The First Records of Canine Babesiosis in Dogs from Dermacentor reticulatus—Free Zone in Poland
Abstract
:1. Introduction
2. Materials and Method
2.1. Dogs-Clinical Examination
2.2. Blood and Ticks Analyses
3. Results
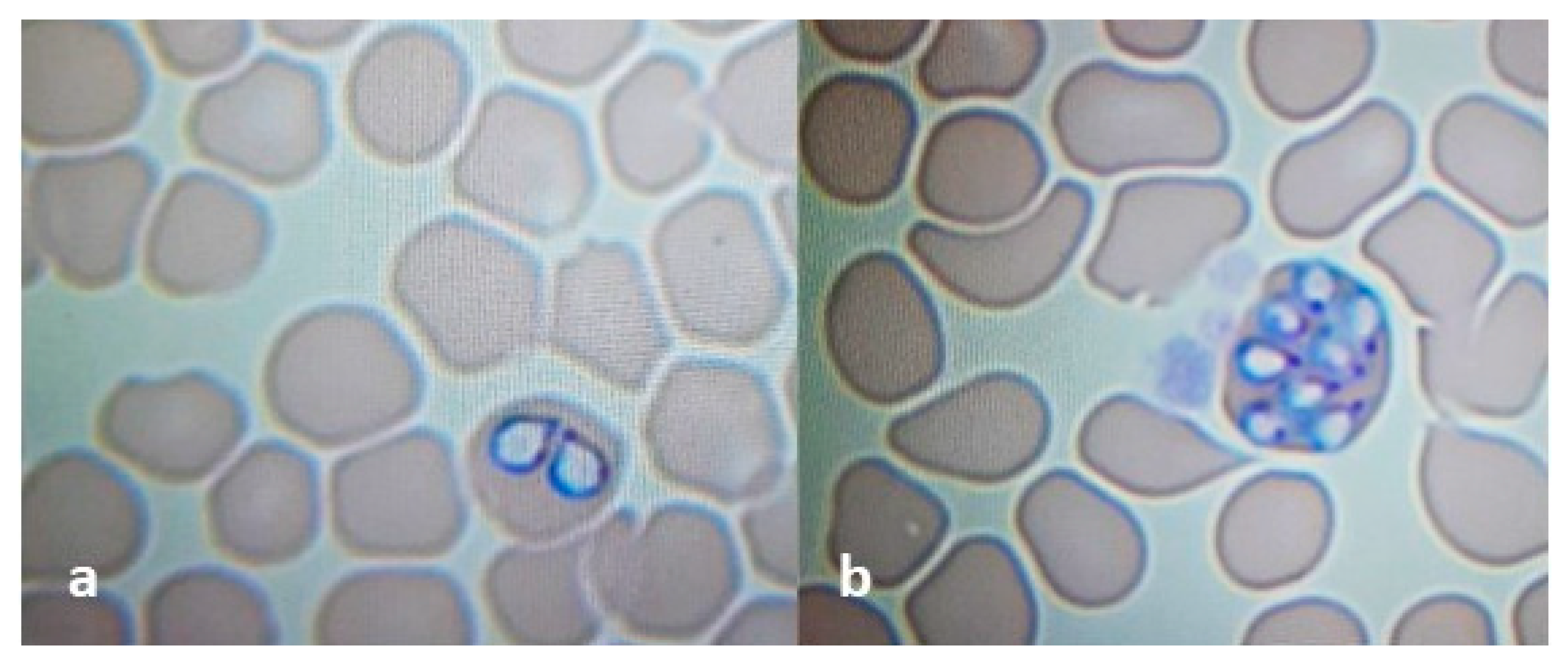
3.1. Dog 1-Clinical Examination and Molecular Analysis
3.2. Dog 2-Clinical Examination and Molecular Analysis
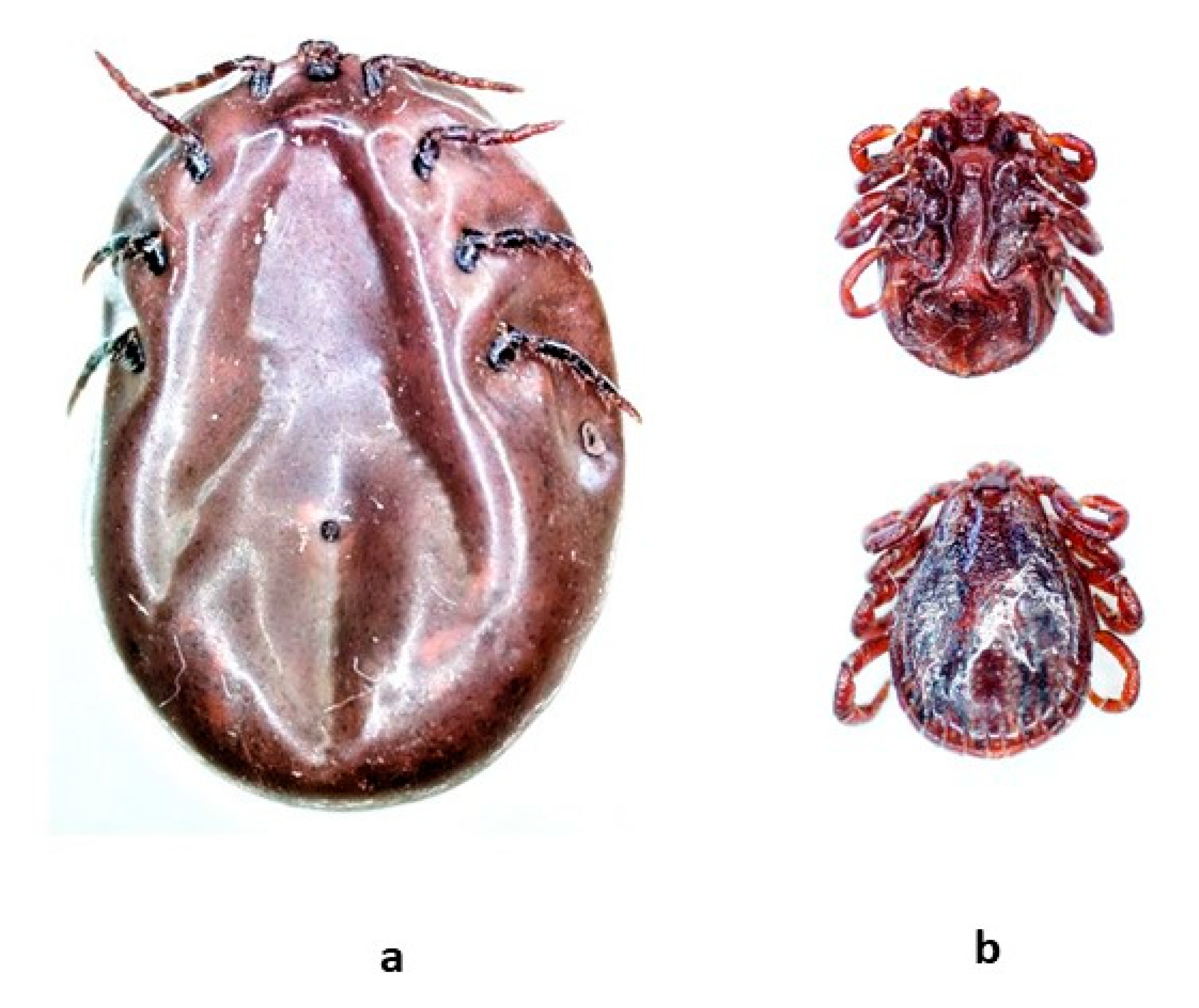
3.3. Ticks-Identification and Molecular Detection of Pathogens
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beugnet, F.; Moreau, Y. Babesiosis. Rev. Sci. Tech. 2015, 34, 627–639. [Google Scholar] [CrossRef]
- Imre, M.; Farkas, R.; Ilie, M.; Imre, K.; Hotea, I.; Morariu, S.; Morar, D.; Dărăbuş, G. Seroprevalence of Babesia canis Infection in Clinically Healthy Dogs from Western Romania. J. Parasitol. 2013, 99, 161–163. [Google Scholar] [CrossRef]
- Simões, P.B.; Cardoso, L.; Araújo, M.; Yisaschar-Mekuzas, Y.; Baneth, G. Babesiosis due to the canine Babesia microti-like small piroplasm in dogs—First report from Portugal and possible vertical transmission. Parasites Vectors 2011, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- Baneth, G.; Florin-Christensen, M.; Cardoso, L.; Schnittger, L. Reclassification of Theileria annae as Babesia vulpes sp. nov. Parasites Vectors 2015, 8, 207. [Google Scholar] [CrossRef] [Green Version]
- Solano-Gallego, L.; Sainz, Á.; Roura, X.; Estrada-Peña, A.; Miró, G. A review of canine babesiosis: The European perspective. Parasites Vectors 2016, 9, 336. [Google Scholar] [CrossRef] [Green Version]
- Adaszek, Ł.; Carbonero Martinez, A.; Winiarczyk, S. The factors affecting the distribution of babesiosis in dogs in Poland. Vet. Parasitol. 2011, 181, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Łyp, P.; Bartnicki, M.; Staniec, M.; Winiarczyk, S.; Adaszek, Ł. Occurrence of different strains of Babesia canis in dogs in eastern Poland. J. Vet. Res. 2016, 60, 423–427. [Google Scholar] [CrossRef] [Green Version]
- Adaszek, Ł.; Łyp, P.; Pobłocki, P.; Skrzypczak, M.; Mazurek, L.; Winiarczyk, S. The first case of Babesia gibsoni infection in a dog in Poland. Vet. Med. 2018, 63, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Teodorowski, O.; Kalinowski, M.; Skrzypczak, M.; Witt, K.; Madany, J.; Winiarczyk, S.; Adaszek, Ł. Babesia gibsoni infection in dogs in Poland. Pol. J. Vet. Sci. 2020, 23, 469–471. [Google Scholar] [CrossRef]
- De Marco, M.d.M.F.; Hernández-Triana, L.M.; Phipps, L.P.; Hansford, K.; Mitchell, E.S.; Cull, B.; Swainsbury, C.S.; Fooks, A.R.; Medlock, J.M.; Johnson, N. Emergence of Babesia canis in southern England. Parasites Vectors 2017, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Petra, B.; Josipa, K.; Renata, B.R.; Vladimir, M. Canine babesiosis: Where do we stand? Acta Vet. 2018, 68, 127–160. [Google Scholar] [CrossRef] [Green Version]
- Helm, C.S.; Weingart, C.; Ramünke, S.; Schäfer, I.; Müller, E.; von Samson-Himmelstjerna, G.; Kohn, B.; Krücken, J. High genetic diversity of Babesia canis (Piana & Galli-Valerio, 1895) in a recent local outbreak in Berlin/Brandenburg, Germany. Transbound. Emerg. Dis. 2022, 69, e3336–e3345. [Google Scholar] [CrossRef]
- Halos, L.; Lebert, I.; Abrial, D.; Danlois, F.; Garzik, K.; Rodes, D.; Schillmeier, M.; Ducrot, C.; Guillot, J. Questionnaire-based survey on the distribution and incidence of canine babesiosis in countries of Western Europe. Parasite 2014, 21, 13. [Google Scholar] [CrossRef] [Green Version]
- Kubelová, M.; Sedlák, K.; Panev, A.; Široký, P. Conflicting results of serological, PCR and microscopic methods clarify the various risk levels of canine babesiosis in Slovakia: A complex approach to Babesia canis diagnostics. Vet. Parasitol. 2013, 191, 353–357. [Google Scholar] [CrossRef]
- Hana, T.; Bronislava, V.; Martina, M.; Andrea, S.; Gad, B.; Miroslav, S. Clinical and Hematologic Findings in Babesia canis Infection in Eastern Slovakia. Acta Parasitol. 2022, 67, 1329–1334. [Google Scholar] [CrossRef]
- Adaszek, Ł.; Łyp, P.; Mazurek, Ł.; Winiarczyk, S. Zmienność genetyczna pierwotniaków Babesia canis izolowanych od psów w Polsce na przestrzeni ostatnich lat. Życie Weter. 2020, 95, 30–33. (In Polish) [Google Scholar]
- Welc-Falęciak, R.; Rodo, A.; Siński, E.; Bajer, A. Babesia canis and other tick-borne infections in dogs in Central Poland. Vet. Parasitol. 2009, 166, 191–198. [Google Scholar] [CrossRef]
- Kostro, K.; Stojecki, K.; Grzybek, M.; Tomczuk, K. Characteristics, immunological events, and diagnostics of Babesia spp. infection, with emphasis on Babesia canis. Bull. Vet. Inst. Pulawy 2015, 59, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Lempereur, L.; Beck, R.; Fonseca, I.; Marques, C.; Duarte, A.; Santos, M.; Zúquete, S.; Gomes, J.; Walder, G.; Domingos, A.; et al. Guidelines for the Detection of Babesia and Theileria Parasites. Vector Borne Zoonotic Dis. 2017, 17, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Silaghi, C.; Weis, L.; Pfister, K. Dermacentor reticulatus and Babesia canis in Bavaria (Germany)—A Georeferenced Field Study with Digital Habitat Characterization. Pathogens 2020, 9, 541. [Google Scholar] [CrossRef]
- Pawełczyk, O.; Góra, S.; Kotela, D.; Solarz, K. Kleszcz pospolity i kleszcz łąkowy jako groźne gatunki wektorowe dla psów domowych. Weter. W Prakt. 2022, 7–8, 55–60. (In Polish) [Google Scholar]
- Dwużnik-Szarek, D.; Mierzejewska, E.J.; Rodo, A.; Goździk, K.; Behnke-Borowczyk, J.; Kiewra, D.; Kartawik, N.; Bajer, A. Monitoring the expansion of Dermacentor reticulatus and occurrence of canine babesiosis in Poland in 2016–2018. Parasites Vectors 2021, 14, 1–18. [Google Scholar] [CrossRef]
- Dwużnik-Szarek, D.; Mierzejewska, E.J.; Kiewra, D.; Czułowska, A.; Robak, A.; Bajer, A. Update on prevalence of Babesia canis and Rickettsia spp. in adult and juvenile Dermacentor reticulatus ticks in the area of Poland (2016–2018). Sci. Rep. 2022, 12, 5755. [Google Scholar] [CrossRef]
- Mierzejewska, E.J.; Estrada-Peña, A.; Alsarraf, M.; Kowalec, M.; Bajer, A. Mapping of Dermacentor reticulatus expansion in Poland in 2012–2014. Ticks Tick-Borne Dis. 2016, 7, 94–106. [Google Scholar] [CrossRef]
- Blaschitz, M.; Narodoslavsky-Gföller, M.; Kanzler, M.; Stanek, G.; Walochnik, J. Babesia species occurring in Austrian Ixodes ricinus ticks. Appl. Environ. Microbiol. 2008, 74, 4841–4846. [Google Scholar] [CrossRef] [Green Version]
- Siuda, K. Kleszcze Polski (Acari: Ixodida). Część II. Systematyka i Rozmieszczenie; Polskie Towarzystwo Parazytologiczne: Warsaw, Poland, 1993. [Google Scholar]
- Nowak-Chmura, M. Fauna Kleszczy (Ixodida) Europy Środkowej; Wydawnictwo Naukowe Uniwersytetu Pedagogicznego: Kraków, Poland, 2013. [Google Scholar]
- Rijpkema, S.; Golubić, D.; Molkenboer, M.; Verbeek-De Kruif, N.; Schellekens, J. Identification of four genomic groups of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in a Lyme borreliosis endemic region of northern Croatia. Exp. Appl. Acarol. 1996, 20, 23–30. [Google Scholar] [CrossRef]
- Grzeszczuk, A.; Stańczak, J.; Kubica-Biernat, B. Serological and molecular evidence of human granulocytic ehrlichiosis focus in the Białowieza Primeval Forest (Puszcza Białowieska), northeastern Poland. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 6–11. [Google Scholar] [CrossRef]
- Wodecka, B.; Skotarczak, B. Genetyczna zmienność Borrelia burgdorferi s.l. u kleszczy Ixodes ricinus w północno-zachodniej Polsce. Wiad. Parazytol. 2000, 46, 475–485. [Google Scholar]
- Regnery, R.L.; Spruill, C.L.; Plikaytis, B.D. Genotypic Identification of Rickettsiae and Estimation of Intraspecies Sequence Divergence for Portions of Two Rickettsial Genes. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef] [Green Version]
- Renesto, P.; Gouvernet, J.; Drancourt, M.; Roux, V.; Raoult, D. Use of rpoB analysis for detection and identification of Bartonella species. J. Clin. Microbiol. 2001, 3, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Rubel, F.; Brugger, K.; Pfeffer, M.; Chitimia-Dobler, L.; Didyk, Y.M.; Leverenz, S.; Dautel, H.; Kahl, O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick-Borne Dis. 2016, 7, 224–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buczek, A.; Buczek, W. Importation of Ticks on Companion Animals and the Risk of Spread of Tick-Borne Diseases to Non-Endemic Regions in Europe. Animals 2021, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Carcy, B.; Randazzo, S.; Depoix, D.; Adaszek, L.; Cardoso, L.; Baneth, G.; Gorenflot, A.; Schetters, T.P. Classification of Babesia canis strains in Europe based on polymorphism of the Bc28.1-gene from the Babesia canis Bc28 multigene family. Vet. Parasitol. 2015, 211, 111–123. [Google Scholar] [CrossRef]
- Bajer, A.; Beck, A.; Beck, R.; Behnke, J.M.; Dwużnik-Szarek, D.; Eichenberger, R.M.; Farkas, R.; Fuehrer, H.-P.; Heddergott, M.; Jokelainen, P.; et al. Babesiosis in Southeastern, Central and Northeastern Europe: An Emerging and Re-Emerging Tick-Borne Disease of Humans and Animals. Microorganisms 2022, 10, 945. [Google Scholar] [CrossRef]
- Geurden, T.; Becskei, C.; Six, R.H.; Maeder, S.; Latrofa, M.S.; Otranto, D.; Farkas, R. Detection of tick-borne pathogens in ticks from dogs and cats in different European countries. Ticks Tick Borne Dis. 2018, 9, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Seleznova, M.; Kivrane, A.; Namina, A.; Krumins, R.; Aleinikova, D.; Lazovska, M.; Akopjana, S.; Capligina, V.; Ranka, R. Babesiosis in Latvian domestic dogs, 2016–2019. Ticks Tick-Borne Dis. 2020, 11, 101459. [Google Scholar] [CrossRef] [PubMed]
- Mitkova, B.; Hrazdilova, K.; Novotna, M.; Jurankova, J.; Hofmannova, L.; Forejtek, P.; Modry, D. Autochthonous Babesia canis, Hepatozoon canis and imported Babesia gibsoni infection in dogs in the Czech Republic. Vet. Med. 2017, 62, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.O.; Tolf, C.; Tamba, P.; Stefanache, M.; Radbea, G.; Rubel, F.; Waldenström, J.; Dobler, G.; Chițimia-Dobler, L. Babesia, Theileria, and Hepatozoon species in ticks infesting animal hosts in Romania. Parasitol. Res. 2017, 116, 2291–2297. [Google Scholar] [CrossRef]
- Radzijevskaja, J.; Mardosaitė-Busaitienė, D.; Aleksandravičienė, A.; Karvelienė, B.; Razgūnaitė, M.; Stadalienė, I.; Paulauskas, A. Genetic Diversity of Babesia canis Strains in Dogs in Lithuania. Microorganisms 2022, 10, 1446. [Google Scholar] [CrossRef]
- Kocoń, A.; Asman, M.; Nowak-Chmura, M.; Witecka, J.; Kłyś, M.; Solarz, K. Molecular detection of tick-borne pathogens in ticks collected from pets in selected mountainous areas of Tatra County (Tatra Mountains, Poland). Sci. Rep. 2020, 10, 15865. [Google Scholar] [CrossRef]
- Michalski, M.M.; Kubiak, K.; Szczotko, M.; Chajęcka, M.; Dmitryjuk, M. Molecular Detection of Borrelia burgdorferi Sensu Lato and Anaplasma phagocytophilum in Ticks Collected from Dogs in Urban Areas of North-Eastern Poland. Pathogens 2020, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Pawełczyk, O.; Asman, M.; Solarz, K.; Jakubas-Zawalska, J.; Janikowski, T.; Mazurek, U. The PCR detection of Anaplasma phagocytophilum, Babesia microti and Borrelia burgdorferi sensu lato in ticks and fleas collected from pets in the Będzin district area (Upper Silesia, Poland)—The preliminary studies. In Stawonogi: Zagrożenie Zdrowia Człowieka i Zwierząt; Wyd. Koliber: Lublin, Poland, 2014; pp. 111–119. [Google Scholar]
- Zygner, W.; Górski, P.; Wędrychowicz, H. Detection of the DNA of Borrelia afzelii, Anaplasma phagocytophilum and Babesia canis in blood samples from dogs in Warsaw. Vet. Rec. 2009, 164, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Król, N.; Obiegala, A.; Pfeffer, M.; Lonc, E.; Kiewra, D. Detection of selected pathogens in ticks collected from cats and dogs in the Wrocław Agglomeration, South-West Poland. Parasites Vectors 2016, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Kiewra, D.; Czulowska, A. Evidence for an increased distribution range of Dermacentor reticulatus in south-west Poland. Exp. Appl. Acarol. 2013, 59, 501–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiewra, D.; Szymanowski, M.; Czułowska, A.; Kolanek, A. The local-scale expansion of Dermacentor reticulatus ticks in Lower Silesia, SW Poland. Ticks Tick-Borne Dis. 2021, 12, 101599. [Google Scholar] [CrossRef]
- Cuber, P.; Solarz, K.; Mosiałek, A.; Jakubiec-Spanier, M.; Spanier, A. The first record and occurrence of the ornate cow tick Dermacentor reticulatus (Fabricius, 1794) in south-western Poland. Ann. Parasitol. 2013, 59, 49–51. [Google Scholar]
- Mitková, B.; Hrazdilová, K.; D’Amico, G.; Duscher, G.G.; Suchentrunk, F.; Forejtek, P.; Gherman, C.M.; Matei, I.A.; Ionică, A.M.; Daskalaki, A.A.; et al. Eurasian golden jackal as host of canine vector-borne protists. Parasites Vectors 2017, 10, 183. [Google Scholar] [CrossRef] [Green Version]
- Adaszek, L.; Winiarczyk, S. Molecular characterization of Babesia canis isolates from naturally infected dogs in Poland. Vet. Parasitol. 2008, 152, 235–241. [Google Scholar] [CrossRef]
- Beck, R.; Vojta, L.; Mrljak, V.; Marinculić, A.; Beck, A.; Živičnjak, T.; Cacciò, S.M. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int. J. Parasitol. 2009, 39, 843–848. [Google Scholar] [CrossRef]
- Neelawala, D.; Dissanayake, D.R.A.; Prasada, D.V.P.; Silva, I.D. Analysis of risk factors associated with recurrence of canine babesiosis caused by Babesia gibsoni. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101572. [Google Scholar] [CrossRef]
- Villa, L.; Zanzani, S.A.; Mortarino, M.; Gazzonis, A.L.; Olivieri, E.; Manfredi, M.T. Molecular Prevalence of Selected Tick-Borne Pathogens in Dermacentor reticulatus Collected in a Natural Park in Italy. Pathogens 2022, 11, 887. [Google Scholar] [CrossRef] [PubMed]
- Liberska, J.; Michalik, J.; Pers-Kamczyc, E.; Wierzbicka, A.; Lane, R.S.; Rączka, G.; Opalińska, P.; Skorupski, M.; Dabert, M. Prevalence of Babesia canis DNA in Ixodes ricinus ticks collected in forest and urban ecosystems in west-central Poland. Ticks Tick Borne Dis. 2021, 12, 101786. [Google Scholar] [CrossRef] [PubMed]
- Welc-Falęciak, R.; Bajer, A.; Paziewska-Harris, A.; Baumann-Popczyk, A.; Siński, E. Diversity of Babesia in Ixodes ricinus ticks in Poland. Adv. Med. Sci. 2012, 57, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Cieniuch, S.; Stańczak, J.; Ruczaj, A. The first detection of Babesia EU1 and Babesia canis in Ixodes ricinus ticks (Acari, Ixodidae) collected in urban and rural areas in northern Poland. Pol. J. Microbiol. 2009, 58, 231–236. [Google Scholar]
- Cacciò, S.M.; Antunovic, B.; Moretti, A.; Mangili, V.; Marinculic, A.; Baric, R.R.; Slemenda, S.B.; Pieniazek, N.J. Molecular characterisation of Babesia canis and Babesia canis vogeli from naturally infected European dogs. Vet. Parasitol. 2002, 106, 285–292. [Google Scholar] [CrossRef]
- Rybarova, M.; Honsová, M.; Papousek, I.; Siroky, P. Variability of species of Babesia starcovici, 1893 in three sympatric ticks (Ixodes ricinus, Dermacentor reticulatus and Haemaphysalis concinna) at the edge of Pannonia in the Czech Republic and Slovakia. Folia Parasitol. 2017, 64, 028. [Google Scholar] [CrossRef]
| Symptoms | Dog 1 | Dog 2 |
|---|---|---|
| Presence of ticks | X | X |
| Fever | X | |
| Lethargy | X | X |
| Weakness | X | X |
| Dehydration | X | |
| Anorexia | X | X |
| Splenomegaly | X | |
| Hemoglobinuria | X | |
| Malaise | X | X |
| Fatigue | X | |
| Pale mucous membranes | X | |
| Vomiting | X | |
| Diarrhea | X | X |
| Parameters CBC | Day 1 | Day 2 | Day 4 | Day 6 | Day 7 | Day 10 | Reference Range |
|---|---|---|---|---|---|---|---|
| WBC [109/L] | 3.09 | 3.42 | 27.94 | 40.62 | 50.21 | 23.37 | 6.00–17.00 |
| LYM [109/L] | 0.45 | 0.57 | 5.14 | 2.98 | No data | 3.34 | 0.80–5.30 |
| MONO [109/L] | 0.19 | 0.29 | 2.88 | 2.20 | No data | 2.17 | 0.00–1.50 |
| NEU [109/L] | 2.40 | 2.33 | 19.08 | 17.50 | No data | 17.46 | 3.20–12.30 |
| EOS [109/L] | 0.05 | 0.23 | 0.84 | 0.70 | No data | 0.40 | 0.00–1.50 |
| HCT [%] | 48.7 | 32.4 | 10.9 | 16.5 | 17.4 | 33.7 | 32.5–58.00 |
| RBC [1012/L] | 7.54 | 4.94 | 1.46 | 2.3 | 2.32 | 3.86 | 5.10–8.50 |
| HGB [g/dL] | 17.6 | 11.5 | 5.1 | 6.7 | 6.9 | 10.5 | 11.00–19.5 |
| PLT [109/L] | 30.00 | 13.00 | 36.00 | 110 | 165 | 338 | 117.00–490.00 |
| MCV [fL] | 64.6 | 65.6 | 74.4 | 71.7 | 74.9 | 87.1 | 60.00–76.00 |
| MCHC [g/dL] | 36.2 | 35.5 | 47.00 | 40.3 | 39.8 | 31.3 | 30.00–38.00 |
| MCH [pg] | 23.4 | 23.3 | 34.9 | 28.9 | 29.8 | 27.3 | 20.00–27.00 |
| Parameters CBC | Day 1 | Day 4 | Day 9 | Reference Range |
|---|---|---|---|---|
| WBC [109/L] | 6.55 | 19.55 | 12.37 | 6.00–17.00 |
| LYM [109/L] | No data | No data | No data | 0.80–5.30 |
| MONO [109/L] | No data | No data | No data | 0.00–1.50 |
| NEU [109/L] | No data | No data | No data | 3.20–12.30 |
| EOS [109/L] | 0.2 | 2.1 | 0.67 | 0.00–1.50 |
| HCT [%] | 34.8 | 40.2 | 44.9 | 32.5–58.00 |
| RBC [1012/L] | 5.34 | 6.04 | 6.69 | 5.10–8.50 |
| HGB [g/dL] | 12.4 | 14.1 | 15.7 | 11.00–19.5 |
| PLT [109/L] | 25.00 | 67.00 | 376.00 | 117.00–490.00 |
| MCV [fL] | 65.2 | 66.6 | 67.00 | 60.00–76.00 |
| MCHC [g/dL] | 35.6 | 35.1 | 35.00 | 30.00–38.00 |
| MCH [pg] | 23.2 | 23.4 | 23.4 | 20.00–27.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawełczyk, O.; Kotela, D.; Asman, M.; Witecka, J.; Wilhelmsson, P.; Bubel, P.; Solarz, K. The First Records of Canine Babesiosis in Dogs from Dermacentor reticulatus—Free Zone in Poland. Pathogens 2022, 11, 1329. https://doi.org/10.3390/pathogens11111329
Pawełczyk O, Kotela D, Asman M, Witecka J, Wilhelmsson P, Bubel P, Solarz K. The First Records of Canine Babesiosis in Dogs from Dermacentor reticulatus—Free Zone in Poland. Pathogens. 2022; 11(11):1329. https://doi.org/10.3390/pathogens11111329
Chicago/Turabian StylePawełczyk, Olga, Damian Kotela, Marek Asman, Joanna Witecka, Peter Wilhelmsson, Paulina Bubel, and Krzysztof Solarz. 2022. "The First Records of Canine Babesiosis in Dogs from Dermacentor reticulatus—Free Zone in Poland" Pathogens 11, no. 11: 1329. https://doi.org/10.3390/pathogens11111329
APA StylePawełczyk, O., Kotela, D., Asman, M., Witecka, J., Wilhelmsson, P., Bubel, P., & Solarz, K. (2022). The First Records of Canine Babesiosis in Dogs from Dermacentor reticulatus—Free Zone in Poland. Pathogens, 11(11), 1329. https://doi.org/10.3390/pathogens11111329








