Epidemiology and Genotype Distribution of Hepatitis C Virus in Russia
Abstract
1. Introduction
2. Materials and Methods
2.1. Retrospective Epidemiological Analysis
2.2. Collection and Storage of Serum Samples
2.3. Isolation of HCV RNA
2.4. PCR Analysis and Genotyping of HCV
2.5. Sanger Sequencing
2.6. Epidemiological Characterization of Samples from HCV-Infected Individuals
2.7. Statistical Analysis
3. Results
3.1. Epidemiology of HCV Infection in Russia
3.2. HCV Genotype and Subgenotype Distribution
3.3. Distribution of RF1_2k/1b Recombinant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Global Hepatitis Report, 2017; World Health Organization: Geneva, Switzerland, 2017; 83p, ISBN 978-92-4-156545-5. Available online: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ (accessed on 20 September 2022).
- World Health Organization; Hepatitis, C. Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 1 October 2022).
- Haykal, M.; Matsumori, A.; Saleh, A.; Fayez, M.; Negm, H.; Shalaby, M.; Bassuony, S. Diagnosis and treatment of HCV heart diseases. Expert Rev. Cardiovasc. Ther. 2021, 19, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Izopet, J.; Alric, L.; Guilbeaud-Frugier, C.; Rostaing, L. Hepatitis C virus-related kidney disease: An overview. Clin. Nephrol. 2008, 69, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Hadziyannis, S.J. Nonhepatic manifestations and combined diseases in HCV infection. Dig. Dis. Sci. 1996, 41, 63S–74S. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferri, C.; Fallahi, P.; Pampana, A.; Ferrari, S.M.; Barani, L.; Marchi, S.; Ferrannini, E. Thyroid cancer in HCV-related chronic hepatitis patients: A case-control study. Thyroid 2007, 17, 447–451. [Google Scholar] [CrossRef]
- Viswanatha, D.S.; Dogan, A. Hepatitis C virus and lymphoma. J Clin Pathol. 2007, 60, 1378–1383. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Engels, E.A.; Landgren, O.; Chiao, E.; Henderson, L.; Amaratunge, H.C.; Giordano, T.P. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology 2009, 49, 116–123. [Google Scholar] [CrossRef]
- Zarębska-Michaluk, D.; Jaroszewicz, J.; Parfieniuk-Kowerda, A.; Janczewska, E.; Dybowska, D.; Pawłowska, M.; Halota, W.; Mazur, W.; Lorenc, B.; Janocha-Litwin, J.; et al. Effectiveness and Safety of Pangenotypic Regimens in the Most Difficult to Treat Population of Genotype 3 HCV Infected Cirrhotics. J. Clin. Med. 2022, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis. Available online: https://www.who.int/publications/i/item/WHO-HIV-2016.06 (accessed on 1 October 2022).
- World Health Organizaion. Final Global Health Sector Strategies on Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections, 2022–2030. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/strategies/global-health-sector-strategies/developing-ghss-2022-2030 (accessed on 1 October 2022).
- World Health Organization. Regional Office for Europe. 2017. Action Plan for the Health Sector Response to Viral Hepatitis in the WHO European Region. World Health Organization. Regional Office for Europe. Available online: https://apps.who.int/iris/handle/10665/344154 (accessed on 1 October 2022).
- Borgia, S.M.; Hedskog, C.; Parhy, B.; Hyland, R.H.; Stamm, L.M.; Brainard, D.M.; Subramanian, M.G.; McHutchison, J.G.; Mo, H.; Svarovskaia, E.; et al. Identification of a Novel Hepatitis C Virus Genotype From Punjab, India: Expanding Classification of Hepatitis C Virus Into 8 Genotypes. J. Infect. Dis. 2018, 218, 1722–1729. [Google Scholar] [CrossRef]
- Blach, S.; Zeuzem, S.; Manns, M.; Altraif, I.; Duberg, A.-S.; Muljono, D.H.; Waked, I.; Alavian, S.M.; Lee, M.; Negro, F.; et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef]
- Pawlotsky, J.M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Cox, A.L.; El-Sayed, M.H.; Kao, J.-H.; Lazarus, J.V.; Lemoine, M.; Lok, A.S.; Zoulim, F. Progress towards elimination goals for viral hepatitis. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 533–542. [Google Scholar] [CrossRef]
- Blach, S.; Terrault, N.A.; Tacke, F.; Gamkrelidze, I.; Craxi, A.; Tanaka, J.; Waked, I.; Dore, G.J.; Abbas, Z.; Abdallah, A.R.; et al. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef] [PubMed]
- Federal State Statistics Service. Russian Statistical Yearbook 2020. Available online: https://eng.rosstat.gov.ru/Publications/document/74811 (accessed on 2 August 2022).
- Lovo, D.K.; Samokhvalov, E.I.; Tsuda, F.; Selivanov, N.A.; Okamoto, H.; Stakhanova, V.M.; Stakhgildyan, I.V.; Doroshenko, N.V.; Yashina, T.L.; Kuzin, N.S.; et al. Prevalence of hepatitis C virus and distribution of its genotypes in Northern Eurasia. Arch Virol. 1996, 141, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Hayakawa, E.; Sminov, A.V.; Rossina, A.L.; Ding, X.; Huy, T.T.-T.; Sata, T.; Uchaikin, V.F. Molecular epidemiology of hepatitis B, C, D and E viruses among children in Moscow, Russia. J. Clin. Virol. 2004, 30, 57–61. [Google Scholar] [CrossRef]
- Kartashev, V.; Döring, M.; Nieto, L.; Coletta, E.; Kaiser, R.; Sierra, S.; Guerrero, A.; Stoiber, H.; Paar, C.; Vandamme, A.; et al. New findings in HCV genotype distribution in selected West European, Russian and Israeli regions. J. Clin. Virol. 2016, 81, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, O.; Norder, H.; Vetrov, T.; Zhdanov, K.; Barzunova, M.; Plotnikova, V.; Mukomolov, S.; Magnius, L.O. Shift in predominating subtype of HCV from 1b to 3a in St. Petersburg mediated by increase in injecting drug use. J. Med. Virol. 2001, 65, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Zakalashvili, M.; Zarkua, J.; Gish, R.G.; Zhamutashvili, M.; Sartania, V.; Weizenegger, M.; Bartel, J.; Raabe, M.; Gvinjilia, L.; Metreveli, S.; et al. Assessment of treatment options for patients with hepatitis C virus recombinant form 2k/1b. Hepatol Res. 2021, 51, 156–165. [Google Scholar] [CrossRef]
- Hedskog, C.; Doehle, B.; Chodavarapu, K.; Gontcharova, V.; Crespo Garcia, J.; de Knegt, R.; Drenth, J.P.H.; McHutchison, J.G.; Brainard, D.; Stamm, L.M.; et al. Characterization of hepatitis C virus intergenotypic recombinant strains and associated virological response to sofosbuvir/ribavirin. Hepatology 2015, 61, 471–480. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Screening, Care and Treatment of Persons with Chronic Hepatitis C Infection. 2016. Available online: https://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2016/en/ (accessed on 1 October 2022).
- Kalinina, O.; Norder, H.; Mukomolov, S.; Magnius, L.O. A natural intergenotypic recombinant of hepatitis C virus identified in St. Petersburg. J. Virol. 2002, 76, 4034–4043. [Google Scholar] [CrossRef]
- Raghwani, J.; Thomas, X.V.; Koekkoek, S.M.; Schinkel, J.; Molenkamp, R.; van de Laar, T.J.; Takebe, Y.; Tanaka, Y.; Mizokami, M.; Rambaut, A.; et al. Origin and evolution of the unique hepatitis C virus circulating recombinant form 2k/1b. J. Virol. 2012, 86, 2212–2220. [Google Scholar] [CrossRef]
- Tallo, T.; Norder, H.; Tefanova, V.; Krispin, T.; Schmidt, J.; Ilmoja, M.; Orgulas, K.; Pruunsild, K.; Priimagi, L.; Magnius, L.O. Genetic characterization of hepatitis C virus strains in Estonia: Fluctuations in the predominating subtype with time. J. Med. Virol. 2007, 79, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Kurbanov, F.; Tanaka, Y.; Avazova, D.; Khan, A.; Sugauchi, F.; Kan, N.; Kurbanova-Khudayberganova, D.; Khikmatullaeva, A.; Musabaev, E.; Mizokami, M. Detection of hepatitis C virus natural recombinant RF1_2k/1b strain among intravenous drug users in Uzbekistan. Hepatol. Res. 2008, 38, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Viazov, S.; Kuzin, S.; Paladi, N.; Tchernovetsky, M.; Isaeva, E.; Mazhul, L.; Vasychova, F.; Widell, A.; Roggendorf, M. Hepatitis C virus genotypes in different regions of the former Soviet Union (Russia, Belarus, Moldova, and Uzbekistan). J. Med. Virol. 1997, 53, 36–40. [Google Scholar] [CrossRef]
- Paolucci, S.; Premoli, M.; Ludovisi, S.; Mondelli, M.U.; Baldanti, F. HCV intergenotype 2k/1b recombinant detected in a DAA-treated patient in Italy. Antivir. Ther. 2017, 22, 365–368. [Google Scholar] [CrossRef]
- Kassela, K.; Karakasiliotis, I.; Kokkiou, E.; Souvalidou, F.; Mimidis, P.; Veletza, S.; Panopoulou, M.; Koskinas, J.; Mimidis, K.; Mavromara, P. Intergenotypic 2k/1b hepatitis C virus recombinants in the East Macedonia and Thrace region of Greece. Ann. Gastroenterol. 2019, 32, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Stelzl, E.; Haas, B.; Bauer, B.; Zhang, S.; Fiss, E.H.; Hillman, G.; Hamilton, A.T.; Mehta, R.; Heil, M.L.; Marins, E.G.; et al. First identification of a recombinant form of hepatitis C virus in Austrian patients by full-genome next generation sequencing. PLoS ONE. 2017, 12, e0181273. [Google Scholar] [CrossRef]
- Zakalashvili, M.; Zarkua, J.; Weizenegger, M.; Bartel, J.; Raabe, M.; Zangurashvili, L.; Kankia, N.; Jashiashvili, N.; Lomidze, M.; Telia, T.; et al. Identification of hepatitis C virus 2k/1b intergenotypic recombinants in Georgia. Liver. Int. 2018, 38, 451–457. [Google Scholar] [CrossRef]
- Kurbanov, F.; Tanaka, Y.; Chub, E.; Maruyama, I.; Azlarova, A.; Kamitsukasa, H.; Ohno, T.; Bonetto, S.; Moreau, I.; Fanning, L.J.; et al. Molecular epidemiology and interferon susceptibility of the natural recombinant hepatitis C virus strain RF1_2k/1b. J. Infect. Dis. 2008, 198, 1448–1456. [Google Scholar] [CrossRef]
- Federal State Statistics Service. Federal Statistical form “Infectious and Parasitic Diseases”. Available online: https://eng.rosstat.gov.ru/ (accessed on 2 August 2022).
- Nelson, P.; Mathers, B.; Cowie, B.; Hagan, H.; Des Jarlais, D.; Horyniak, D.; Degenhardt, L. The epidemiology of viral hepatitis among people who inject drugs: Results of global systematic reviews. Lancet 2011, 378, 571–583. [Google Scholar] [CrossRef]
- Isakov, V.; Hedskog, C.; Wertheim, J.O.; Hostager, R.E.; Parhy, B.; Schneider, A.D.B.; Suri, V.; Mo, H.; Geivandova, N.; Morozov, V.; et al. Prevalence of resistance-associated substitutions and phylogenetic analysis of hepatitis C virus infection in Russia. Int. J. Infect. Dis. 2021, 113, 36–42. [Google Scholar] [CrossRef]
- Olinger, C.M.; Lazouskaya, N.V.; Eremin, V.F.; Muller, C.P. Multiple genotypes and subtypes of hepatitis B and C viruses in Belarus: Similarities with Russia and western European influences. Clin. Microbiol. Infect. 2008, 14, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Aibekova, L.; Bexeitova, A.; Aldabergenova, A.; Hortelano, G.; Ge, Z.; Yi, F.; Shao, Y.; DeHovitz, J.; Vermund, S.H.; Ali, S. Transmission of HIV and HCV within Former Soviet Union Countries. Can. J. Gastroenterol. Hepatol. 2020, 2020, 9701920. [Google Scholar] [CrossRef] [PubMed]
- Marascio, N.; Quirino, A.; Barreca, G.S.; Galati, L.; Costa, C.; Pisani, V.; Mazzitelli, M.; Matera, G.; Liberto, M.C.; Focà, A.; et al. Discussion on critical points for a tailored therapy to cure hepatitis C virus infection. Clin. Mol. Hepatol. 2019, 25, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Krassenburg, L.A.P.; Maan, R.; Ramji, A.; Manns, M.P.; Cornberg, M.; Wedemeyer, H.; de Knegt, R.J.; Hansen, B.E.; Janssen, H.L.A.; de Man, R.A.; et al. Clinical outcomes following DAA therapy in patients with HCV-related cirrhosis depend on disease severity. J. Hepatol. 2021, 74, 1053–1063. [Google Scholar] [CrossRef]
- Hayes, C.N.; Imamura, M.; Tanaka, J.; Chayama, K. Road to elimination of HCV: Clinical challenges in HCV management. Liver Int. 2022, 42, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Marcellusi, A.; Simonelli, C.; Mennini, F.S.; Kondili, L.A. Economic Consequences of Anti-HCV Treatment of Patients Diagnosed Through Screening in Italy: A Prospective Modelling Analysis. Appl. Health Econ. Health Policy 2022, 20, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of the Russian Federation; Chronic Viral Hepatitis, C. National Clinical Guidelines. 2021. Available online: https://cr.minzdrav.gov.ru/schema/516_2 (accessed on 3 October 2022).

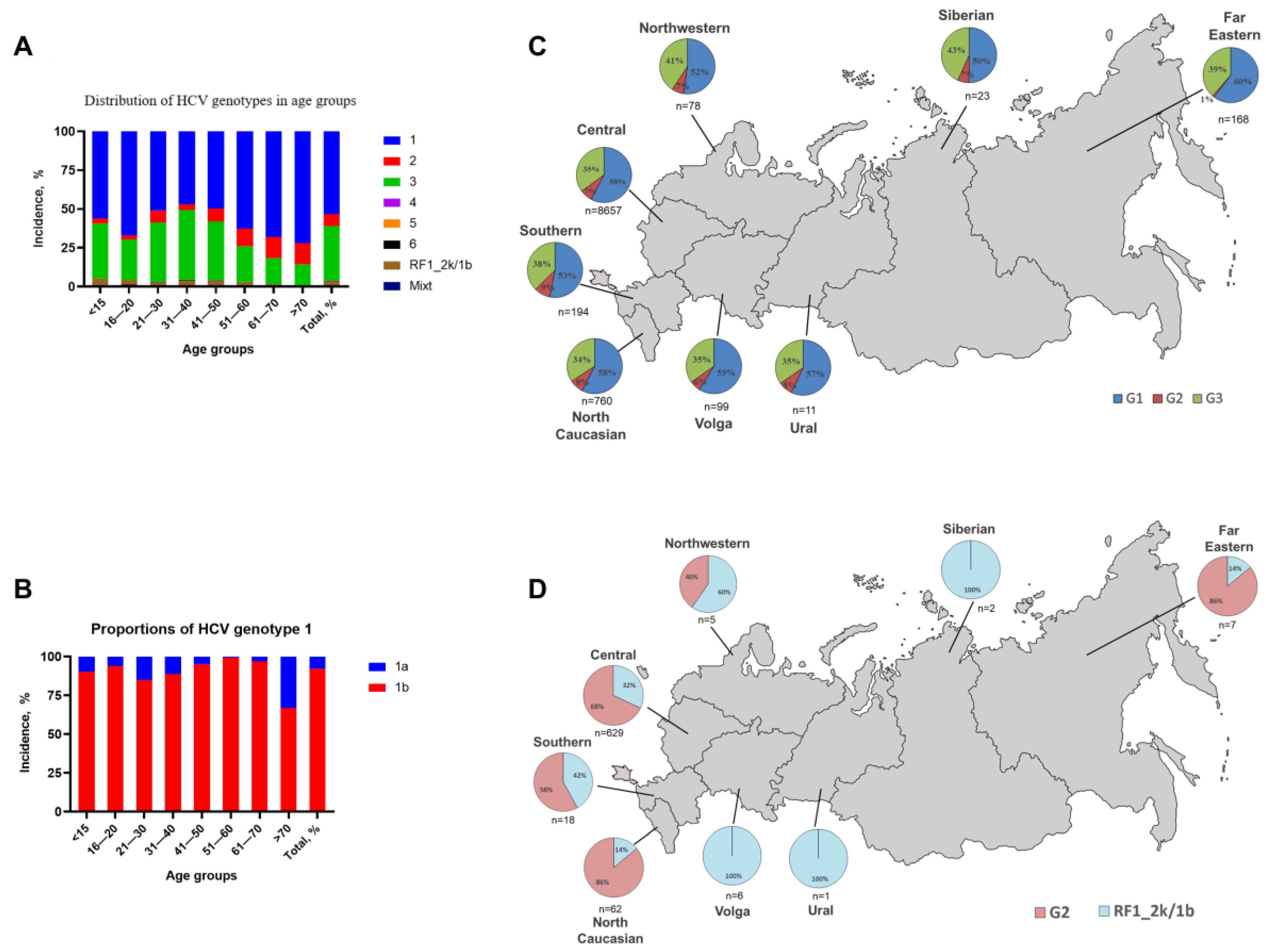

| Age Groups | Genotype (or Subtype) Total Value (Percentage %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | RF1_2k/1b | Mixed | ||
| 1a | 1b | ||||||||
| <15 | 7 (5, 4) | 66 (50, 8) | 4 (3, 1) | 46 (35, 4) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 7 (5, 4) | 0 (0, 0) |
| 16–20 | 3 (4, 1) | 46 (63, 0) | 2 (2, 7) | 19 (26, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 2 (2, 7) | 1 (1, 4) |
| 21–30 | 83(7, 7) | 463 (43, 2) | 82 (7, 7) | 412 (38, 5) | 2 (0, 2) | 0 (0, 0) | 0 (0, 0) | 29 (2, 7) | 0 (0, 0) |
| 31–40 | 188 (5, 3) | 1493 (41, 8) | 129 (3, 6) | 1604 (44, 9) | 8 (0, 2) | 0 (0, 0) | 6 (0, 2) | 134 (3, 8) | 7 (0, 2) |
| 41–50 | 54 (2, 4) | 1078 (47, 4) | 189 (8, 3) | 866 (38, 1) | 5 (0, 2) | 0 (0, 0) | 0 (0, 0) | 81 (3, 6) | 1 (0, 0) |
| 51–60 | 9 (0, 5) | 1107 (62, 2) | 202 (11, 3) | 409 (23, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 51 (2, 9) | 2 (0, 1) |
| 61–70 | 19 (2, 2) | 570 (66, 0) | 116 (13, 4) | 145 (16, 8) | 1 (0, 1) | 0 (0, 0) | 0 (0, 0) | 12 (1, 4) | 1 (0, 1) |
| >70 | 55 (23, 9) | 111 (48, 3) | 31 (13, 5) | 31 (13, 5) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 2 (0, 9) | 0 (0, 0) |
| Total | 418 (4, 2) | 4934 (49, 4) | 755 (7, 6) | 3532 (35, 4) | 16 (0, 2) | 0 (0, 0) | 6 (0, 1) | 318 (3, 2) | 12 (0, 1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimenov, N.; Kostyushev, D.; Komarova, S.; Fomicheva, A.; Urtikov, A.; Belaia, O.; Umbetova, K.; Darvina, O.; Tsapkova, N.; Chulanov, V. Epidemiology and Genotype Distribution of Hepatitis C Virus in Russia. Pathogens 2022, 11, 1482. https://doi.org/10.3390/pathogens11121482
Pimenov N, Kostyushev D, Komarova S, Fomicheva A, Urtikov A, Belaia O, Umbetova K, Darvina O, Tsapkova N, Chulanov V. Epidemiology and Genotype Distribution of Hepatitis C Virus in Russia. Pathogens. 2022; 11(12):1482. https://doi.org/10.3390/pathogens11121482
Chicago/Turabian StylePimenov, Nikolay, Dmitry Kostyushev, Svetlana Komarova, Anastasia Fomicheva, Alexander Urtikov, Olga Belaia, Karina Umbetova, Olga Darvina, Natalia Tsapkova, and Vladimir Chulanov. 2022. "Epidemiology and Genotype Distribution of Hepatitis C Virus in Russia" Pathogens 11, no. 12: 1482. https://doi.org/10.3390/pathogens11121482
APA StylePimenov, N., Kostyushev, D., Komarova, S., Fomicheva, A., Urtikov, A., Belaia, O., Umbetova, K., Darvina, O., Tsapkova, N., & Chulanov, V. (2022). Epidemiology and Genotype Distribution of Hepatitis C Virus in Russia. Pathogens, 11(12), 1482. https://doi.org/10.3390/pathogens11121482








