Assessment of the Susceptibility of Clinical Gram-Negative and Gram-Positive Bacterial Strains to Fosfomycin and Significance of This Antibiotic in Infection Treatment
Abstract
:1. Introduction
2. Materials and Methods
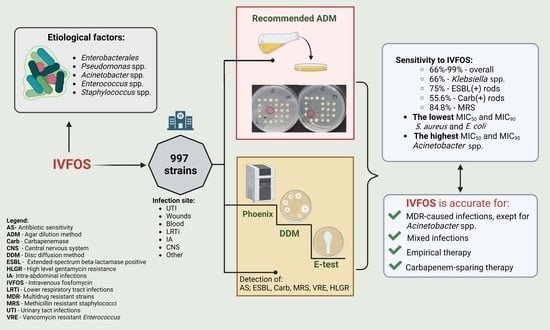
2.1. Bacterial Strains
- -
- Gram-negative bacilli from the order Enterobacterales (n = 546):
- Klebsiella (n = 250);
- E. coli (n = 181);
- Enterobacter (n = 47);
- Proteus (n = 41);
- Citrobacter (n = 13);
- Serratia (n = 11);
- Providencia (n = 3).
- -
- Non-fermenting bacilli: P. aeruginosa (n = 153);
- -
- Rods of the genus Acinetobacter (n = 87);
- -
- Gram-positive cocci (n = 211):
- S. aureus (n = 104);
- Coagulase-negative Staphylococcus spp. (n = 57);
- Enterococcus spp. (n = 50).
2.2. Microbiological Assays
2.3. Identification of Strains and Determination of Susceptibility to Antibiotics Other Than Fosfomycin
2.4. Identification of β-Lactamases
2.5. Diffusion Methods
2.6. Agar Dilution Method (Reference Method)
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marino, A.; Stracquadani, S.; Bellanca, C.M.; Augello, E.; Ceccarelli, M.; Cantarella, G.; Bernardini, R.; Nunnari, G.; Cacopardo, B. Oral Fosfomycin Formulation in Bacterial Prostatitis: New Role for an Old Molecule-Brief Literature Review and Clinical Considerations. Infect. Dis. Rep. 2022, 14, 621–634. [Google Scholar] [CrossRef]
- Karaiskos, I.; Galani, L.; Sakka, V.; Gkoufa, A.; Sopilidis, O.; Chalikopoulos, D.; Alivizatos, G.; Giamarellou, E. Oral fosfomycin for the treatment of chronic bacterial prostatitis. J. Antimicrob. Chemother. 2019, 74, 1430–1437. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Assessment Report. EMA (Online). Available online: https://www.ema.europa.eu/en/documents/referral/fosfomycin-article-31-referralassessment-report_en.pdf (accessed on 12 April 2022).
- Zhanel, G.G.; Zhanel, M.A.; Karlowsky, J.A. Intravenous Fosfomycin: An Assessment of Its Potential for Use in the Treatment of Systemic Infections in Canada. Can. J. Infect. Dis. Med. Microbiol. 2019, 2018, 8912039. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, M.; Emoto, Y.; Emoto, M. A Simple, Reproducible, Inexpensive, Yet Old-Fashioned Method for Determining Phagocytic and Bactericidal Activities of Macrophages. Yonsei Med. J. 2016, 57, 283–290. [Google Scholar] [CrossRef]
- Meena, V.D.; Dotaniya, M.L.; Saha, J.K.; Patra, A.K. Antibiotics and antibiotic resistant bacteria in wastewater: Impact on environment, soil microbial activity and human health. Afr. J. Microbiol. Res. 2015, 9, 965–978. [Google Scholar]
- Díez-Aguilar, M.; Cantón, R. New microbiological aspects of fosfomycin. Rev. Esp. Quimioter. 2019, 32 (Suppl. 1), 8–18. [Google Scholar]
- Dijkmans, A.C.; Zacarías, N.V.O.; Burggraaf, J.; Mouton, J.W.; Wilms, E.B.; van Nieuwkoop, C.; Touw, D.J.; Stevens, J.; Kamerling, I.M.C. Fosfomycin: Pharmacological, Clinical and Future Perspectives. Antibiotics 2017, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Peng, Q.; Li, S.; Deng, Z.; Gao, J. The intriguing biology and chemistry of fosfomycin: The only marketed phosphonate antibiotic. RSC Adv. 2019, 9, 42204–42218. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.C.M. Potential of fosfomycin in treating multidrug-resistant infections in children. J. Paediatr. Child Health 2020, 56, 864–872. [Google Scholar] [CrossRef] [Green Version]
- AD Fosfomycin 0.25-256. Device for Fosfomycin Susceptibility Testing with the Agar Dilution Method. Liofilchem (Online). Available online: http://www.liofilchem.net/login/pd/ifu/77061_IFU.pdf (accessed on 28 April 2020).
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf (accessed on 1 January 2022).
- Van den Bijllaardt, W.; Schijffelen, M.J.; Bosboom, R.W.; Cohen Stuart, J.; Diederen, B.; Kampinga, G.; Muller, A.E. Susceptibility of ESBL Escherichia coli and Klebsiella pneumoniae to fosfomycin in the Netherlands and comparison of several testing methods including Etest, MIC test strip, Vitek2, Phoenix and disc diffusion. J. Antimicrob. Chemother. 2018, 73, 2380–2387. [Google Scholar] [CrossRef] [Green Version]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Routine and Extended Internal Quality Control for MIC Determination and Disk Diffusion as Recommended by EUCAST. Version 11.0. 2021. Available online: http://www.eucast.org (accessed on 8 February 2021).
- EUCAST General Consultation on Fosfomycin IV Breakpoints. Consultation Period 14 May to (Extension) 15 July 2022. Available online: https://www.eucast.org/publications_and_documents/consultations/ (accessed on 11 August 2022).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI Supplement M100S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Doyle, D.; Peirano, G.; Lascols, C.; Lloyd, T.; Church, D.L.; Pitout, J.D. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J. Clin. Microbiol. 2012, 50, 3877–3880. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, L.V.; Chibabhai, V. Evaluation of the RESIST-4 O.K.N.V immunochromatographic lateral flow assay for the rapid detection of OXA-48, KPC, NDM and VIM carbapenemases from cultured isolates. Access Microbiol. 2019, 1, e000031. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 2000, 6, 509–515. [CrossRef] [Green Version]
- WHO Regional Office for Europe; European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2022–2020 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Data from the ECDC Surveillance Atlas Antimicrobial Resistance. The Surveillance Atlas of Infectious Diseases. 2022–2020.2022 Data. Available online: https://atlas.ecdc.europa.eu/public/index.aspx?Dataset=27&HealthTopic=4 (accessed on 1 September 2022).
- Elham, B.; Fawzia, A. Colistin resistance in Acinetobacter baumannii isolated from critically ill patients: Clinical characteristics, antimicrobial susceptibility and outcome. Afr. Health Sci. 2019, 19, 2400–2406. [Google Scholar] [CrossRef]
- Papathanakos, G.; Andrianopoulos, I.; Papathanasiou, A.; Priavali, E.; Koulenti, D.; Koulouras, V. Colistin-Resistant Acinetobacter Baumannii Bacteremia: A Serious Threat for Critically Ill Patients. Microorganisms 2020, 8, 287. [Google Scholar] [CrossRef] [Green Version]
- Cafiso, V.; Stracquadanio, S.; Lo Verde, F.; Gabriele, G.; Mezzatesta, M.L.; Caio, C.; Pigola, G.; Ferro, A.; Stefani, S. Colistin Resistant A. baumannii: Genomic and Transcriptomic Traits Acquired Under Colistin Therapy. Front. Microbiol. 2019, 7, 3195. [Google Scholar] [CrossRef]
- Malik, S.; Kaminski, M.; Landman, D.; Quale, J. Cefiderocol Resistance in Acinetobacter baumannii: Roles of β-Lactamases, Siderophore Receptors, and Penicillin Binding Protein 3. Antimicrob. Agents Chemother. 2020, 64, e01221-20. [Google Scholar] [CrossRef] [PubMed]
- Smoke, S.M.; Brophy, A.; Reveron, S.; Iovleva, A.; Kline, E.G.; Marano, M.; Miller, L.P.; Shields, R.K. Evolution and Transmission of Cefiderocol-Resistant Acinetobacter baumannii During an Outbreak in the Burn Intensive Care Unit. Clin. Infect. Dis. 2022, ciac647. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Rousaki, M.; Kritsotakis, E.I. Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance. Antibiotics 2022, 11, 723. [Google Scholar] [CrossRef]
- Putensen, C.; Ellger, B.; Sakka, S.G.; Weyland, A.; Schmidt, K.; Zoller, M.; Weiler, N.; Kindgen-Milles, D.; Jaschinski, U.; Weile, J.; et al. Current clinical use of intravenous fosfomycin in ICU patients in two European countries. Infection 2019, 47, 827–836. [Google Scholar] [CrossRef] [Green Version]
- Zirpe, K.G.; Mehta, Y.; Pandit, R.; Pande, R.; Deshmukh, A.M.; Patil, S.; Bhagat, S.; Barkate, H. A Real-world Study on Prescription Pattern of Fosfomycin in Critical Care Patients. Indian J. Crit. Care Med. 2021, 25, 1055–1058. [Google Scholar] [CrossRef]
- Pontikis, K.; Karaiskos, I.; Bastani, S.; Dimopoulos, G.; Kalogirou, M.; Katsiari, M.; Oikonomou, A.; Poulakou, G.; Roilides, E.; Giamarellou, H. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int. J. Antimicrob. Agents 2014, 43, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Tsegka, K.G.; Voulgaris, G.L.; Kyriakidou, M.; Falagas, M.E. Intravenous fosfomycin for the treatment of patients with central nervous system infections: Evaluation of the published evidence. Expert Rev. Anti Infect. Ther. 2020, 18, 657–668. [Google Scholar] [CrossRef]
- Tsegka, K.G.; Voulgaris, G.L.; Kyriakidou, M.; Kapaskelis, A.; Falagas, M.E. Intravenous fosfomycin for the treatment of patients with bone and joint infections: A review. Expert Rev. Anti Infect. Ther. 2022, 20, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Stracquadanio, S.; Musso, N.; Costantino, A.; Lazzaro, L.M.; Stefani, S.; Bongiorno, D. Staphylococcus aureus Internalization in Osteoblast Cells: Mechanisms, Interactions and Biochemical Processes. What Did We Learn from Experimental Models? Pathogens 2021, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Zelmer, A.R.; Nelson, R.; Richter, K.; Atkins, G.J. Can intracellular Staphylococcus aureus in osteomyelitis be treated using current antibiotics? A systematic review and narrative synthesis. Bone Res. 2022, 10, 53. [Google Scholar] [CrossRef]
- Valour, F.; Trouillet-Assant, S.; Riffard, N.; Tasse, J.; Flammier, S.; Rasigade, J.P.; Chidiac, C.; Vandenesch, F.; Ferry, T.; Laurent, F. Antimicrobial activity against intraosteoblastic Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 2029–2036. [Google Scholar] [CrossRef] [Green Version]
- Morata, L.; Soriano, A. The role of fosfomycin in osteoarticular infection. Rev. Esp. Quimioter. 2019, 32 (Suppl. 1), 30–36. [Google Scholar]
- Li, G.; Standing, J.F.; Bielicki, J.; Hope, W.; van den Anker, J.; Heath, P.T.; Sharland, M. The Potential Role of Fosfomycin in Neonatal Sepsis Caused by Multidrug-Resistant Bacteria. Drugs 2017, 77, 941–950. [Google Scholar] [CrossRef]
- Baquero, F.; Hortelano, J.G.; Navarro, M.; Scarpellini, A.; Jara, P.; Cañedo, T.; Rodríguez, A. Antibiotherapy of Serratia marcescens septicemia in children. Chemotherapy 1977, 23 (Suppl. 1), 416–422. [Google Scholar] [CrossRef]
- Corti, N.; Sennhauser, F.H.; Stauffer, U.G.; Nadal, D. Fosfomycin for the initial treatment of acute haematogenous osteomyelitis. Arch. Dis. Child. 2003, 88, 512–516. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zheng, B.; Li, Y.; Zhu, S.; Xue, F.; Liu, J. Antimicrobial Susceptibility and Molecular Mechanisms of Fosfomycin Resistance in Clinical Escherichia coli Isolates in Mainland China. PLoS ONE 2015, 10, e0135269. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Sengupta, M.; Sarker, T.K. Fosfomycin susceptibility among multidrug-resistant, extended-spectrum beta-lactamase-producing, carbapenem-resistant uropathogens. Indian J. Urol. 2017, 33, 149–154. [Google Scholar] [CrossRef]
- Fajfr, M.; Louda, M.; Paterová, P.; Ryšková, L.; Pacovský, J.; Košina, J.; Žemličková, H.; Broďák, M. The susceptibility to fosfomycin of Gram-negative bacteria isolates from urinary tract infection in the Czech Republic: Data from a unicentric study. BMC Urol. 2017, 17, 33. [Google Scholar] [CrossRef] [Green Version]
- Parisio, E.M.; Camarlinghi, G.; Coppi, M.; Niccolai, C.; Antonelli, A.; Nardone, M.; Vettori, C.; Giani, T.; Mattei, R.; Rossolini, G.M. Evaluation of commercial AD Fosfomycin test for susceptibility testing of multidrug-resistant Enterobacterales and Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2021, 27, e5–e788.e9. [Google Scholar] [CrossRef]
- Xu, W.; Chen, T.; Wang, H.; Zeng, W.; Wu, Q.; Yu, K.; Xu, Y.; Zhang, X.; Zhou, T. Molecular Mechanisms and Epidemiology of Fosfomycin Resistance in Staphylococcus aureus Isolated from Patients at a Teaching Hospital in China. Front. Microbiol. 2020, 11, 1290. [Google Scholar] [CrossRef]
- Souza, R.B.; Trevisol, D.J.; Schuelter-Trevisol, F. Bacterial sensitivity to fosfomycin in pregnant women with urinary infection. Braz. J. Infect. Dis. 2015, 19, 319–323. [Google Scholar] [CrossRef] [Green Version]
- Falagas, M.E.; Roussos, N.; Gkegkes, I.D.; Rafailidis, P.I.; Karageorgopoulos, D.E. Fosfomycin for the treatment of infections caused by Gram-positive cocci with advanced antimicrobial drug resistance: A review of microbiological, animal and clinical studies. Expert Opin. Investig. Drugs 2009, 18, 921–944. [Google Scholar] [CrossRef]
- Perez Fernandez, P.; Herrera, I.; Martinez, P.; Gómez-Lus, M.L.; Prieto, J. Enhancement of the susceptibility of Staphylococcus aureus to phagocytosis after treatment with fosfomycin compared with other antimicrobial agents. Chemotherapy 1995, 41, 45–49. [Google Scholar] [CrossRef]
- Trautmann, M.; Meincke, C.; Vogt, K.; Ruhnke, M.; Lajous-Petter, A.M. Intracellular bactericidal activity of fosfomycin against staphylococci: A comparison with other antibiotics. Infection 1992, 20, 350–354. [Google Scholar] [CrossRef]
- Krause, R.; Patruta, S.; Daxbock, F.; Fladere, P.; Wenisch, C. The effect of fosfomycin on neutrophil function. J. Antimicrob. Chemother. 2001, 47, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tullio, V.; Cuffini, A.M.; Banche, G.; Mandras, N.; Allizond, V.; Roana, J.; Giacchino, F.; Bonello, F.; Ungheri, D.; Carlone, N.A. Role of fosfomycin tromethamine in modulating non-specific defence mechanisms in chronic uremic patients towards ESBL-producing Escherichia coli. Int. J. Immunopathol. Pharmacol. 2008, 21, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Honda, J.; Yoshimuta, T.; Fumimori, T.; Okamoto, M.; Aizawa, H. Fosfomycin inhibits neutrophil function via a protein kinase C-dependent signaling pathway. Int. Immunopharmacol. 2002, 2, 511–518. [Google Scholar] [CrossRef]
- Castaneda-García, A.; Rodríguez-Rojas, A.; Guelfo, J.R.; Blazquez, J. The Glycerol-3-Phosphate Permease GlpT Is the Only Fosfomycin Transporter in Pseudomonas aeruginosa. J. Bacteriol. 2009, 191, 6968–6974. [Google Scholar] [CrossRef] [Green Version]
- European Committee on Antimicrobial Susceptibility Testing. MIC and Zone Diameter Distributions and ECOFFs. Available online: https://www.eucast.org/mic_distributions_and_ecoffs/ (accessed on 14 April 2021).
- Mirakhur, A.; Gallagher, M.J.; Ledson, M.J.; Hart, C.A.; Walshaw, M.J. Fosfomycin therapy for multiresistant Pseudomonas aeruginosa in cystic fibrosis. J. Cyst. Fibros. 2003, 2, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Zeiser, E.T.; Becka, S.A.; Park, S.; Wilson, B.M.; Winkler, M.L.; D’Souza, R.; Singh, I.; Sutton, G.; Fouts, D.E.; et al. Ceftazidime-Avibactam in Combination with Fosfomycin: A Novel Therapeutic Strategy Against Multidrug-Resistant Pseudomonas aeruginosa. J. Infect. Dis. 2019, 220, 666–676. [Google Scholar] [CrossRef] [Green Version]
- Di, X.; Wang, R.; Liu, B.; Zhang, X.; Ni, W.; Wang, J.; Liang, B.; Cai, Y.; Liu, Y. In vitro activity of fosfomycin in combination with colistin against clinical isolates of carbapenem-resistant Pseudomas aeruginosa. J. Antibiot. 2015, 68, 551–555. [Google Scholar] [CrossRef]
- Albiero, J.; Mazucheli, J.; Barros, J.P.D.R.; Szczerepa, M.M.D.A.; Nishiyama, S.A.B.; CarraraMarroni, F.E.; Sy, S.; Fidler, M.; Sy, S.K.B.; Tognim, M.C.B. Pharmacodynamic attainment of the synergism of meropenem and fosfomycin combination against Pseudomonas aeruginosa producing metallo-β-lactamase. Antimicrob. Agents Chemother. 2019, 63, e00126-19. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C.C.; McIntosh, M.P.; Peleg, A.Y.; Kirkpatrick, C.M.; Bergen, P.J. In vitro pharmacodynamics of fosfomycin against clinical isolates of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2015, 70, 3042–3050. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.C.M.; Waichungo, J.; Gordon, N.C.; Sharland, M.; Murunga, S.; Kamau, A.; Berkley, J.A. The potential of fosfomycin for multi-drug resistant sepsis: An analysis of in vitro activity against invasive paediatric Gram-negative bacteria. J. Med. Microbiol. 2019, 68, 711–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez-Aguilar, M.; Morosini, M.I.; del Campo, R.; García-Castillo, M.; Zamora, J.; Cantón, R. In vitro activity of fosfomycin against a collection of clinical Pseudomonas aeruginosa isolates from 16 Spanish hospitals: Establishing the validity of standard broth microdilution as susceptibility testing method. Antimicrob. Agents Chemother. 2013, 57, 5701–5703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopichand, P.; Agarwal, G.; Natarajan, M.; Mandal, J.; Deepanjali, S.; Parameswaran, S.; Dorairajan, L.N. In vitro effect of fosfomycin on multi-drug resistant gram-negative bacteria causing urinary tract infections. Infect. Drug Resist. 2019, 12, 2005–2013. [Google Scholar] [CrossRef]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1. [Google Scholar] [CrossRef]
- Lan, P.; Jiang, Y.; Zhou, J.; Yu, Y. A global perspective on the convergence of hypervirulence and carbapenem resistance in Klebsiella pneumoniae. J. Glob. Antimicrob. Resist. 2021, 25, 26–34. [Google Scholar] [CrossRef]
- Hawkey, P.M.; Warren, R.E.; Livermore, D.M.; McNulty, C.A.M.; Enoch, D.A.; Otter, J.A.; Wilson, A.P.R. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: Report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J. Antimicrob. Chemother. 2018, 73 (Suppl. 3), iii2–iii78. [Google Scholar] [CrossRef] [Green Version]
- Mączyńska, B.; Paleczny, J.; Oleksy-Wawrzyniak, M.; Choroszy-Król, I.; Bartoszewicz, M. In Vitro Susceptibility of Multi-Drug Resistant Klebsiella pneumoniae Strains Causing Nosocomial Infections to Fosfomycin. A Comparison of Determination Methods. Pathogens 2021, 10, 512. [Google Scholar] [CrossRef]
- Demirci-Duarte, S.; Unalan-Altintop, T.; Gulay, Z.; Sari Kaygisiz, A.N.; Cakar, A.; Gur, D. In vitro susceptibility of OXA-48, NDM, VIM and IMP enzyme- producing Klebsiella spp. and Escherichia coli to Fosfomycin. J. Infect. Dev. Ctries. 2020, 14, 394–397. [Google Scholar] [CrossRef]
- Flamm, R.K.; Rhomberg, P.R.; Watters, A.A.; Sweeney, K.; Ellis-Grosse, E.J.; Shortridge, D. Activity of fosfomycin when tested against US contemporary bacterial isolates. Diagn. Microbiol. Infect. Dis. 2019, 93, 143–146. [Google Scholar] [CrossRef]
- Grabein, B.; Graninger, W.; Rodríguez Bańo, J.; Dinh, A.; Liesenfeld, D.B. Intravenous fosfomycin—Back to the future. Systematic review and meta-analysis of the clinical literature. Clin. Microbiol. Infect. 2017, 23, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Bulman, Z.P.; Lenhard, J.R.; Satlin, M.J.; Kreiswirth, B.N.; Walsh, T.J.; Marrocco, A.; Bergen, P.J.; Nation, R.L.; Li, J.; et al. Pharmacodynamics of colistin and fosfomycin: A ‘treasure trove’ combination combats KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2017, 72, 1985–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdigão Neto, L.V.; Oliveira, M.S.; Martins, R.C.R.; Marchi, A.P.; Gaudereto, J.J.; da Costa, L.A.T.J.; de Lima, L.F.A.; Takeda, C.F.V.; Costa, S.F.; Levin, A.S. Fosfomycin in severe infections due to genetically distinct pan-drug-resistant Gram-negative microorganisms: Synergy with meropenem. J. Antimicrob. Chemother. 2019, 74, 177–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folgori, L.; Ellis, S.J.; Bielicki, J.A.; Heath, P.T.; Sharland, M.; Balasegaram, M. Tackling antimicrobial resistance in neonatal sepsis. Lancet Glob. Health 2017, 5, e1066–e1068. [Google Scholar] [CrossRef]
- Rieg, S.; Ernst, A.; Peyerl-Hoffmann, G.; Joost, I.; Camp, J.; Hellmich, M.; Kern, W.V.; Kaasch, A.J.; Seifert, H. Combination therapy with rifampicin or fosfomycin in patients with Staphylococcus aureus bloodstream infection at high risk for complications or relapse: Results of a large prospective observational cohort. J. Antimicrob. Chemother. 2020, 75, 2282–2290. [Google Scholar] [CrossRef]
- AL-Quraini, M.; Rizvi, M.; AL-Jabri, Z.; Sami, H.; AL-Muzahmi, M.; AL-Muharrmi, Z.; Taneja, N.; AL-Busaidi, I.; Soman, R. Assessment of In-Vitro Synergy of Fosfomycin with Meropenem, Amikacin and Tigecycline in Whole Genome Sequenced Extended and Pan Drug Resistant Klebsiella Pneumoniae: Exploring A Colistin Sparing Protocol. Antibiotics 2022, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Singkham-in, U.; Chatsuwan, T. Synergism of imipenem with fosfomycin associated with the active cell wall recycling and heteroresistance in Acinetobacter calcoaceticus-baumannii complex. Sci. Rep. 2022, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Ramos, J.; Salavert Lletí, M. Fosfomycin in infections caused by multidrug-resistant Gram-negative pathogens. Rev. Esp. Quimioter. 2019, 32 (Suppl. 1), 45–54. [Google Scholar]
- Yusuf, E.; Bax, H.I.; Verkaik, N.J.; van Westreenen, M. An Update on Eight “New” Antibiotics against Multidrug-Resistant Gram-Negative Bacteria. J. Clin. Med. 2021, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Nwabor, O.F.; Terbtothakun, P.; Voravuthikunchai, S.P.; Chusri, S. Evaluation of the Synergistic Antibacterial Effects of Fosfomycin in Combination with Selected Antibiotics against Carbapenem-Resistant Acinetobacter baumannii. Pharmaceuticals 2021, 14, 185. [Google Scholar] [CrossRef]
- Bassetti, M.; Garau, J. Current and future perspectives in the treatment of multidrug-resistant Gram-negative infections. J. Antimicrob. Chemother. 2021, 76 (Suppl. 4), iv23–iv37. [Google Scholar] [CrossRef]
- Abbott, I.J.; van Gorp, E.; van der Meijden, A.; Wijma, R.A.; Meletiadis, J.; Roberts, J.A.; Mouton, J.W.; Peleg, A.Y. Oral Fosfomycin Treatment for Enterococcal Urinary Tract Infections in a Dynamic In Vitro Model. Antimicrob. Agents Chemother. 2020, 64, e00342-20. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tomich, A.D.; McElheny, C.L.; Cooper, V.S.; Tait-Kamradt, A.; Wang, M.; Hu, F.; Rice, L.B.; Sluis-Cremer, N.; Doi, Y. High-Level Fosfomycin Resistance in Vancomycin-Resistant Enterococcus faecium. Emerg. Infect. Dis. 2017, 23, 1902–1904. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Legakis, N.J.; Triarides, N.; Falagas, M.E. Susceptibility of contemporary isolates to fosfomycin: A systematic review of the literature. Int. J. Antimicrob. Agents 2016, 47, 269–285. [Google Scholar] [CrossRef]





| Bacterial Strains | Number of Strains | S * | Only ESBL * | ESBL + MBL/KPC/OXA-48 * | Only MBL/KPC/OXA-48 * | Other MDR * | MRS * | Only VRE * | Only HLGR | VRE + HLGR | HLGR + LIN-R |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 997 | 519 | 149 | 26 | 82 | 122 | 79 | 6 | 8 | 6 | |
| Enterobacterales | 546 | 306 | 149 | 26 | 60 | 5 | |||||
| Klebsiella spp. | 250 | 78 | 86 | 26 | 58 | 2 | |||||
| E. coli | 181 | 146 | 34 | 1 | |||||||
| Enterobacter spp. | 47 | 27 | 17 | 1 | 2 | ||||||
| Proteus spp. | 41 | 30 | 10 | 1 | |||||||
| Citrobacter spp. | 13 | 11 | 2 | ||||||||
| Serratia spp. | 11 | 11 | |||||||||
| Providencia spp. | 3 | 3 | |||||||||
| Pseudomonas spp. | 153 | 99 | 21 | 33 | |||||||
| Acinetobacter spp. | 87 | 2 | 1 | 84 | |||||||
| Staphylococcus spp. | 161 | 82 | 79 | ||||||||
| S. aureus | 104 | 64 | 40 | ||||||||
| CNS | 57 | 18 | 39 | ||||||||
| Enterococcus spp. | 50 | 30 | 6 | 7 | 6 | 1 | |||||
| E. faecalis | 32 | 25 | 5 | 1 | 1 | ||||||
| E. faecium | 18 | 5 | 6 | 2 | 5 |
| Bacterial Strains | n | Susceptibility to IVFOS | |
|---|---|---|---|
| n | % | ||
| Total | 860 | 714 | 83.00% |
| Total Enterobacterales | 546 | 429 | 78.60% |
| Klebsiella spp. | 250 | 165 | 66.00% |
| E. coli | 181 | 170 | 93.90% |
| Enterobacter spp. | 47 | 34 | 72.30% |
| Proteus spp. | 41 | 34 | 82.90% |
| other | 27 | 26 | 96.30% |
| Pseudomonas spp. | 153 | 139 | 90.80% |
| Total Staphylococcus spp. | 161 | 146 | 90.70% |
| S. aureus | 104 | 103 | 99.00% |
| CNS | 57 | 43 | 75.40% |
| Sources of Strains– Clinical Samples | n | Susceptibility to IVFOS | |
|---|---|---|---|
| n | % | ||
| Urine | 240 | 190 | 79.20% |
| Pus and wound swabs | 238 | 211 | 88.70% |
| Blood | 203 | 158 | 77.80% |
| From lover respiratory tract | 97 | 83 | 85.40% |
| Intra-abdominal samples | 43 | 37 | 86.00% |
| Other | 36 | 32 | 88.90% |
| Cerebrospinal fluid (CSF) | 3 | 3 | 100.00% |
| Mechanism of Resistance | n | Susceptibility to IVFOS | |
|---|---|---|---|
| n | % | ||
| Susceptible | 487 | 442 | 90.80% |
| Only ESBL | 149 | 113 | 75.80% |
| Only MBL/KPC/OXA-48 | 81 | 45 | 55.60% |
| ESBL + MBL/KPC/OXA-48 | 26 | 16 | 61.60% |
| Other MDR Gram-negative rods | 38 | 31 | 81.60% |
| MRS | 79 | 67 | 84.80% |
| Bacterial Strains | Values (mg/L) | |
|---|---|---|
| MIC50 | MIC90 | |
| Total Enterobacterales | 8 | 128 |
| Klebsiella spp. | 32 | 512 |
| E. coli | 1 | 32 |
| Enterobacter spp. | 16 | 128 |
| Proteus spp. | 8 | 64 |
| Other Enterobacterales | 2 | 32 |
| Acinetobacter spp. | 256 | 256 |
| Pseudomonas spp. | 64 | 128 |
| Total Staphylococcus spp. | 1 | 32 |
| S. aureus | 0.5 | 4 |
| CNS | 16 | 64 |
| Total Enterococcus spp. | 32 | 64 |
| E. faecalis | 32 | 64 |
| E. faecium | 64 | 64 |
| Pathogens | S/R | Susceptibility to Other Antibiotics (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amikacin | Gentamicin | Imipenem | Meropenem | Ceftazidime | Cefepime | Piperacillin-Tazobactam | Ciprofloxacin | Levofloxacin | Tigecycline | ||
| Enterobacterales | Total | 86 | 68.3 | 80 | 81.4 | 59.2 | 58.6 | 67 | 45.7 | 47.4 | 39.8 |
| S | 91.4 | 74.4 | 86.2 | 87.3 | 66.4 | 65.8 | 74.6 | 52.3 | 54.1 | 47 | |
| R | 66.7 | 46.2 | 56.9 | 59.6 | 32.5 | 33.3 | 39.3 | 21.4 | 23.1 | 13.8 | |
| Pseudomonas spp. | Total | 82.4 | NT | 63.6 | 73 | 71.2 | NT | 73.7 | 58.6 | 58.9 | NT |
| S | 83.5 | NT | 66.4 | 75.5 | 71.9 | NT | 74.6 | 60.9 | 61.3 | NT | |
| R | 71.4 | NT | 35.7 | 46.2 | 64.3 | NT | 64.3 | 35.7 | 35.7 | NT | |
| Acinetobacter spp. | Total | 5.7 | 4.6 | 5.7 | 5.9 | NT | NT | 4.6 | 2.3 | 2.5 | NT |
| Pathogens | S/R | Susceptibility to Other Antibiotics (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Methicillin (MSS) | Gentamicin | Ceftaroline | Ciprofloxacin | Trimethoprim-Sulfamethoxazole | Vancomycin | Linezolid | Tigecycline | ||
| S. aureus | Total | 61.5 | 96.1 | 98.9 | 49 | 100 | 100 | 100 | NT |
| S | 62 | 97 | 98.9 | 49.5 | 100 | 100 | 100 | NT | |
| R | n = 0 | n = 0 | n = 1 | n = 0 | n = 0 | n = 1 | n = 1 | NT | |
| CNS | Total | 26 | 59.6 | NT | 50 | 66.1 | 98.2 | 92.7 | NT |
| S | 34.9 | 67.4 | NT | 53.5 | 71.4 | 97.6 | 95 | NT | |
| R | 0 | 35.7 | NT | 38.5 | 50 | 100 | 85.7 | NT | |
| Enterococcus spp. | Total | NT | 72 | NT | NT | NT | 76 | 98 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska-Krochmal, B.; Mączyńska, B.; Rurańska-Smutnicka, D.; Secewicz, A.; Krochmal, G.; Bartelak, M.; Górzyńska, A.; Laufer, K.; Woronowicz, K.; Łubniewska, J.; et al. Assessment of the Susceptibility of Clinical Gram-Negative and Gram-Positive Bacterial Strains to Fosfomycin and Significance of This Antibiotic in Infection Treatment. Pathogens 2022, 11, 1441. https://doi.org/10.3390/pathogens11121441
Kowalska-Krochmal B, Mączyńska B, Rurańska-Smutnicka D, Secewicz A, Krochmal G, Bartelak M, Górzyńska A, Laufer K, Woronowicz K, Łubniewska J, et al. Assessment of the Susceptibility of Clinical Gram-Negative and Gram-Positive Bacterial Strains to Fosfomycin and Significance of This Antibiotic in Infection Treatment. Pathogens. 2022; 11(12):1441. https://doi.org/10.3390/pathogens11121441
Chicago/Turabian StyleKowalska-Krochmal, Beata, Beata Mączyńska, Danuta Rurańska-Smutnicka, Anna Secewicz, Grzegorz Krochmal, Małgorzata Bartelak, Aleksandra Górzyńska, Klaudyna Laufer, Krystyna Woronowicz, Joanna Łubniewska, and et al. 2022. "Assessment of the Susceptibility of Clinical Gram-Negative and Gram-Positive Bacterial Strains to Fosfomycin and Significance of This Antibiotic in Infection Treatment" Pathogens 11, no. 12: 1441. https://doi.org/10.3390/pathogens11121441
APA StyleKowalska-Krochmal, B., Mączyńska, B., Rurańska-Smutnicka, D., Secewicz, A., Krochmal, G., Bartelak, M., Górzyńska, A., Laufer, K., Woronowicz, K., Łubniewska, J., Łappo, J., Czwartos, M., & Dudek-Wicher, R. (2022). Assessment of the Susceptibility of Clinical Gram-Negative and Gram-Positive Bacterial Strains to Fosfomycin and Significance of This Antibiotic in Infection Treatment. Pathogens, 11(12), 1441. https://doi.org/10.3390/pathogens11121441