Immunoprotective Analysis of the NFA49590 Protein from Nocardia farcinica IFM 10152 Demonstrates Its Potential as a Vaccine Candidate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Bacterial Strains, Plasmid, and Cells
2.3. LC–MS/MS and In Silico Analysis
2.4. Preparation of Recombinant NFA49590 Protein
2.5. Preparation of rNFA49590 and Whole Bacteria Antiserum
2.6. Subcellular Localization
2.7. Antigenicity Determination by Western Blot
2.8. Mitogen-Activated Protein Kinase (MAPK) and NF-κB Analysis
2.9. Cytokine Measurements
2.10. Mouse Immunization
2.11. Whole Blood and Neutrophil Killing Assay
2.12. Mouse Infection
2.13. Statistical Analysis
3. Results
3.1. nfa49590 Was Conserved in N. farcinica Strains with Potential Antigenicity
3.2. Expression and Purification of rNFA49590 Protein
3.3. Subcellular Localization of Native NFA49590 Protein
3.4. Antigenicity of rNFA49590 Protein
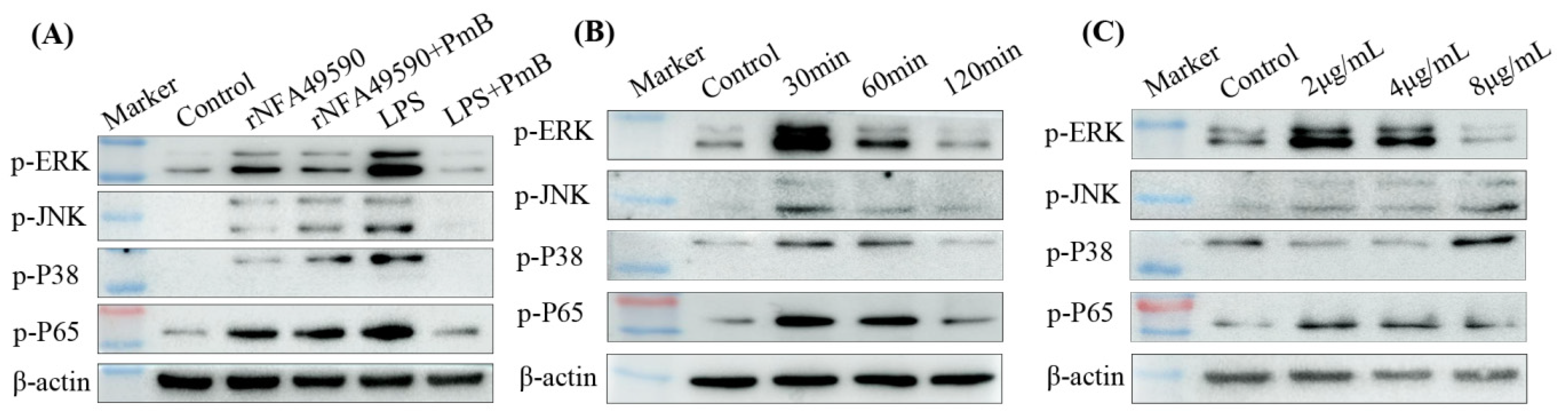
3.5. rNFA49590 Activated the MAPK and NF-κB Pathways in RAW264.7 Cells
3.6. rNFA49590 Stimulated Cytokine Secretion through Activation of the MAPK and NF-κB Pathways in RAW264.7 Cells
3.7. rNFA49590 Induces a High Humoral Response in Mice
3.8. Enhanced Bacterial Clearance Ability of Whole Blood and Neutrophils in rNFA49590-Immunized Mice
3.9. rNFA49590 Protein Protects Mice against Challenge with N. farcinica
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown-Elliott, B.A.; Brown, J.M.; Conville, P.S.; Wallace, R.J. Clinical and Laboratory Features of the Nocardia spp. Based on Current Molecular Taxonomy. Clin. Microbiol. Rev. 2006, 19, 259–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.W. Nocardiosis: Updates and Clinical Overview. Mayo Clin. Proc. 2012, 87, 403–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosioni, J.; Lew, D.; Garbino, J. Nocardiosis: Updated Clinical Review and Experience at a Tertiary Center. Infection 2010, 38, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budzik, J.M.; Hosseini, M.; Mackinnon, A.C.; Taxy, J.B. Disseminated Nocardia farcinica: Literature Review and Fatal Outcome in an Immunocompetent Patient. Surg. Infect. 2012, 13, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardak, E.; Yigla, M.; Berger, G.; Sprecher, H.; Oren, I. Clinical Spectrum and Outcome of Nocardia Infection: Experience of 15-Year Period from a Single Tertiary Medical Center. Am. J. Med Sci. 2012, 343, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Conville, P.S.; Witebsky, F.G. Organisms Designated as Nocardia asteroides Drug Pattern Type VI Are Members of the Species Nocardia cyriacigeorgica. J. Clin. Microbiol. 2007, 45, 2257–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Z.; Al-Sayer, H.; Das Chugh, T.; Chandy, R.; Provost, F.; Boiron, P. Antimicrobial susceptibility profile of soil isolates of Nocardia asteroides from Kuwait. Clin. Microbiol. Infect. 2000, 6, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Larruskain, J.; Idigoras, P.; Marimón, J.M.; Pérez-Trallero, E. Susceptibility of 186 Nocardia sp. Isolates to 20 Antimicrobial Agents. Antimicrob. Agents Chemother. 2011, 55, 2995–2998. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, Y.; Fujii, S.; Shinonaga, H.; Arai, K.; Saito, F.; Fukai, T.; Satoh, H.; Miyazaki, Y.; Ishikawa, J. Monooxygenation of rifampicin catalyzed by the rox gene product of Nocardia farcinica: Structure elucidation, gene identification and role in drug resistance. J. Antibiot. 2010, 63, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Kogure, T.; Shimada, R.; Ishikawa, J.; Yazawa, K.; Brown, J.M.; Mikami, Y.; Gonoi, T. Homozygous Triplicate Mutations in Three 16S rRNA Genes Responsible for High-Level Aminoglycoside Resistance in Nocardia farcinica Clinical Isolates from a Canada-Wide Bovine Mastitis Epizootic. Antimicrob. Agents Chemother. 2010, 54, 2385–2390. [Google Scholar] [CrossRef]
- Mehta, H.; Weng, J.; Prater, A.; Elworth, R.A.L.; Han, X.; Shamoo, Y. Pathogenic Nocardia cyriacigeorgica and Nocardia nova Evolve To Resist Trimethoprim-Sulfamethoxazole by both Expected and Unexpected Pathways. Antimicrob. Agents Chemother. 2018, 62, e00364-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, J.; Yamashita, A.; Mikami, Y.; Hoshino, Y.; Kurita, H.; Hotta, K.; Shiba, T.; Hattori, M. The complete genomic sequence of Nocardia farcinica IFM 10152. Proc. Natl. Acad. Sci. USA 2004, 101, 14925–14930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.; Tan, X.; Hou, X.; Si, C.; Xu, S.; Tang, L.; Yuan, X.; Li, Z. Cloning, Expression, Invasion, and Immunological Reactivity of a Mammalian Cell Entry Protein Encoded by the mce1 Operon of Nocardia farcinica. Front. Microbiol. 2017, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zhang, X.; Li, H.; Sun, L.; Hou, X.; Song, H.; Han, L.; Xu, S.; Qiu, X.; Wang, X.; et al. Nfa34810 Facilitates Nocardia farcinica Invasion of Host Cells and Stimulates Tumor Necrosis Factor Alpha Secretion through Activation of the NF-κB and Mitogen-Activated Protein Kinase Pathways via Toll-Like Receptor 4. Infect. Immun. 2020, 88, e00831-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Bourexis, D.; Brister, J.R.; Canese, K.; Comeau, D.C.; Funk, K.; Kim, S.; Klimke, W.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2021, 49, D10–D17. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.Y.; Wagner, J.R.; Laird, M.R.; Melli, G.; Rey, S.; Lo, R.; Dao, P.; Sahinalp, S.C.; Ester, M.; Foster, L.J.; et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar] [CrossRef] [Green Version]
- Tusnady, G.E.; Simon, I. The HMMTOP transmembrane topology prediction server. Bioinformatics 2001, 17, 849–850. [Google Scholar] [CrossRef] [Green Version]
- UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Garcia-Boronat, M.; Diez-Rivero, C.M.; Reinherz, E.L.; Reche, P.A. PVS: A web server for protein sequence variability analysis tuned to facilitate conserved epitope discovery. Nucleic Acids Res. 2008, 36, W35–W41. [Google Scholar] [CrossRef]
- Derungs, T.; Leo, F.; Loddenkemper, C.; Schneider, T. Treatment of disseminated nocardiosis: A host–pathogen approach with adjuvant interferon gamma. Lancet Infect. Dis. 2021, 21, e334–e340. [Google Scholar] [CrossRef]
- Saullo, J.L.; Miller, R.A. Update on Nocardia infections in solid-organ transplantation. Curr. Opin. Organ Transplant. 2020, 25, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Agterof, M.J.; Van Der Bruggen, T.; Tersmette, M.; Ter Borg, E.J.; Bosch, J.M.M.V.D.; Biesma, D.H. Nocardiosis: A case series and a mini review of clinical and microbiological features. Neth. J. Med. 2007, 65, 199–202. [Google Scholar] [PubMed]
- Davis-Scibienski, C.; Beaman, B.L. Interaction of Nocardia asteroides with Rabbit Alveolar Macrophages: Association of Virulence, Viability, Ultrastructural Damage, and Phagosome-Lysosome Fusion. Infect. Immun. 1980, 28, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Beaman, B.L. In vitro response of rabbit alveolar macrophages to infection with Nocardia asteroides. Infect. Immun. 1977, 15, 925–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, S.K.; Shibasaki, Y.; Nakanishi, T. Immune responses to live and inactivated Nocardia seriolae and protective effect of recombinant interferon gamma (rIFN γ) against nocardiosis in ginbuna crucian carp, Carassius auratus langsdorfii. Fish Shellfish Immunol. 2014, 39, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Z.; Wang, W.; Hou, S.; Cai, J.; Xia, L.; Lu, Y. Development of DNA vaccines encoding ribosomal proteins (RplL and RpsA) against Nocardia seriolae infection in fish. Fish Shellfish Immunol. 2020, 96, 201–212. [Google Scholar] [CrossRef]
- Hoang, H.H.; Wang, P.-C.; Chen, S.-C. Recombinant resuscitation-promoting factor protein of Nocardia seriolae, a promissing vaccine candidate for largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2021, 111, 127–139. [Google Scholar] [CrossRef]
- Choi, C.-W.; Lee, Y.G.; Kwon, S.-O.; Kim, H.-Y.; Lee, J.C.; Chung, Y.-H.; Yun, C.-Y.; Kim, S.I. Analysis of Streptococcus pneumoniae secreted antigens by immuno-proteomic approach. Diagn. Microbiol. Infect. Dis. 2012, 72, 318–327. [Google Scholar] [CrossRef]
- Shukla, M.; Rohatgi, S. Vaccination with Secreted Aspartyl Proteinase 2 Protein from Candida parapsilosis Can Enhance Survival of Mice during C. tropicalis -Mediated Systemic Candidiasis. Infect. Immun. 2020, 88, e00312-20. [Google Scholar] [CrossRef]
- Allen, K.J.; Rogan, D.; Finlay, B.B.; Potter, A.A.; Asper, D.J. Vaccination with type III secreted proteins leads to decreased shedding in calves after experimental infection with Escherichia coli O157. Can. J. Veter. Res. 2011, 75, 98–105. [Google Scholar]
- Han, L.; Ji, X.; Liu, X.; Xu, S.; Li, F.; Che, Y.; Qiu, X.; Sun, L.; Li, Z. Estradiol Aggravate Nocardia farcinica Infections in Mice. Front. Immunol. 2022, 13, 858609. [Google Scholar] [CrossRef] [PubMed]





| No. | Gene Code | Protein ID | Score | Matches | Protein Description |
|---|---|---|---|---|---|
| 1 | NFA49580 | BAD59810 | 8485 | 376 (268) | hypothetical protein |
| 2 | NFA15900 | BAD56436 | 7802 | 345 (252) | hypothetical protein |
| 3 | NFA56390 | BAD60491 | 6316 | 320 (204) | hypothetical protein |
| 4 | NFA47630 | BAD59615 | 4636 | 123 (96) | hypothetical protein |
| 5 | NFA1840 | BAD55026 | 3856 | 208 (134) | putative esterase |
| 6 | NFA49590 | BAD59811 | 3686 | 181 (129) | hypothetical protein |
| 7 | NFA54170 | BAD60269 | 2837 | 100 (74) | putative protease |
| 8 | NFA54590 | BAD60311 | 2622 | 131 (82) | hypothetical protein |
| 9 | NFA49620 | BAD59814 | 2438 | 124 (75) | putative protease |
| 10 | NFA50960 | BAD59948 | 2353 | 100 (84) | hypothetical protein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Ji, X.; Yang, C.; Zhao, S.; Fan, S.; Zhao, L.; Qiu, X.; Yao, J.; Liu, X.; Li, F.; et al. Immunoprotective Analysis of the NFA49590 Protein from Nocardia farcinica IFM 10152 Demonstrates Its Potential as a Vaccine Candidate. Pathogens 2022, 11, 1488. https://doi.org/10.3390/pathogens11121488
Han L, Ji X, Yang C, Zhao S, Fan S, Zhao L, Qiu X, Yao J, Liu X, Li F, et al. Immunoprotective Analysis of the NFA49590 Protein from Nocardia farcinica IFM 10152 Demonstrates Its Potential as a Vaccine Candidate. Pathogens. 2022; 11(12):1488. https://doi.org/10.3390/pathogens11121488
Chicago/Turabian StyleHan, Lichao, Xingzhao Ji, Caixin Yang, Shuo Zhao, Shihong Fan, Lijun Zhao, Xiaotong Qiu, Jiang Yao, Xueping Liu, Fang Li, and et al. 2022. "Immunoprotective Analysis of the NFA49590 Protein from Nocardia farcinica IFM 10152 Demonstrates Its Potential as a Vaccine Candidate" Pathogens 11, no. 12: 1488. https://doi.org/10.3390/pathogens11121488
APA StyleHan, L., Ji, X., Yang, C., Zhao, S., Fan, S., Zhao, L., Qiu, X., Yao, J., Liu, X., Li, F., & Li, Z. (2022). Immunoprotective Analysis of the NFA49590 Protein from Nocardia farcinica IFM 10152 Demonstrates Its Potential as a Vaccine Candidate. Pathogens, 11(12), 1488. https://doi.org/10.3390/pathogens11121488






