Prevalence, Infection Intensity and Molecular Diagnosis of Mixed Infections with Metastrongylus spp. (Metastrongylidae) in Wild Boars in Uzbekistan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens and Morphological Comparisons
2.1.1. Collection of Adult Lungworms from Wild Boars
2.1.2. Collection of Third-Stage Larval Lungworms from Earthworms
2.2. DNA Extraction, PCR Amplification and Sequencing
2.3. Phylogenetic Analysis
2.4. Statistical Analysis
3. Results
3.1. Prevalence, Intensity and Species Composition
3.2. Molecular Characterization of Metastrongylus spp. from Uzbekistan
3.3. Seasonality of Infection
3.4. Age Dynamics of Infections with Metastrongylids
3.5. Prevalence of Metastrongylid larvae in Different Oligochaete Species
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission, 2nd ed.; CAB International: Wallingford, UK, 2000; p. 650. [Google Scholar]
- Boev, S.N. Protostrongylids, Fundamentals of Nematology; Nauka Publishers: Moscow, Russia, 1975; Volume 25, p. 265. [Google Scholar]
- Durette-Desset, M.C.; Beveridge, I.; Spratt, D.M. The origins and evolutionary expansion of the Strongylida (Nematoda). Int. J. Parasitol. 1994, 24, 1139–1165. [Google Scholar] [CrossRef]
- Kontrimavicius, V.L.; Delyamure, S.L.; Boev, S.N. Fundamentals of Nematodology. In Metastrongyloids of Domestic and Wild Animals. M.; Publishing Nauka: Moscow, Russia, 1976; Volume 26, 239p. [Google Scholar]
- Tokobaev, M.M. Helminths of wild mammals of Central Asia; Publishing Ilim: Frunze, Kyrgyzstan, 1976; p. 179. [Google Scholar]
- Kuchboev, A.E.; Krücken, J.; Ruziev, B.H.; Von Samson-Himmelstjerna, G. Molecular phylogeny and diagnosis of species of the family Protostrongylidae from caprine hosts in Uzbekistan. Parasitol. Res. 2015, 114, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, I.; Spratt, D.M.; Durette-Desset, M.C. Order Strongylida (Railliet & Henry, 1913) Handbook of Zoology; Schmidt-Rhaesa, A., Ed.; De Gruyter: Berlin, Germany, 2014; Volume 2, pp. 557–608. [Google Scholar]
- Khrustalev, A.V. On the species composition of the genus Metastrongylus-parasites of the lungs of pigs and wild boars in the USSR. Parasitology 1981, 15, 420–423. [Google Scholar]
- Forrester, D.J.; Porter, J.H.; Belden, R.C.; Frankenberger, W.B. Lungworms of feral swine in Florida. J. Am. Vet. Med. Assoc. 1982, 181, 1278–1280. [Google Scholar]
- Morita, T.; Haruta, K.; Shibata-Haruta, A.; Kanda, E.; Imai, S.; Ike, K. Lung worms of wild boars in the western region of Tokyo, Japan. J. Vet. Med. Sci. 2007, 69, 417–420. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, D.; Müller, G. Parasites of the respiratory tract of Sus scrofa scrofa (wild boar) from commercial breeder in southern Brazil and its relationship with Ascaris suum. Parasitol. Res. 2013, 112, 1353–1356. [Google Scholar] [CrossRef]
- Nagy, G.; Varga, G.; Csivincsik, A.; Sugar, L. Occurrence of Metastrongylus asymmetricus (Noda, 1973) in Hungary. Magy. Allatorv. Lapja 2013, 135, 308–312. [Google Scholar]
- Ruziev, B.K.; Kuchboev, A.E.; Karimova, R.R. Ecology of nematodes of the genus Metastrongylus Molin, 1861-parasites of the wild boar of Uzbekistan. Bull. Karshi State Univ. 2020, 4, 58–61. [Google Scholar]
- Baitursunov, K.K. To the study of the ecology of wild boar helminths (Sus scrofa Linnaeus, 1758) in Kazakhstan. In Proceedings of the National Academy of Sciences of the Republic of Kazakhstan; Biological Series; Publisher Ғылым: Almaty, Kazakhstan, 2010; Volume 2, pp. 26–29. [Google Scholar]
- Kotlan, A. Die Helminthosen der Haus- und Nutztiere unter Berücksichtigung der Helminthosen des Menschen; Akadémiai Kiadó: Budapest, Hungary, 1960; p. 631. [Google Scholar]
- Gassó, D.L.; Rossi, G.; Mentaberre, E.; Casas, R.; Velarde, P.; Nosal, E.; Serrano, J.; Segales, P.; Fernandez-Llario, P.; Feliu, C. An identification key for the five most common species of Metastrongylus. Parasitol. Res. 2014, 113, 3495–3500. [Google Scholar] [CrossRef]
- Miloshev, B. Case of triple infection with Metastrongylus elongatus, Thaeniarhynchus saginatus and Enterobius vermicularis. Suvrem. Meditsina 1956, 7, 94–97. [Google Scholar]
- Beaver, P.C.; Jung, R.C.; Cupp, E.W. Clinical Parasitology, 9th ed.; Lea and Febiger: Philadelphia, PA, USA, 1984; pp. 291–292. [Google Scholar]
- Calvopina, M.; Caballero, H.; Morita, T.; Korenaga, M. Human pulmonary infection by the zoonotic Metastrongylus salmi nematode. The first reported case in the Americas. Am. J. Trop. Med. Hyg. 2016, 95, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Shol, V.A. Helminthiases of pigs in Kazakhstan; Publisher Ғылым: Almaty, Kazakhstan, 1964; 45p. [Google Scholar]
- Melnikova, T.G. Some information about the helminth fauna of the wild boar Sus scroga nigripes in Tajikistan. Collection Natural foci of diseases and questions of parasitology. Alma-Ata 1961, 3, 285–287. [Google Scholar]
- Koshchanov, E.K. Helminthes of wild mammals of Uzbekistan. Ph.D. Thesis, Institute of Zoology, Tashkent, Uzbekistan, 1972; p. 36. [Google Scholar]
- Shapolatov, Z.S. Pig Parasites in Uzbekistan (Helminths); Publisher Fan: Tashkent, Uzbekistan, 1979; p. 118. [Google Scholar]
- Karimova, R.R.; Kuchboev, A.E.; Umarov, D.K. Pulmonary nematodes of wild mammals of Uzbekistan. In Proceedings of the Actual Problems of Studying and Preserving the Animal World of Uzbekistan, Tashkent, Uzbekistan, 16–17 April 2011; p. 87. [Google Scholar]
- Kuchboev, A.E.; Umarov, D.K.; Karimova, R.R.; Ruziev, B.K. Metastrongilids of wild boars in Uzbekistan. In Proceedings of the European Multicolloquium of Parasitology: Program&Abstract Book EMOP XI, Cluj-Napoca, Romania, 25–29 July 2012; pp. 468–469. [Google Scholar]
- Alicata, J.E. Life history of Metastrongylus salmi and remarks on the eggs of the swine lungworm. Proc. Helminthol. Soc. Wash. 1934, 1, 12–13. [Google Scholar]
- Hobmaier, A.; Hobmaier, M. Die Entwicklung der Larve des Lungenwurmes Metastrongylus elongatus (Strongylus paradoxus) des Schweines und ihr Invasionsweg, sowie vorläufige Mitteilung über die Entwicklung von Choerostrongylus brevivaginatus. Münch. Tierärtzl. Wschr. 1929, 80, 365–369. [Google Scholar]
- Breza, M.K. Epizootologickemu vyznamu horizontsnej migraete dazdoviek (najna E. foetida) pri metastrongylosach osipanych. Folia Vet. 1961, 5, 287–293. [Google Scholar]
- Rose, J. Metastrongylus apri the pig lungworm: Observations on the free-living embryonated egg and the larva in the intermediate host. Parasitology 1959, 49, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Bobkova, A.F. The study of the epizootology of pig metastrongilosis in Belarus and the search for new methods of therapy—Achievements of veterinary science-in production. Trans. Belarusian Sci. Vet. 1966, 5, 74–79. [Google Scholar]
- Kolevatova, A.I. Infection of lumbricides metastrongylus. Mater. Vses. Obs. Helminthol. 1972, 24, 79–86. [Google Scholar]
- Tiunov, V.I. Some questions of the relationship between metastrongylus and their intermediate hosts. Teach materials. Trans. Vses. Inst. Helminthol. 1966, 1, 275–281. [Google Scholar]
- Ustinov, I.D. Infection of various species of earthworms with metastrongylid larvae in farms that is unfavorable for pig metastrongilosis. Trans. Vses. Inst. Helminthol. 1963, 10, 68–81. [Google Scholar]
- Burenkov, S.N.; Krotenkov, V.P. Ecological and epizootological features of Lumbricidae-intermediate hosts of wild boar metastrongylids. Russ. Parasitol. J. 2013, 1, 34–38. [Google Scholar]
- Antipov, A.A.; Bakhur, T.I.; Feshchenko, D.V.; Romanishina, T.A.; Avramenko, N.V.; Goncharenko, V.P.; Zghozinska, O.A.; Solovyova, L.M.; Koziy, N.V.; Pidborska, R.V.; et al. Earthworms (Lumbricidae) as Intermidiate Hosts of Lung Nematodes (Metastrongylidae) of Swine in Kyiv and Zhytomyr Regions of Ukraine. Vestn. Zool. 2018, 52, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Gasser, R.B.; Chilton, N.B.; Hoste, H.; Beveridge, I. Rapid sequencing of rDNA from single worms and eggs of parasitic helminths. Nucleic Acids Res. 1993, 21, 2525–2526. [Google Scholar] [CrossRef] [Green Version]
- Conole, J.C.; Chilton, N.B.; Jarvis, T.; Gasser, R.B. Mutation scanning analysis of microsatellite variability in the second internal transcribed spacer (precursor ribosomal RNA) for three species of Metastrongylus (Strongylida: Metastrongyloidea). Parasitology 2001, 122, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Santín-Durán, M.; de la Fuente, C.; Alunda, J.M.; Rosenthal, B.M.; Hoberg, E.P. Identical ITS-1 and ITS-2 sequences suggest Spiculopteragia asymmetrica and Spiculopteragia quadrispiculata (Nematoda: Trichostrongylidae) constitute morphologically distinct variants of a single species. J. Parasitol. 2002, 88, 417–418. [Google Scholar] [CrossRef]
- Chilton, N.B.; Huby-Chilton, F.; Gasser, R.B.; Beveridge, I. The evolutionary origins of nematodes within the order Strongylida are related to predilection sites within hosts. Mol. Phylogenetics Evol. 2006, 40, 118–128. [Google Scholar] [CrossRef]
- Kutz, S.J.; Asmundsson, I.; Hoberg, E.P.; Appleyard, G.D.; Jenkins, E.J.; Beckmen, K.; Branigan, M.; Butler, L.; Chilton, N.B.; Cooley, D.; et al. Serendipitous discovery of a novel protostrongylid (Nematoda: Metastrongyloidea) in caribou (Rangifer tarandus), muskoxen (Ovibos moschatus) and moose (Alces alces) from high latitudes of North America based on DNA sequence comparisons. Can. J. Zool. 2007, 85, 1143–1156. [Google Scholar] [CrossRef]
- Kuchboev, A.; Sobirova, K.; Karimova, R.; Amirov, O.; Samson-Himmelstjerna, G.; Krücken, J. Molecular analysis of polymorphic species of the genus Marshallagia (Nematoda: Ostertagiinae). Parasites Vectors 2020, 13, 411. [Google Scholar] [CrossRef]
- Leignel, V.; Humbert, J.F.; Elard, L. Study by ribosomal DNA ITS 2 sequencing and RAPD analysis on the systematics of four Metastrongylus species (Nematoda: Metastrongyloidea). J. Parasitol. 1997, 83, 606–611. [Google Scholar] [CrossRef]
- Carreno, R.A.; Nadler, S.A. Phylogenetic analysis of the Metastrongyloidea (Nematoda: Strongylida) inferred from ribosomal RNA gene sequences. J. Parasitol. 2003, 89, 965–973. [Google Scholar] [CrossRef]
- Malevich, I.I. Collection and Study of Earthworms-Soil-Formers; Publisher Selkhozizdat: Moscow, Russia, 1950; p. 156. [Google Scholar]
- Gilyarov, M.S. Accounting for large invertebrates (mesofauna). In Quantitative Methods in Soil Zoology; Nauka: Moscow, Russia, 1987; pp. 9–26. [Google Scholar]
- Perel, T.S. Distribution and Patterns of Distribution of Earthworms in the Fauna of the USSR; Publisher Nauka: Moscow, Russia, 1979; p. 272. [Google Scholar]
- Kotelnikov, G.A. Diagnosis of Helminthiases in Animals; Publisher Selkhozizdat: Moscow, Russia, 1974; p. 240. [Google Scholar]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefimgs Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.; Schmidt, H.A.; Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [Green Version]
- Kalyaanamoorthy, S.; Minh, B.; Wong, T.; Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [Green Version]
- Hoang, D.T.; Chernomor, O.; Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 2518–2522. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [Green Version]
- Anisimova, M.; Gil, M.; Dufayard, J.-F.; Dessimoz, C.; Gascuel, O. Survey of Branch Support Methods Demonstrates Accuracy, Power, and Robustness of Fast Likelihood-based Approximation Schemes. Syst. Biol. 2011, 60, 685–699. [Google Scholar] [CrossRef] [Green Version]
- Regina, M.M.; Marycruz, Á.J.; Alix, D.; Frédérique, R.; Manuel, B.; José, A.G.; Carlos, R.C.; Roger, G.; Luc, V.; Isabelle, B. Earthworms Building Up Soil Microbiota, a Review. Front. Environ. Sci. Eng. 2019, 7, 81. [Google Scholar] [CrossRef] [Green Version]
- Thamsborg, S.M.; Roepstorff, A.; Larsen, M. Integrated and biological control of parasites in organic and conventional production systems. Vet. Parasitol. 1999, 84, 169–186. [Google Scholar] [CrossRef]
- Wallgren, P.; Pettersson, E. Lungworms (Metastrongylus spp.) demonstrated in domestic pigs with respiratory disease: Was there a clinical relevance? Porc. Health Manag. 2022, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-de-Mera, I.G.; Gortazar, C.; Vicente, J.; Hofle, U.; Fierro, Y. Wild boar helminths: Risks in animal translocations. Vet. Parasitol. 2003, 115, 335–341. [Google Scholar] [CrossRef]
- Jarvis, T.; Kapel, C.; Moks, E.; Talvik, H.; Magi, E. Helminths of wild boar in the isolated population close to the northern border of its habitat area. Vet. Parasitol. 2007, 150, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Förster, M.; Klimpel, M.; Sievert, K. The house fly (Musca domestica) as a potential vector of metazoan parasites caught in a pig-pen in Germany. Vet. Parasitol. 2009, 160, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Senlik, B.; Cirak, V.; Girisgin, O.; Akyol, C. Helminth infections of wild boars (Sus scrofa) in the Bursa province of Turkey. J. Helminthol. 2011, 85, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Sarkari, B.; Mowlavi, G.R. Helminth parasites of wild boars. Iran. J. Parasitol. 2016, 11, 377–382. [Google Scholar]
- Cleveland, C.A.; DeNicola, A.; Dubey, J.P.; Hill, D.E.; Berghaus, R.D.; Yabsley, M.J. Survey for selected pathogens in wild pigs (Sus scrofa) from Guam, Marianna Islands, USA. Vet. Microbiol. 2017, 205, 22–25. [Google Scholar] [CrossRef]
- Li, K.; Luo, H.; Zhang, H.; Lan, Y.; Han, Z.; Shahzad, M.; Wang, X.; Qiu, G.; Huang, S.; Jiang, W.; et al. First report of Metastrongylus pudendotectus by the genetic characterization of mitochondria genome of cox1 in pigs from Tibet, China. Vet. Parasitol. 2016, 223, 91–95. [Google Scholar] [CrossRef]
- Panayotova-Pencheva, M.; Dakova, V. Studies on the gastrointestinal and lung parasite fauna of wild boars (Sus scrofa scrofa L.) from Bulgaria. Ann. Parasitol. 2018, 64, 379–384. [Google Scholar] [PubMed]
- Andreyanov, O.N. Helminth fauna of wild boar in the Ryazan region. Russ. Parasitol. J. 2013, 4, 6–8. [Google Scholar]
- Samoilovskaya, N.A. Parasite fauna of wild boars in the “Losiny Ostrov” National Park (Moscow). Russ. Parasitol. J. 2011, 4, 6–8. [Google Scholar]
- Kaplich, V.M.; Yakubovsky, M.V.; Tereshkina, N.V. About the helminthes fauna of wild boar (Sus scrofa) in the subzone of oak-dark coniferous forests of Belarus. In Proceedings of Belarusian State Technological University; BSTU: Minsk, Belarus, 2013; Volume 1, pp. 70–72. [Google Scholar]
- Dakova, V.; Panayotova-Pencheva, M. Morphometric Features of Three Lungworms in Materials from Wild Boars from Bulgaria. Acta Morphol. Anthropol. 2017, 24, 21–29. [Google Scholar]
- Nosal, P.; Kowal, J.; Nowosad, B. Structure of Metastrongylidae in wild boars from southern Poland. Helminthologia 2010, 47, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Spieler, N.; Schnyder, M. Lungworms (Metastrongylus spp.) and intestinal parasitic stages of two separated Swiss wild boar populations north and south of the Alps: Similar parasite spectrum with regional idiosyncrasies. Int. J. Parasitol. Parasites Wildl. 2021, 14, 202–210. [Google Scholar] [CrossRef]
- Nagy, G.; Csivincsik, A.; Sugár, L. Wild boar density drives Metastrongylus infection in earthworm. Acta Parasitol. 2014, 60, 25–29. [Google Scholar] [CrossRef]
- Hoglund, J.; Wilhelmsson, E.; Christensson, D.; Morner, T.; Waller, P.G.; Mattsson, J. ITS2 sequences of Dictyocaulus species from cattle, roe deer and moose in Sweden: Molecular evidence for a new species. Int. J. Parasitol. 1999, 29, 607–611. [Google Scholar] [CrossRef]
- von Samson-Himmelstjerna, G.; Janssen, I.J.I.; Ramünke, S.; Goday, C.; Borges, F.A.; Koudela, B.; Niedźwiedź, A.; Tomczuk, K.; Studzińska, M.B.; Kornas, S.; et al. Very low intraspecific sequence variation in selected nuclear and mitochondrial Parascaris univalens genes. Infect. Genet. Evol. 2021, 95, 105035. [Google Scholar] [CrossRef]
- Louro, M.; Kuzmina, T.A.; Bredtmann, C.M.; Diekmann, I.; de Carvalho, L.M.M.; von Samson-Himmelstjerna, G.; Krücken, J. Genetic variability, cryptic species and phylogenetic relationship of six cyathostomin species based on mitochondrial and nuclear sequences. Sci. Rep. 2021, 11, 8245. [Google Scholar] [CrossRef]
- Ramünke, S.; de Almeida, B.F.; von Son-de, F.E.; von Samson-Himmelstjerna, G.; Krücken, J. Molecular marker sequences of cattle Cooperia species identify Cooperia spatulata as a morphotype of Cooperia punctata. PLoS ONE 2018, 13, e0200390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roeber, F.; Kahn, L. The specific diagnosis of gastrointestinal nematode infections in livestock: Larval culture technique, its limitations and alternative DNA-based approaches. Vet. Parasitol. 2014, 205, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Krücken, J.; Martinez-Valladares, M.; Pepe, P.; Maurelli, M.P.; de Queiroz, C.; Castilla Gómez, A.V.; Wang, T.; Cringoli, G.; Charlier, J.; et al. Advances in diagnosis of gastrointestinal nematodes in livestock and companion animals. Adv. Parasitol. 2022, 118, 85–176. [Google Scholar] [CrossRef] [PubMed]
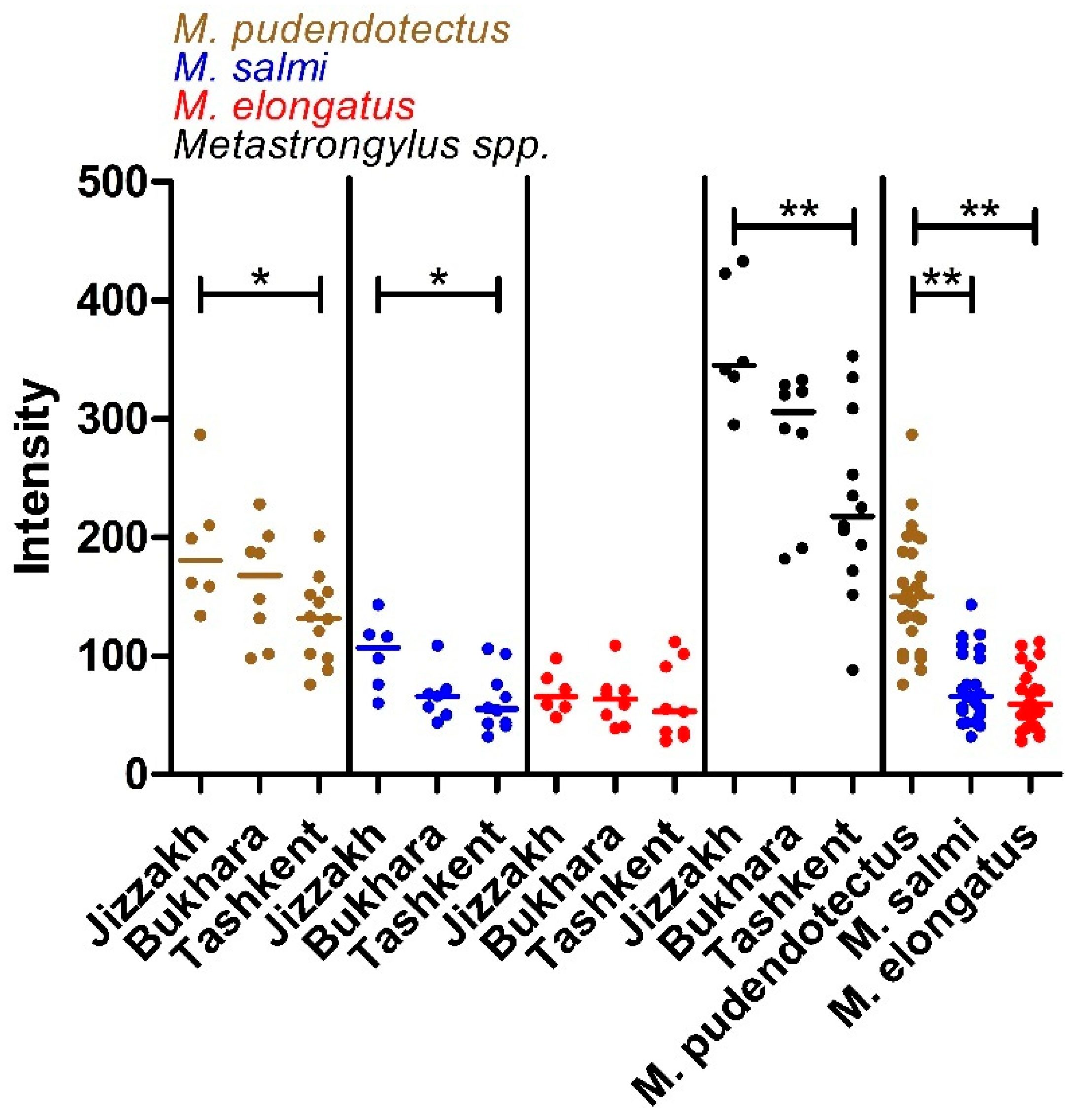
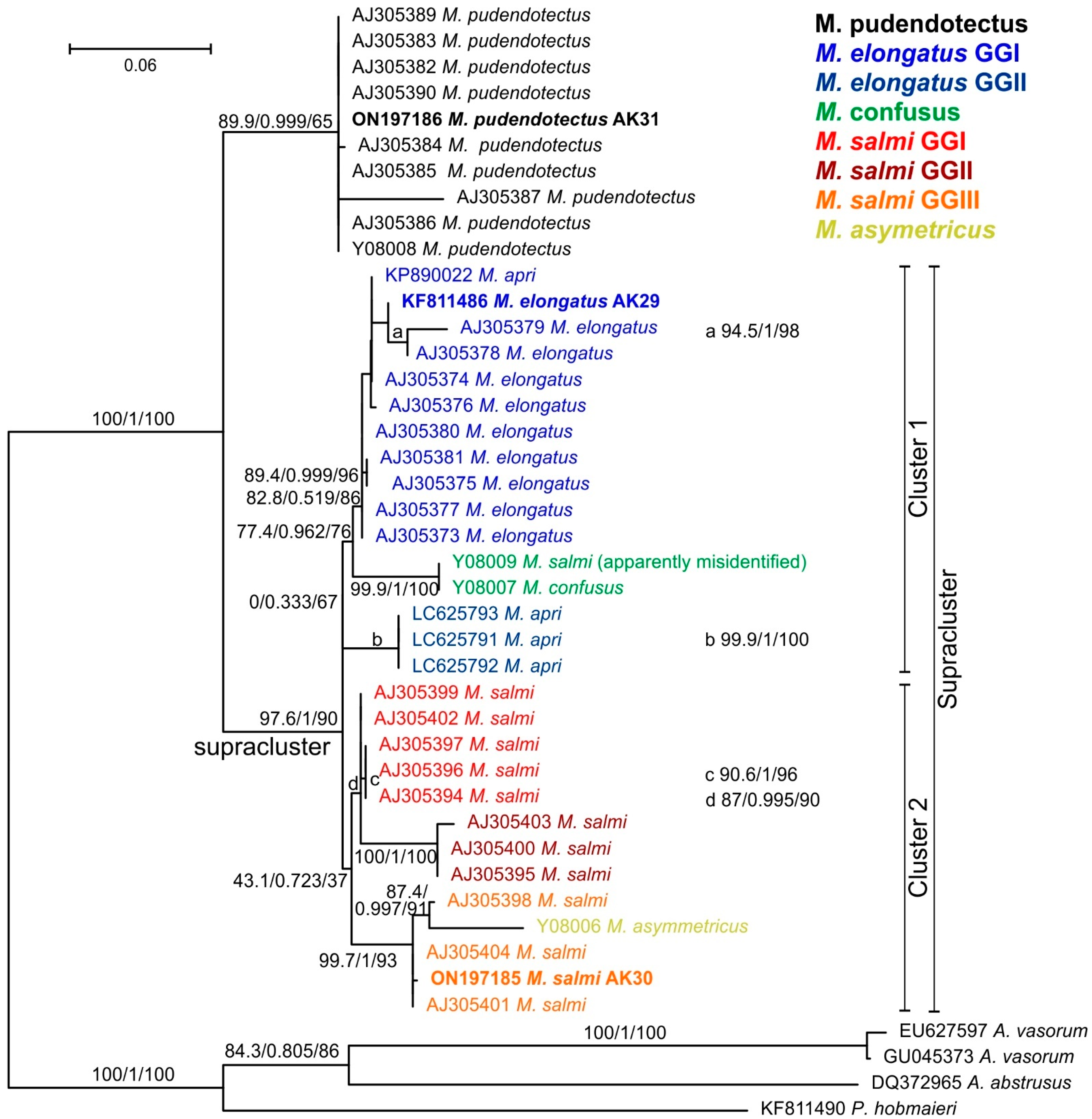
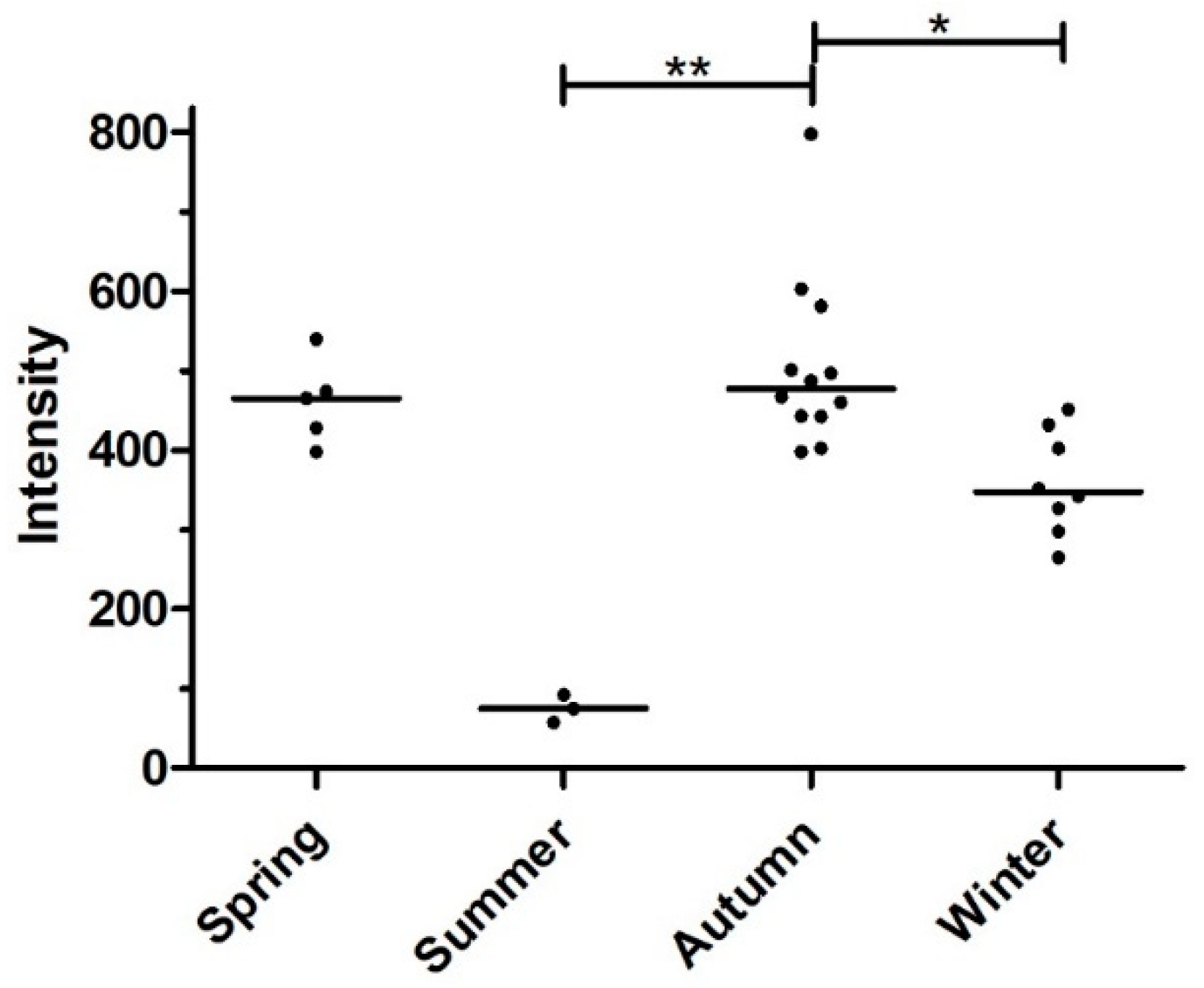
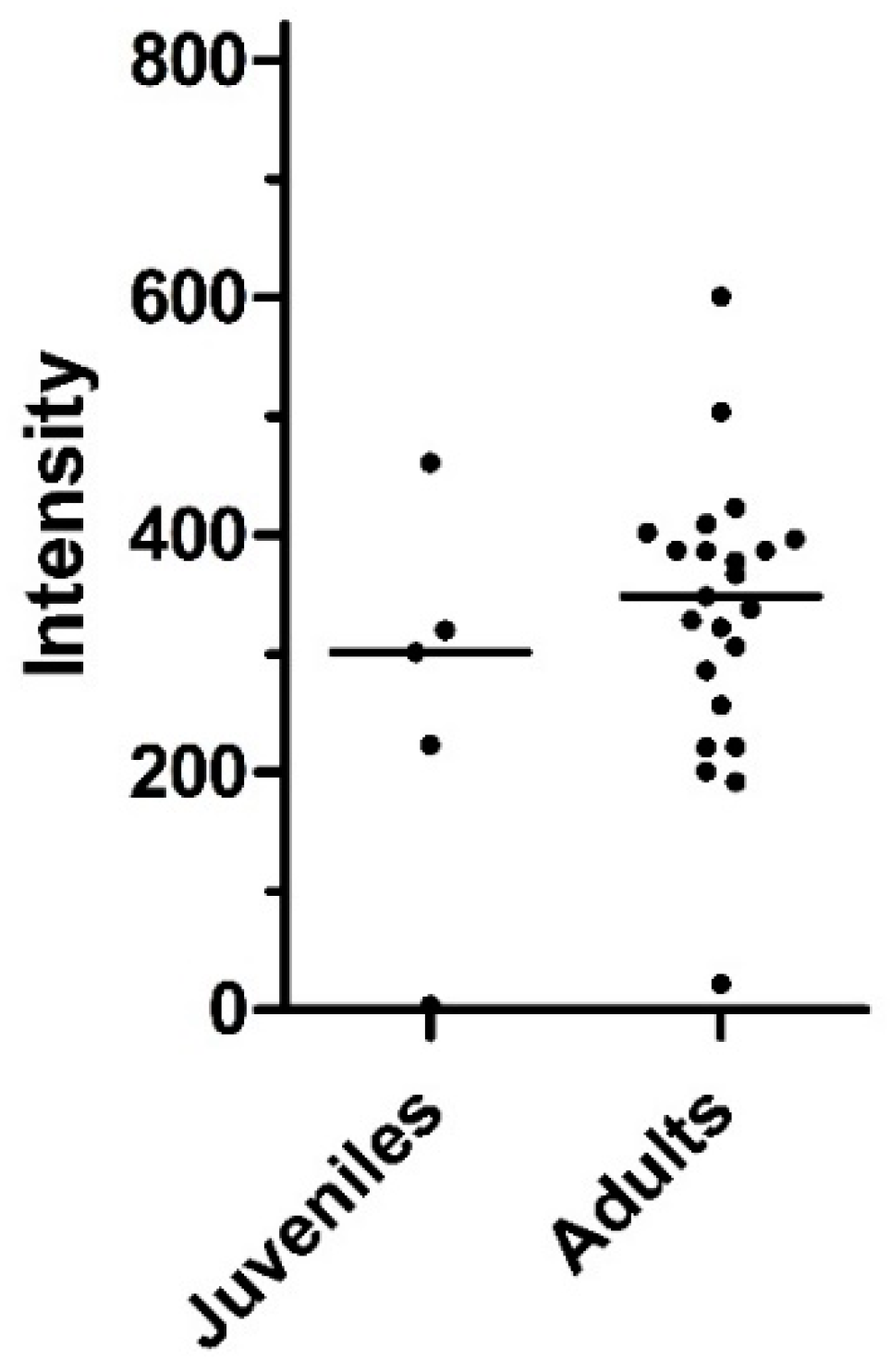
| Metastrongylus spp. | Metastrongylus pudendotectus | Metastrongylus salmi | Metastrongylus elongatus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | N | n | % Prevalence (95% CI) | n | % Prevalence (95% CI) | n | % Prevalence (95% CI) | n | % Prevalence (95% CI) |
| Jizzakh | 9 | 6 | 66.7 (35.4–87.8) | 6 | 66.7 (35.4–87.8) | 6 | 66.7 (35.4–87.8) | 6 | 66.7 (35.4–87.8) |
| Bukhara | 11 | 8 | 72.7 (43.4–90.3) | 8 | 72.7 (43.4–90.3) | 7 | 63.6 (35.4–84.8) | 8 | 72.7 (43.4–90.3) |
| Tashkent | 13 | 12 | 92.3 (66.7–98.6) | 12 | 92.3 (66.7–98.6) | 11 | 84.6 (57.8–95.7) | 11 | 84.6 (57.8–95.7) |
| Total | 33 | 26 | 78.8 (62.2–89.3) | 26 | 78.8 (62.2–89.3) | 24 | 72.7 (55.8–84.9) | 25 | 75.7 (59.0–87.2) |
| Species | Specimen ID | GenBank Accession No. | Stage | Host and Locality |
|---|---|---|---|---|
| Metastrongylus elongatus | 29 | KF811486 | adult | Sus scrofa, Tashkent, Uzbekistan |
| Metastrongylus salmi | 30 | ON197185 | adult | Sus scrofa, Tashkent, Uzbekistan |
| Metastrongylus pudendotectus | 31 | ON197186 | adult | Sus scrofa, Tashkent, Uzbekistan |
| Season | N | n | % Prevalence (95% CI) |
|---|---|---|---|
| Spring | 5 | 4 | 80 (37.6–96.4) |
| Summer | 6 | 3 | 50 (18.8–81.2) |
| Autumn | 13 | 12 | 92.3 (66.7–98.6) |
| Winter | 9 | 8 | 88.8 (56.5–98.0) |
| Age Group | N | n | % Prevalence (95% CI) |
|---|---|---|---|
| Juveniles | 6 | 5 | 83.3 (43.6–97.0) |
| Adults | 27 | 21 | 77.8 (59.2–89.4) |
| Oligochaeta Species | N | n | % Prevalence (95% CI) | Significant Differences | Ecology § |
|---|---|---|---|---|---|
| Aporrectodea caliginosa trapezoides | 96 | 16 | 16.7 (10.5–25.4) | a,d,e,g,j,l | Endogeic |
| Aporrectodea jassyensis | 46 | 0 | 0 (0–7.7) | b,c,e,h,j,k | Endogeic |
| Octolasium lacteum | 48 | 5 | 10.4 (4.5–22.2) | a,d,e,g,j,l | Endogeic |
| Eisenia fetida | 64 | 8 | 12.5 (6.5–22.8) | a,d,e,g,j,l | Epigeic, epiendogeic |
| Eisenia veneta | 96 | 0 | 0 (0–3.8) | b,c,f,h,i,k | Epigeic |
| Dendrobaena byblica | 88 | 0 | 0 (0–4.2) | b,c,f,h,i,k | Epigeic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuchboev, A.E.; Krücken, J. Prevalence, Infection Intensity and Molecular Diagnosis of Mixed Infections with Metastrongylus spp. (Metastrongylidae) in Wild Boars in Uzbekistan. Pathogens 2022, 11, 1316. https://doi.org/10.3390/pathogens11111316
Kuchboev AE, Krücken J. Prevalence, Infection Intensity and Molecular Diagnosis of Mixed Infections with Metastrongylus spp. (Metastrongylidae) in Wild Boars in Uzbekistan. Pathogens. 2022; 11(11):1316. https://doi.org/10.3390/pathogens11111316
Chicago/Turabian StyleKuchboev, Abdurakhim E., and Jürgen Krücken. 2022. "Prevalence, Infection Intensity and Molecular Diagnosis of Mixed Infections with Metastrongylus spp. (Metastrongylidae) in Wild Boars in Uzbekistan" Pathogens 11, no. 11: 1316. https://doi.org/10.3390/pathogens11111316
APA StyleKuchboev, A. E., & Krücken, J. (2022). Prevalence, Infection Intensity and Molecular Diagnosis of Mixed Infections with Metastrongylus spp. (Metastrongylidae) in Wild Boars in Uzbekistan. Pathogens, 11(11), 1316. https://doi.org/10.3390/pathogens11111316








