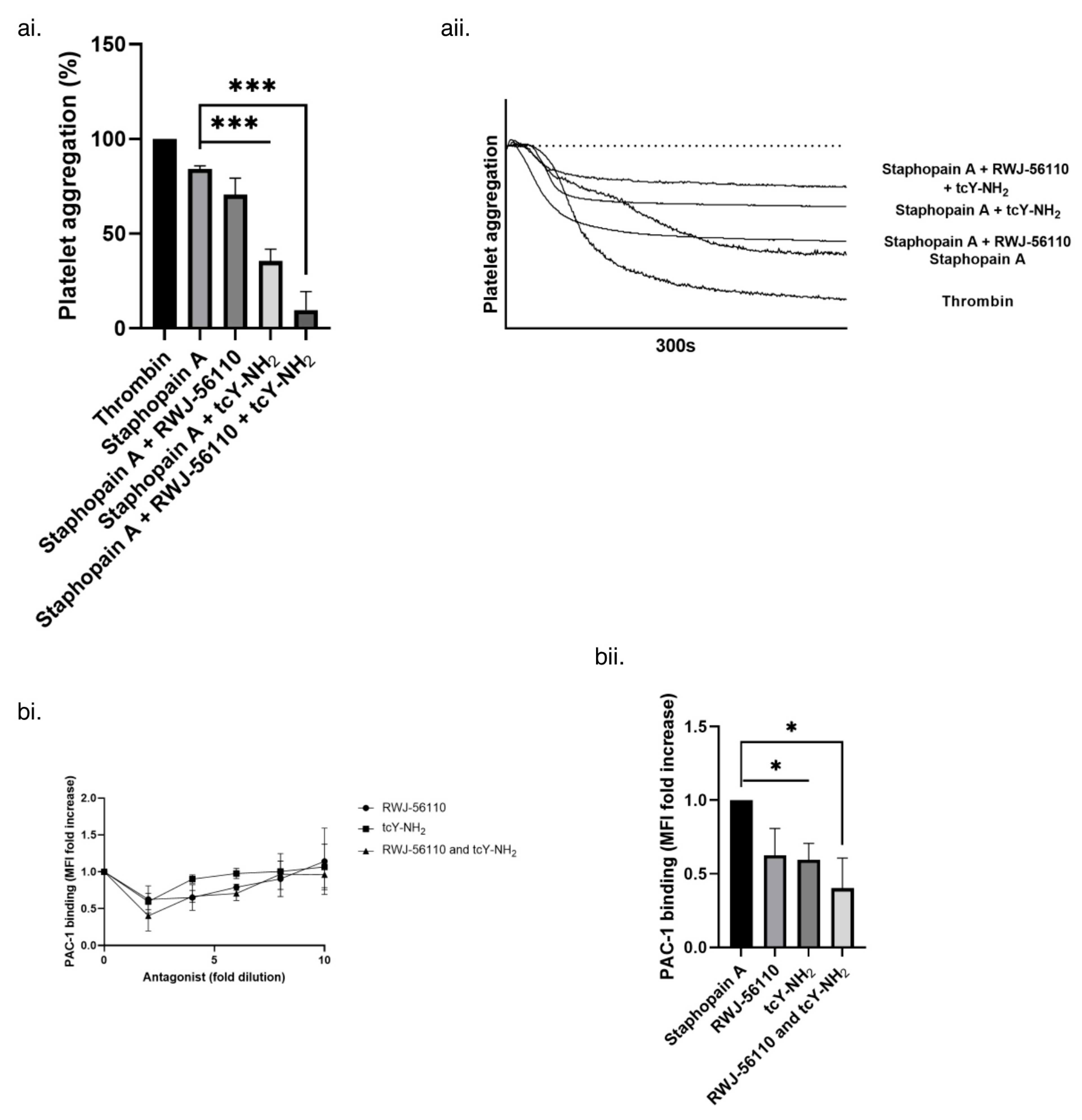
3.4. Platelet Activation by Staphopain A Occurs via Protease Activated Receptors 1 and 4 (PAR-1 and -4)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sullam, P.M.; Bayer, A.S.; Foss, W.M.; Cheung, A.L. Diminished platelet binding in vitro by Staphylococcus aureus is associated with reduced virulence in a rabbit model of infective endocarditis. Infect. Immun. 1996, 64, 4915–4921. [Google Scholar] [CrossRef]
- Fitzgerald, J.R.; Foster, T.J.; Cox, D. The interaction of bacterial pathogens with platelets. Nat. Rev. Microbiol. 2006, 4, 445–457. [Google Scholar] [CrossRef]
- Cabell, C.H.; Pond, K.K.; Peterson, G.E.; Durack, D.T.; Corey, G.R.; Anderson, D.J.; Ryan, T.; Lukes, A.S.; Sexton, D.J. The risk of stroke and death in patients with aortic and mitral valve endocarditis. Am. Heart J. 2001, 142, 75–80. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, W.; Liu, Z.; Pei, Y.; Zhu, Y.; Chen, F.; Zou, L.; Jiang, Y.; Liu, X.; Huang, J. Early predictive value of platelet function for clinical outcome in sepsis. J. Infect. 2022, 84, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Polzin, A.; Dannenberg, L.; M’Pembele, R.; Mourikis, P.; Naguib, D.; Zako, S.; Helten, C.; Petzold, T.; Levkau, B.; Hohlfeld, T.; et al. Staphylococcus aureus increases platelet reactivity in patients with infective endocarditis. Sci. Rep. 2022, 12, 12933. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.E.; Becker, R.E.; Sailer, A.; Turner, J.R.; Wardenburg, J.B. Synergistic Action of Staphylococcus aureus A-Toxin on Platelets and Myeloid Lineage Cells Contributes to Lethal Sepsis. Cell Host Microbe 2015, 17, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Surewaard, B.G.; Thanabalasuriar, A.; Zeng, Z.; Tkaczyk, C.; Cohen, T.S.; Bardoel, B.W.; Jorch, S.K.; Deppermann, C.; Wardenburg, J.B.; Davis, R.P. A-Toxin induces platelet aggregation and liver injury during Staphylococcus aureus Sepsis. Cell Host Microbe 2018, 24, 271–284.e3. [Google Scholar] [CrossRef] [PubMed]
- Vandijck, D.M.; Blot, S.I.; De Waele, J.J.; Hoste, E.A.; Vandewoude, K.H.; Decruyenaere, J.M. Thrombocytopenia and outcome in critically ill patients with bloodstream infection. Heart Lung 2010, 39, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh-Cognasse, H.; Damien, P.; Chabert, A.; Pozzetto, B.; Cognasse, F.; Garraud, O. Platelets and infections–complex interactions with bacteria. Front. Immunol. 2015, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, F.; Massberg, S. Patrolling the vascular borders: Platelets in immunity to infection and cancer. Nat. Rev. Immunol. 2019, 19, 747–760. [Google Scholar] [CrossRef]
- Mcdevitt, D.; Nanavaty, T.; House-Pompeo, K.; Bell, E.; Turner, N.; Mcintire, L.; Foster, T.; HööK, M. Characterization of the interaction between the Clumping factor (ClfA) and fibrinogen. Eur. J. Biochem. 1997, 247, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Hartleib, J.; Köhler, N.; Dickinson, R.B.; Chhatwal, G.S.; Sixma, J.J.; Hartford, O.M.; Foster, T.J.; Peters, G.; Kehrel, B.E.; Herrmann, M. Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood J. Am. Soc. Hematol. 2000, 96, 2149–2156. [Google Scholar]
- Siboo, I.R.; Cheung, A.L.; Bayer, A.S.; Sullam, P.M. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect. Immun. 2001, 69, 3120–3127. [Google Scholar] [CrossRef]
- Que, Y.-A.; François, P.; Haefliger, J.-A.; Entenza, J.-M.; Vaudaux, P.; Moreillon, P. Reassessing the role of Staphylococcus aureus clumping factor and fibronectin-binding protein by expression in Lactococcus lactis. Infect. Immun. 2001, 69, 6296–6302. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.; Kerrigan, S.W.; Kaw, G.; Hogan, M.; Penadés, J.; Litt, D.; Fitzgerald, D.J.; Foster, T.J.; Cox, D. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: Roles for the clumping factors ClfA and ClfB, the serine–aspartate repeat protein SdrE and protein A. Mol. Microbiol. 2002, 44, 1033–1044. [Google Scholar] [CrossRef]
- Loughman, A.; Fitzgerald, J.R.; Brennan, M.P.; Higgins, J.; Downer, R.; Cox, D.; Foster, T.J. Roles for fibrinogen, immunoglobulin and complement in platelet activation promoted by Staphylococcus aureus clumping factor A. Mol. Microbiol. 2005, 57, 804–818. [Google Scholar] [CrossRef]
- Fitzgerald, J.R.; Loughman, A.; Keane, F.; Brennan, M.; Knobel, M.; Higgins, J.; Visai, L.; Speziale, P.; Cox, D.; Foster, T.J. Fibronectin-binding proteins of Staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin GPIIb/IIIa and IgG binding to the FcγRIIa receptor. Mol. Microbiol. 2006, 59, 212–230. [Google Scholar] [CrossRef]
- O’Seaghdha, M.; van Schooten, C.J.; Kerrigan, S.W.; Emsley, J.; Silverman, G.J.; Cox, D.; Lenting, P.J.; Foster, T.J. Staphylococcus aureus protein A binding to von Willebrand factor A1 domain is mediated by conserved IgG binding regions. FEBS J. 2006, 273, 4831–4841. [Google Scholar] [CrossRef]
- Procopio Evagrio George, N.; Wei, Q.; Kyun Shin, P.; Konstantopoulos, K.; Ross, J.M. Staphylococcus aureus adhesion via Spa, ClfA, and SdrCDE to immobilized platelets demonstrates shear-dependent behavior. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2394–2400. [Google Scholar] [CrossRef]
- Kerrigan, S.W.; Clarke, N.; Loughman, A.; Meade, G.; Foster, T.J.; Cox, D. Molecular basis for Staphylococcus aureus–mediated platelet aggregate formation under arterial shear in vitro. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 335–340. [Google Scholar] [CrossRef]
- Miajlovic, H.; Zapotoczna, M.; Geoghegan, J.A.; Kerrigan, S.W.; Speziale, P.; Foster, T.J. Direct interaction of iron-regulated surface determinant IsdB of Staphylococcus aureus with the GPIIb/IIIa receptor on platelets. Microbiology 2010, 156, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.; Muhly, M.; Mannhardt, U.; Hugo, F.; Klapettek, K.; Mueller-Eckhardt, C.; Roka, L. Staphylococcal α toxin promotes blood coagulation via attack on human platelets. J. Exp. Med. 1988, 168, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.S.; Ramos, M.D.; Menzies, B.E.; Yeaman, M.R.; Shen, A.J.; Cheung, A.L. Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: A host defense role for platelet microbicidal proteins. Infect. Immun. 1997, 65, 4652–4660. [Google Scholar] [CrossRef] [PubMed]
- Dubin, G. Extracellular proteases of Staphylococcus spp. Biol. Chem. 2002, 383, 1075–1086. [Google Scholar] [CrossRef]
- Stach, N.; Kaszycki, P.; Władyka, B.; Dubin, G. Extracellular proteases of Staphylococcus spp. In Pet-to-Man Travelling Staphylococci; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–145. [Google Scholar]
- Moraes, L.A.; Spyridon, M.; Kaiser, W.J.; Jones, C.; Sage, T.; Atherton, R.; Gibbins, J.M. Non-genomic effects of PPARγ ligands: Inhibition of GPVI-stimulated platelet activation. J. Thromb. Haemost. 2010, 8, 577–587. [Google Scholar] [CrossRef][Green Version]
- Novick, R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 1967, 33, 155–166. [Google Scholar] [CrossRef]
- O’Reilly, M.; de Azavedo, J.C.; Kennedy, S.; Foster, T.J. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb. Pathog. 1986, 1, 125–138. [Google Scholar] [CrossRef]
- Shaw, L.; Golonka, E.; Potempa, J.; Foster, S.J. The role and regulation of the extracellular proteases of Staphylococcus aureus. Microbiology 2004, 150, 217–228. [Google Scholar] [CrossRef]
- Kantyka, T.; Potempa, J. Chapter sixteen—Human SCCA Serpins Inhibit Staphylococcal Cysteine Proteases by Forming Classic “Serpin-Like” Covalent Complexes. In Methods in Enzymology; Whisstock, J.C., Bird, P.I., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 499, pp. 331–345. [Google Scholar]
- Kalińska, M.; Kantyka, T.; Greenbaum, D.C.; Larsen, K.S.; Władyka, B.; Jabaiah, A.; Bogyo, M.; Daugherty, P.S.; Wysocka, M.; Jaros, M. Substrate specificity of Staphylococcus aureus cysteine proteases–Staphopains A, B and C. Biochimie 2012, 94, 318–327. [Google Scholar] [CrossRef]
- Waller, A.K.; Sage, T.; Kumar, C.; Carr, T.; Gibbins, J.M.; Clarke, S.R. Staphylococcus aureus lipoteichoic acid inhibits platelet activation and thrombus formation via the Paf receptor. J. Infect. Dis. 2013, 208, 2046–2057. [Google Scholar] [CrossRef]
- Nickerson, N.N.; Prasad, L.; Jacob, L.; Delbaere, L.T.; McGavin, M.J. Activation of the SspA Serine Protease Zymogen of Staphylococcus aureus Proceeds through Unique Variations of a Trypsinogen-like Mechanism and Is Dependent on Both Autocatalytic and Metalloprotease-specific Processing. J. Biol. Chem. 2007, 282, 34129–34138. [Google Scholar] [CrossRef] [PubMed]
- Sabat, A.J.; Wladyka, B.; Kosowska-Shick, K.; Grundmann, H.; van Dijl, J.M.; Kowal, J.; Appelbaum, P.C.; Dubin, A.; Hryniewicz, W. Polymorphism, genetic exchange and intragenic recombination of the aureolysin gene among Staphylococcus aureus strains. BMC Microbiol. 2008, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Shattil, S.J.; Hoxie, J.A.; Cunningham, M.; Brass, L.F. Changes in the platelet membrane glycoprotein IIb. IIIa complex during platelet activation. J. Biol. Chem. 1985, 260, 11107–11114. [Google Scholar] [CrossRef]
- Massaguer, A.; Engel, P.; Pérez-del-Pulgar, S.; Bosch, J.; Pizcueta, P. Production and characterization of monoclonal antibodies against conserved epitopes of P-selectin (CD62P). Tissue Antigens 2000, 56, 117–128. [Google Scholar] [CrossRef]
- Shattil, S.J.; Cunningham, M.; Hoxie, J.A. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood 1987, 70, 307–315. [Google Scholar] [CrossRef]
- Dubin, G.; Krajewski, M.; Popowicz, G.; Stec-Niemczyk, J.; Bochtler, M.; Potempa, J.; Dubin, A.; Holak, T.A. A novel class of cysteine protease inhibitors: Solution structure of staphostatin A from Staphylococcus aureus. Biochemistry 2003, 42, 13449–13456. [Google Scholar] [CrossRef]
- Rzychon, M.; Sabat, A.; Kosowska, K.; Potempa, J.; Dubin, A. Staphostatins: An expanding new group of proteinase inhibitors with a unique specificity for the regulation of staphopains, Staphylococcus spp. cysteine proteinases. Mol. Microbiol. 2003, 49, 1051–1066. [Google Scholar] [CrossRef]
- Potempa, J.; Golonka, E.; Filipek, R.; Shaw, L.N. Fighting an enemy within: Cytoplasmic inhibitors of bacterial cysteine proteases. Mol. Microbiol. 2005, 57, 605–610. [Google Scholar] [CrossRef]
- Marjoram, R.J.; Voss, B.; Pan, Y.; Dickeson, S.K.; Zutter, M.M.; Hamm, H.E.; Santoro, S.A. Suboptimal activation of protease-activated receptors enhances α2β1 integrin-mediated platelet adhesion to collagen. J. Biol. Chem. 2009, 284, 34640–34647. [Google Scholar] [CrossRef]
- Quinton, T.M.; Kim, S.; Dangelmaier, C.; Dorsam, R.T.; Jin, J.; Daniel, J.L.; Kunapuli, S.P. Protein kinase C-and calcium-regulated pathways independently synergize with Gi pathways in agonist-induced fibrinogen receptor activation. Biochem. J. 2002, 368, 535–543. [Google Scholar] [CrossRef]
- Jackson, S.P.; Nesbitt, W.S.; Kulkarni, S. Signaling events underlying thrombus formation. J. Thromb. Haemost. 2003, 1, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Woulfe, D. Platelet G protein-coupled receptors in hemostasis and thrombosis. J. Thromb. Haemost. 2005, 3, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Strehl, A.; Munnix, I.C.A.; Kuijpers, M.J.E.; van der Meijden, P.E.J.; Cosemans, J.M.E.M.; Feijge, M.A.H.; Nieswandt, B.; Heemskerk, J.W.M. Dual Role of Platelet Protein Kinase C in Thrombus Formation: Stimulation of Pro-Aggregatory and Suppression of Procoagulant Activity in Platelets. J. Biol. Chem. 2007, 282, 7046–7055. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.T.; Poole, A.W. Diverse functions of protein kinase C isoforms in platelet activation and thrombus formation. J. Thromb. Haemost. 2010, 8, 454–462. [Google Scholar] [CrossRef]
- Voss, B.; McLaughlin, J.N.; Holinstat, M.; Zent, R.; Hamm, H.E. PAR1, but not PAR4, activates human platelets through a Gi/o/phosphoinositide-3 kinase signaling axis. Mol. Pharmacol. 2007, 71, 1399–1406. [Google Scholar] [CrossRef]
- Andrade-Gordon, P.; Maryanoff, B.E.; Derian, C.K.; Zhang, H.-C.; Addo, M.F.; Darrow, A.L.; Eckardt, A.J.; Hoekstra, W.J.; McComsey, D.F.; Oksenberg, D. Design, synthesis, and biological characterization of a peptide-mimetic antagonist for a tethered-ligand receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 12257–12262. [Google Scholar] [CrossRef]
- Ma, L.; Hollenberg, M.D.; Wallace, J.L. Thrombin-induced platelet endostatin release is blocked by a proteinase activated receptor-4 (PAR4) antagonist. Br. J. Pharmacol. 2001, 134, 701–704. [Google Scholar] [CrossRef]
- Voss, B.M.; Hamm, H.E. Calcium Mobilization in Human Platelets is Differentially Modulated by PAR-1 and PAR-4 through Gi/o and PI3K. FASEB J. 2006, 20, A118. [Google Scholar] [CrossRef]
- Azim, A.C.; Barkalow, K.; Chou, J.; Hartwig, J.H. Activation of the small GTPases, rac and cdc42, after ligation of the platelet PAR-1 receptor. Blood 2000, 95, 959–964. [Google Scholar] [CrossRef]
- Wu, C.-C.; Wu, S.-Y.; Liao, C.-Y.; Teng, C.-M.; Wu, Y.-C.; Kuo, S.-C. The roles and mechanisms of PAR4 and P2Y12/phosphatidylinositol 3-kinase pathway in maintaining thrombin-induced platelet aggregation. Br. J. Pharmacol. 2010, 161, 643–658. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, C.; Yu, S.; Liu, P.; Luo, D.; Zhou, Q.; Gao, C.; Hu, H. A critical role of thrombin/PAR-1 in ADP-induced platelet secretion and the second wave of aggregation. J. Thromb. Haemost. 2013, 11, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Holinstat, M.; Voss, B.; Bilodeau, M.L.; McLaughlin, J.N.; Cleator, J.; Hamm, H.E. PAR4, but not PAR1, signals human platelet aggregation via Ca2+ mobilization and synergistic P2Y12 receptor activation. J. Biol. Chem. 2006, 281, 26665–26674. [Google Scholar] [CrossRef] [PubMed]
- Entenza, J.-M.; Moreillon, P.; Senn, M.M.; Kormanec, J.; Dunman, P.M.; Berger-Bächi, B.; Projan, S.; Bischoff, M. Role of σB in the expression of Staphylococcus aureus cell wall adhesins ClfA and FnbA and contribution to infectivity in a rat model of experimental endocarditis. Infect. Immun. 2005, 73, 990–998. [Google Scholar] [CrossRef]
- Que, Y.-A.; Haefliger, J.-A.; Piroth, L.; François, P.; Widmer, E.; Entenza, J.M.; Sinha, B.; Herrmann, M.; Francioli, P.; Vaudaux, P. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J. Exp. Med. 2005, 201, 1627–1635. [Google Scholar] [CrossRef]
- Shannon, O.; Uekötter, A.; Flock, J.-I. Extracellular fibrinogen binding protein, Efb, from Staphylococcus aureus as an antiplatelet agent in vivo. Thromb. Haemost. 2005, 93, 927–931. [Google Scholar] [CrossRef]
- Bertling, A.; Niemann, S.; Hussain, M.; Holbrook, L.; Stanley, R.G.; Brodde, M.F.; Pohl, S.; Schifferdecker, T.; Roth, J.; Jurk, K. Staphylococcal extracellular adherence protein induces platelet activation by stimulation of thiol isomerases. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1979–1990. [Google Scholar] [CrossRef]
- Lourbakos, A.; Yuan, Y.; Jenkins, A.L.; Travis, J.; Andrade-Gordon, P.; Santulli, R.; Potempa, J.; Pike, R.N. Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: A new trait in microbial pathogenicity. Blood J. Am. Soc. Hematol. 2001, 97, 3790–3797. [Google Scholar]
- Ohbayashi, T.; Irie, A.; Murakami, Y.; Nowak, M.; Potempa, J.; Nishimura, Y.; Shinohara, M.; Imamura, T. Degradation of fibrinogen and collagen by staphopains, cysteine proteases released from Staphylococcus Aureus. Microbiol. 2011, 157, 786–792. [Google Scholar] [CrossRef]
- Imamura, T.; Tanase, S.; Szmyd, G.; Kozik, A.; Travis, J.; Potempa, J. Induction of vascular leakage through release of bradykinin and a novel kinin by cysteine proteinases from Staphylococcus aureus. J. Exp. Med. 2005, 201, 1669–1676. [Google Scholar] [CrossRef]
- Laarman, A.J.; Mijnheer, G.; Mootz, J.M.; Van Rooijen, W.J.; Ruyken, M.; Malone, C.L.; Heezius, E.C.; Ward, R.; Milligan, G.; Van Strijp, J.A. Staphylococcus aureus Staphopain A inhibits CXCR2-dependent neutrophil activation and chemotaxis. EMBO J. 2012, 31, 3607–3619. [Google Scholar] [CrossRef]
- Stelzner, K.; Boyny, A.; Hertlein, T.; Sroka, A.; Moldovan, A.; Paprotka, K.; Kessie, D.; Mehling, H.; Potempa, J.; Ohlsen, K. Intracellular Staphylococcus aureus employs the cysteine protease staphopain A to induce host cell death in epithelial cells. PLoS Pathog. 2021, 17, e1009874. [Google Scholar] [CrossRef] [PubMed]
- Kantyka, T.; Shaw, L.N.; Potempa, J. Papain-like proteases of Staphylococcus aureus. Cysteine Proteases Pathog. Org. 2011, 712, 1–14. [Google Scholar]
- Sambrano, G.R.; Huang, W.; Faruqi, T.; Mahrus, S.; Craik, C.; Coughlin, S.R. Cathepsin G activates protease-activated receptor-4 in human platelets. J. Biol. Chem. 2000, 275, 6819–6823. [Google Scholar] [CrossRef] [PubMed]
- Leger, A.J.; Jacques, S.L.; Badar, J.; Kaneider, N.C.; Derian, C.K.; Andrade-Gordon, P.; Covic, L.; Kuliopulos, A. Blocking the protease-activated receptor 1-4 heterodimer in platelet-mediated thrombosis. Circulation 2006, 113, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M.L.; Nakanishi-Matsui, M.; Shapiro, M.J.; Ishihara, H.; Coughlin, S.R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Investig. 1999, 103, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Covic, L.; Gresser, A.L.; Kuliopulos, A. Biphasic kinetics of activation and signaling for PAR1 and PAR4 thrombin receptors in platelets. Biochemistry 2000, 39, 5458–5467. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.J.; Weiss, E.J.; Faruqi, T.R.; Coughlin, S.R. Protease-activated receptors 1 and 4 are shut off with distinct kinetics after activation by thrombin. J. Biol. Chem. 2000, 275, 25216–25221. [Google Scholar] [CrossRef]
- Coughlin, S.R. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 2005, 3, 1800–1814. [Google Scholar] [CrossRef]
- Hamilton, J.R.; Frauman, A.G.; Cocks, T.M. Increased expression of protease-activated receptor-2 (PAR2) and PAR4 in human coronary artery by inflammatory stimuli unveils endothelium-dependent relaxations to PAR2 and PAR4 agonists. Circ. Res. 2001, 89, 92–98. [Google Scholar] [CrossRef]
- Kataoka, H.; Hamilton, J.R.; McKemy, D.D.; Camerer, E.; Zheng, Y.-W.; Cheng, A.; Griffin, C.; Coughlin, S.R. Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood 2003, 102, 3224–3231. [Google Scholar] [CrossRef]
- Laniyonu, A.A.; Hollenberg, M.D. Vascular actions of thrombin receptor-derived polypeptides: Structure-activity profiles for contractile and relaxant effects in rat aorta. Br. J. Pharmacol. 1995, 114, 1680–1686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vidwan, P.; Pathak, A.; Sheth, S.; Huang, J.; Monroe, D.M.; Stouffer, G.A. Activation of Protease-Activated Receptors 3 and 4 Accelerates Tissue Factor–Induced Thrombin Generation on the Surface of Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Vergnolle, N.; Derian, C.K.; D’Andrea, M.R.; Steinhoff, M.; Andrade-Gordon, P. Characterization of thrombin-induced leukocyte rolling and adherence: A potential proinflammatory role for proteinase-activated receptor-4. J. Immunol. 2002, 169, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Otani, H.; Yagi, Y.; Kawai, K.; Araki, H.; Fukuhara, S.; Inagaki, C. Proteinase-activated receptor 4 stimulation-induced epithelial-mesenchymal transition in alveolar epithelial cells. Respir. Res. 2007, 8, 31. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waller, A.K.; Birch, K.; Gibbins, J.M.; Clarke, S.R. Activation of Human Platelets by Staphylococcus aureus Secreted Protease Staphopain A. Pathogens 2022, 11, 1237. https://doi.org/10.3390/pathogens11111237
Waller AK, Birch K, Gibbins JM, Clarke SR. Activation of Human Platelets by Staphylococcus aureus Secreted Protease Staphopain A. Pathogens. 2022; 11(11):1237. https://doi.org/10.3390/pathogens11111237
Chicago/Turabian StyleWaller, Amie K., Katie Birch, Jonathan M. Gibbins, and Simon R. Clarke. 2022. "Activation of Human Platelets by Staphylococcus aureus Secreted Protease Staphopain A" Pathogens 11, no. 11: 1237. https://doi.org/10.3390/pathogens11111237
APA StyleWaller, A. K., Birch, K., Gibbins, J. M., & Clarke, S. R. (2022). Activation of Human Platelets by Staphylococcus aureus Secreted Protease Staphopain A. Pathogens, 11(11), 1237. https://doi.org/10.3390/pathogens11111237





