Depletion of Na+/H+ Exchanger Isoform 1 Increases the Host Cell Resistance to Trypanosoma cruzi Invasion
Abstract
:1. Introduction
2. Results
2.1. Alkalinization of Host Cell Cytosol Inhibits T. cruzi MT Invasion by Interfering with F-Actin Arrangement and Lysosome Distribution
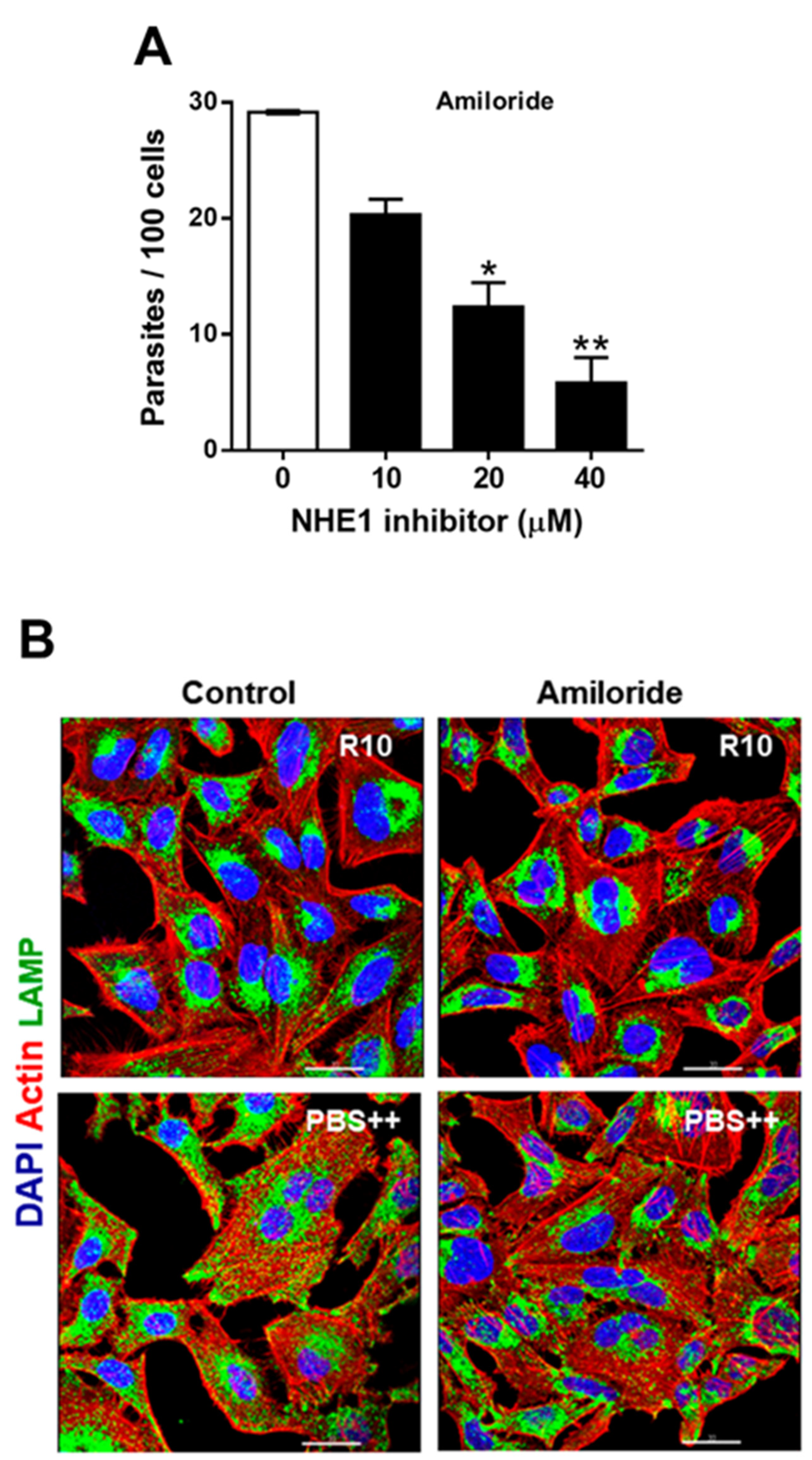
2.2. Treatment of Host Cells with NHE1 Inhibitors Reduces the Susceptibility to Invasion by T. cruzi MT
2.3. Depletion of Host Cell NHE1 Reduces the Susceptibility to MT Invasion by Affecting Actin Cytoskeleton Structure and Lysosome Distribution
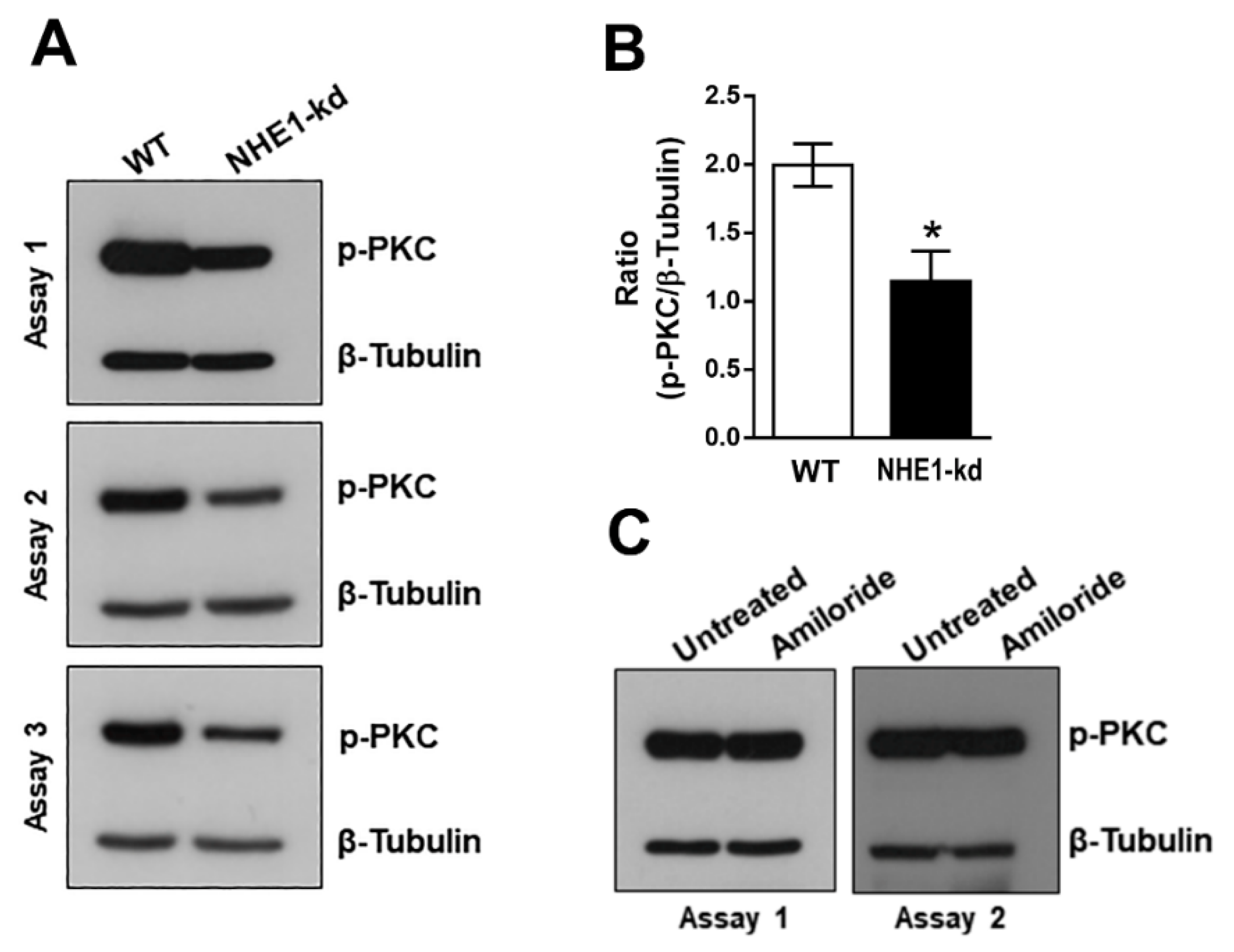
2.4. Phosphorylation Levels of PKC Are Decreased in NHE1-Deficient Cells
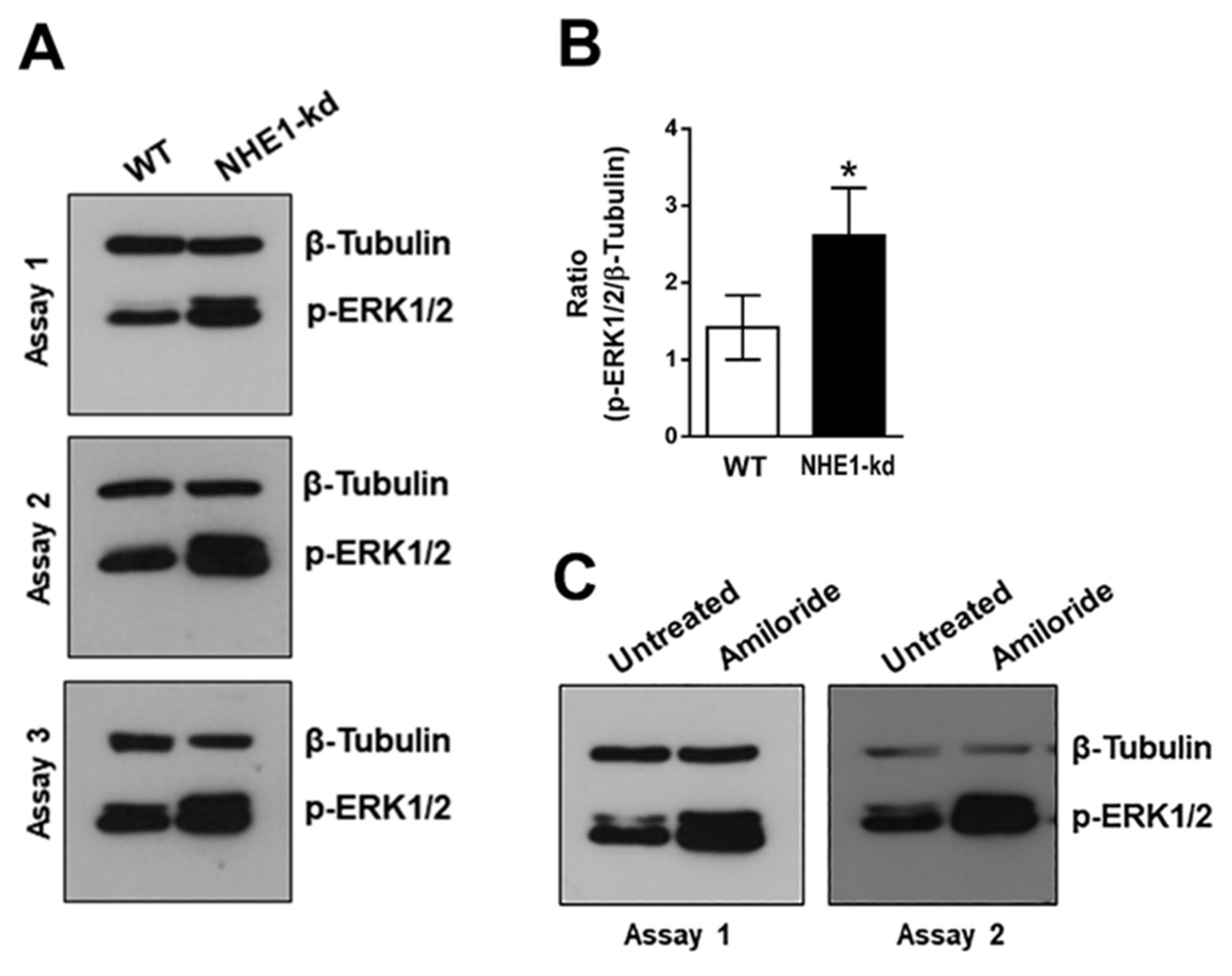
2.5. Phosphorylation Levels of ERK1/2 Are Increased in NHE1-Depleted and in Amiloride-Treated Cells
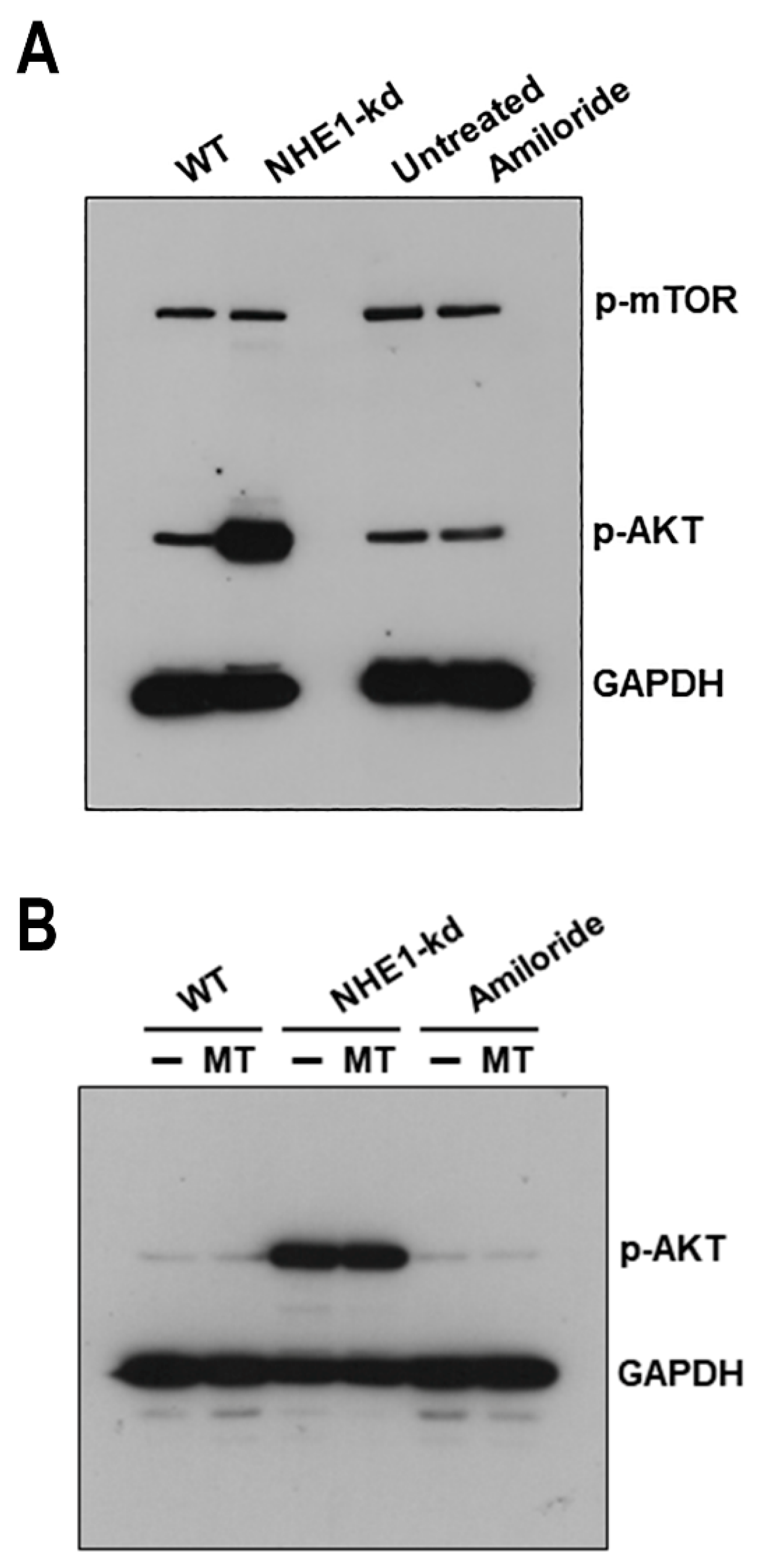
2.6. Akt Is Highly Activated in NHE1-Deficient Cells
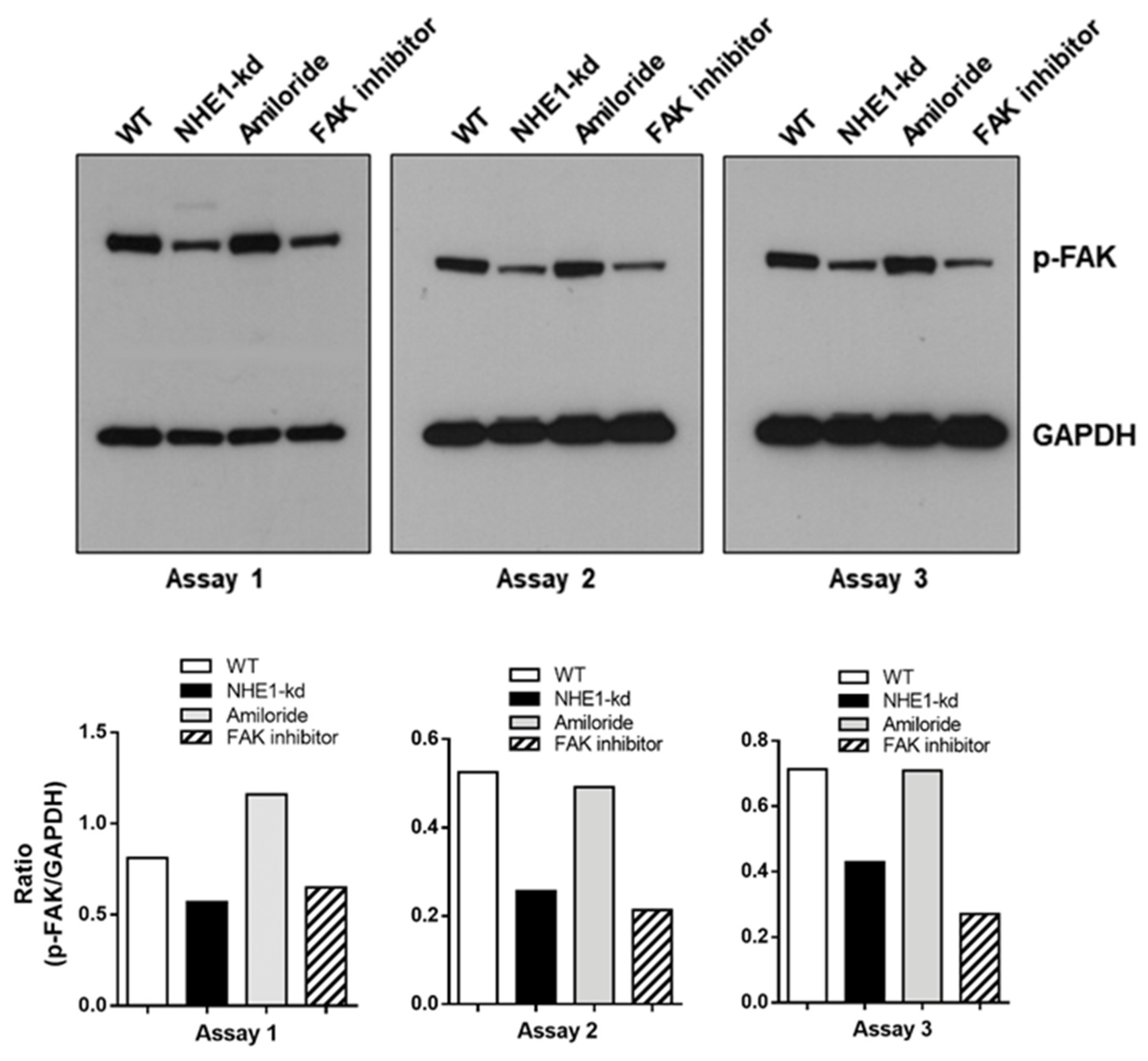
2.7. Focal Adhesion Kinase (FAK) Is Dephosphorylated in NHE1-Depleted Cells
3. Discussion
4. Materials and Methods
4.1. Parasites, Mammalian Cells and Invasion Assay
4.2. Generation of NHE1-Depleted HeLa Cell Lines
4.3. Indirect Immunofluorescence Assay
4.4. Antibodies and Reagents
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wakabayashi, S.; Shigekawa, M.; Pouyssegur, J. Molecular Physiology of Vertebrate Na+/H+ Exchangers. Physiol. Rev. 1997, 77, 51–74. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, J.; Grinstein, S. Na+/H+ Exchangers of Mammalian Cells. J. Biol. Chem. 1997, 272, 22373–22376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, S.; Pang, T.; Su, X.; Shigekawa, M. A Novel Topology Model of the Human Na+/H+ Exchanger Isoform 1. J. Biol. Chem. 2000, 275, 7942–7949. [Google Scholar] [CrossRef] [Green Version]
- Denker, S.P.; Barber, D.L. Cell Migration Requires Both Ion Translocation and Cytoskeletal Anchoring by the Na-H Exchanger NHE1. J. Cell Biol. 2002, 159, 1087–1096. [Google Scholar] [CrossRef]
- Stock, C.; Schwab, A. Role of the Na+/H+ Exchanger NHE1 in Cell Migration. Acta Physiol. 2006, 187, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Denker, S.P.; Huang, D.C.; Orlowski, J.; Furthmayr, H.; Barber, D.L. Direct Binding of the Na-H Exchanger NHE1 to ERM Proteins Regulates the Cortical Cytoskeleton and Cell Shape Independently of H+ Translocation. Mol. Cell 2000, 6, 1425–1436. [Google Scholar] [CrossRef]
- Putney, L.K.; Denker, S.P.; Barber, D.L. The Changing Face of the Na+/H+ Exchanger, NHE1: Structure, Regulation, and Cellular Actions. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 527–552. [Google Scholar] [CrossRef]
- Tardieux, I.; Webster, P.; Ravesloot, J.; Boron, W.; Lunn, J.A.; Heuser, J.E.; Andrews, N.W. Lysosome Recruitment and Fusion Are Early Events Required for Trypanosome Invasion of Mammalian Cells. Cell 1992, 71, 1117–1130. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rioult, M.G.; Ora, A.; Andrews, N.W. A Trypanosome-Soluble Factor Induces IP3 Formation, Intracellular Ca2+ Mobilization and Microfilament Rearrangement in Host Cells. J. Cell Biol. 1995, 129, 1263–1273. [Google Scholar] [CrossRef] [Green Version]
- Cortez, M.; Atayde, V.; Yoshida, N. Host Cell Invasion Mediated by Trypanosoma cruzi Surface Molecule Gp82 Is Associated with F-Actin Disassembly and Is Inhibited by Enteroinvasive Escherichia Coli. Microbes Infect. 2006, 8, 1502–1512. [Google Scholar] [CrossRef]
- Martins, R.M.; Alves, R.M.; Macedo, S.; Yoshida, N. Starvation and Rapamycin Differentially Regulate Host Cell Lysosome Exocytosis and Invasion by Trypanosoma cruzi Metacyclic Forms. Cell. Microbiol. 2011, 13, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Schenkman, S.; Andrews, N.W.; Nussenzweig, V.; Robbins, E.S. Trypanosoma cruzi Invade a Mammalian Epithelial Cell in a Polarized Manner. Cell 1988, 55, 157–165. [Google Scholar] [CrossRef]
- Stuart-Tilley, A.; Sardet, C.; Pouyssegur, J.; Schwartz, M.A.; Brown, D.; Alper, S.L. Immunolocalization of Anion Exchanger AE2 and Cation Exchanger NHE-1 in Distinct Adjacent Cells of Gastric Mucosa. Am. J. Physiol. 1994, 266, C559–C568. [Google Scholar] [CrossRef] [PubMed]
- Pizzonia, J.H.; Biemesderfer, D.; Abu-Alfa, A.K.; Wu, M.S.; Exner, M.; Isenring, P.; Igarashi, P.; Aronson, P.S. Immunochemical Characterization of Na+/H+ Exchanger Isoform NHE4. Am. J. Physiol. 1998, 275, F510–F517. [Google Scholar] [CrossRef]
- Nejsum, L.N.; Praetorius, J.; Nielsen, S. NKCC1 and NHE1 Are Abundantly Expressed in the Basolateral Plasma Membrane of Secretory Coil Cells in Rat, Mouse, and Human Sweat Glands. Am. J. Physiol. Cell Physiol. 2005, 289, C333–C340. [Google Scholar] [CrossRef] [Green Version]
- Orlowski, J.; Kandasamy, R.A.; Shull, G.E. Molecular Cloning of Putative Members of the Na/H Exchanger Gene Family. CDNA Cloning, Deduced Amino Acid Sequence, and MRNA Tissue Expression of the Rat Na/H Exchanger NHE-1 and Two Structurally Related Proteins. J. Biol. Chem. 1992, 267, 9331–9339. [Google Scholar] [CrossRef]
- Hoft, D.F.; Farrar, P.L.; Kratz-Owens, K.; Shaffer, D. Gastric Invasion by Trypanosoma cruzi and Induction of Protective Mucosal Immune Responses. Infect. Immun. 1996, 64, 3800–3810. [Google Scholar] [CrossRef] [Green Version]
- Neira, I.; Silva, F.A.; Cortez, M.; Yoshida, N. Involvement of Trypanosoma cruzi Metacyclic Trypomastigote Surface Molecule Gp82 in Adhesion to Gastric Mucin and Invasion of Epithelial Cells. Infect. Immun. 2003, 71, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Staquicini, D.I.; Martins, R.M.; Macedo, S.; Sasso, G.R.S.; Atayde, V.D.; Juliano, M.A.; Yoshida, N. Role of GP82 in the Selective Binding to Gastric Mucin during Oral Infection with Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2010, 4, e613. [Google Scholar] [CrossRef]
- Yoshida, N. Molecular Basis of Mammalian Cell Invasion by Trypanosoma cruzi. An. Acad. Bras. Cienc. 2006, 78, 87–111. [Google Scholar] [CrossRef]
- Rodrigues, J.P.F.; Souza Onofre, T.; Barbosa, B.C.; Ferreira, É.R.; Bonfim-Melo, A.; Yoshida, N. Host Cell Protein LAMP-2 Is the Receptor for Trypanosoma cruzi Surface Molecule Gp82 That Mediates Invasion. Cell. Microbiol. 2019, 21, e13003. [Google Scholar] [CrossRef] [Green Version]
- Cortez, C.; Real, F.; Yoshida, N. Lysosome Biogenesis/Scattering Increases Host Cell Susceptibility to Invasion by Trypanosoma cruzi Metacyclic Forms and Resistance to Tissue Culture Trypomastigotes. Cell. Microbiol. 2016, 18, 748–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onofre, T.S.; Rodrigues, J.P.F.; Shio, M.T.; Macedo, S.; Juliano, M.A.; Yoshida, N. Interaction of Trypanosoma cruzi Gp82 With Host Cell LAMP2 Induces Protein Kinase C Activation and Promotes Invasion. Front. Cell. Infect. Microbiol. 2021, 11, 627888. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Cortez, M.; Atayde, V.D.; Yoshida, N. Actin Cytoskeleton-Dependent and -Independent Host Cell Invasion by Trypanosoma cruzi Is Mediated by Distinct Parasite Surface Molecules. Infect. Immun. 2006, 74, 5522–5528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enomoto, A.; Murakami, H.; Asai, N.; Morone, N.; Watanabe, T.; Kawai, K.; Murakumo, Y.; Usukura, J.; Kaibuchi, K.; Takahashi, M. Akt/PKB Regulates Actin Organization and Cell Motility via Girdin/APE. Dev. Cell 2005, 9, 389–402. [Google Scholar] [CrossRef] [Green Version]
- Jin, E.J.; Kwang, S.P.; Bang, O.S.; Kang, S.S. Akt Signaling Regulates Actin Organization via Modulation of MMP-2 Activity during Chondrogenesis of Chick Wing Limb Bud Mesenchymal Cells. J. Cell. Biochem. 2007, 102, 252–261. [Google Scholar] [CrossRef]
- Liu, L.; Chen, L.; Chung, J.; Huang, S. Rapamycin Inhibits F-Actin Reorganization and Phosphorylation of Focal Adhesion Proteins. Oncogene 2008, 27, 4998–5010. [Google Scholar] [CrossRef] [Green Version]
- Li, S.Y.; Mruk, D.D.; Cheng, C.Y. Focal Adhesion Kinase Is a Regulator of F-Actin Dynamics. Spermatogenesis 2013, 3, e25385. [Google Scholar] [CrossRef]
- Onofre, T.S.; Rodrigues, J.P.F.; Yoshida, N. Depletion of Host Cell Focal Adhesion Kinase Increases the Susceptibility to Invasion by Trypanosoma cruzi Metacyclic Forms. Front. Cell. Infect. Microbiol. 2019, 9, 231. [Google Scholar] [CrossRef]
- Rodríguez, A.; Samoff, E.; Rioult, M.G.; Chung, A.; Andrews, N.W. Host Cell Invasion by Trypanosomes Requires Lysosomes and Microtubule/Kinesin-Mediated Transport. J. Cell Biol. 1996, 134, 349–362. [Google Scholar] [CrossRef]
- Brandt, D.; Gimona, M.; Hillmann, M.; Haller, H.; Mischak, H. Protein Kinase C Induces Actin Reorganization via a Src- and Rho-Dependent Pathway. J. Biol. Chem. 2002, 277, 20903–20910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Zhang, X.F.; Van Goor, D.; Dunn, A.P.; Hyland, C.; Medeiros, N.; Forscher, P. Protein Kinase C Activation Decreases Peripheral Actin Network Density and Increases Central Nonmuscle Myosin II Contractility in Neuronal Growth Cones. Mol. Biol. Cell 2013, 24, 3097–3114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hatton, G.I. Extracellular Signal-Regulated Protein Kinase 1/2 with Actin Cytoskeleton in Supraoptic Oxytocin Neurons and Astrocytes: Role in Burst Firing. J. Neurosci. 2007, 27, 13822–13834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fliegel, L. Structural and Functional Changes in the Na+/H+ Exchanger Isoform 1, Induced by Erk1/2 Phosphorylation. Int. J. Mol. Sci. 2019, 20, 2378. [Google Scholar] [CrossRef] [Green Version]
- Meima, M.E.; Webb, B.A.; Witkowska, H.E.; Barber, D.L. The Sodium-Hydrogen Exchanger NHE1 Is an Akt Substrate Necessary for Actin Filament Reorganization by Growth Factors. J. Biol. Chem. 2009, 284, 26666–26675. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.M.; Yoshida, N. Stage-Specific Surface Antigens of Metacyclic Trypomastigotes of Trypanosoma cruzi Identified by Monoclonal Antibodies. Mol. Biochem. Parasitol. 1986, 18, 271–282. [Google Scholar] [CrossRef]
- Bonfim-Melo, A.; Zanetti, B.F.; Ferreira, E.R.; Vandoninck, S.; Han, S.W.; Van Lint, J.; Mortara, R.A.; Bahia, D. Trypanosoma cruzi Extracellular Amastigotes Trigger the Protein Kinase D1-Cortactin-Actin Pathway during Cell Invasion. Cell Microbiol. 2015, 17, 1797–1810. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, J.P.F.; Loch, L.; Onofre, T.S.; Yoshida, N. Depletion of Na+/H+ Exchanger Isoform 1 Increases the Host Cell Resistance to Trypanosoma cruzi Invasion. Pathogens 2022, 11, 1294. https://doi.org/10.3390/pathogens11111294
Rodrigues JPF, Loch L, Onofre TS, Yoshida N. Depletion of Na+/H+ Exchanger Isoform 1 Increases the Host Cell Resistance to Trypanosoma cruzi Invasion. Pathogens. 2022; 11(11):1294. https://doi.org/10.3390/pathogens11111294
Chicago/Turabian StyleRodrigues, João Paulo Ferreira, Leonardo Loch, Thiago Souza Onofre, and Nobuko Yoshida. 2022. "Depletion of Na+/H+ Exchanger Isoform 1 Increases the Host Cell Resistance to Trypanosoma cruzi Invasion" Pathogens 11, no. 11: 1294. https://doi.org/10.3390/pathogens11111294




