A Familiar Outbreak of Monophasic Salmonella serovar Typhimurium (ST34) Involving Three Dogs and Their Owner’s Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbiological Methods for Bacterial Identification and Serotyping
2.1.1. Sample Collection
2.1.2. Identification and Isolation
2.1.3. Human Strains Collection
2.1.4. Serotyping
2.1.5. Antimicrobial Resistance
2.2. Data Collection of Epidemiological Investigation
2.3. Whole-Genome Sequencing and In Silico Analysis
3. Results
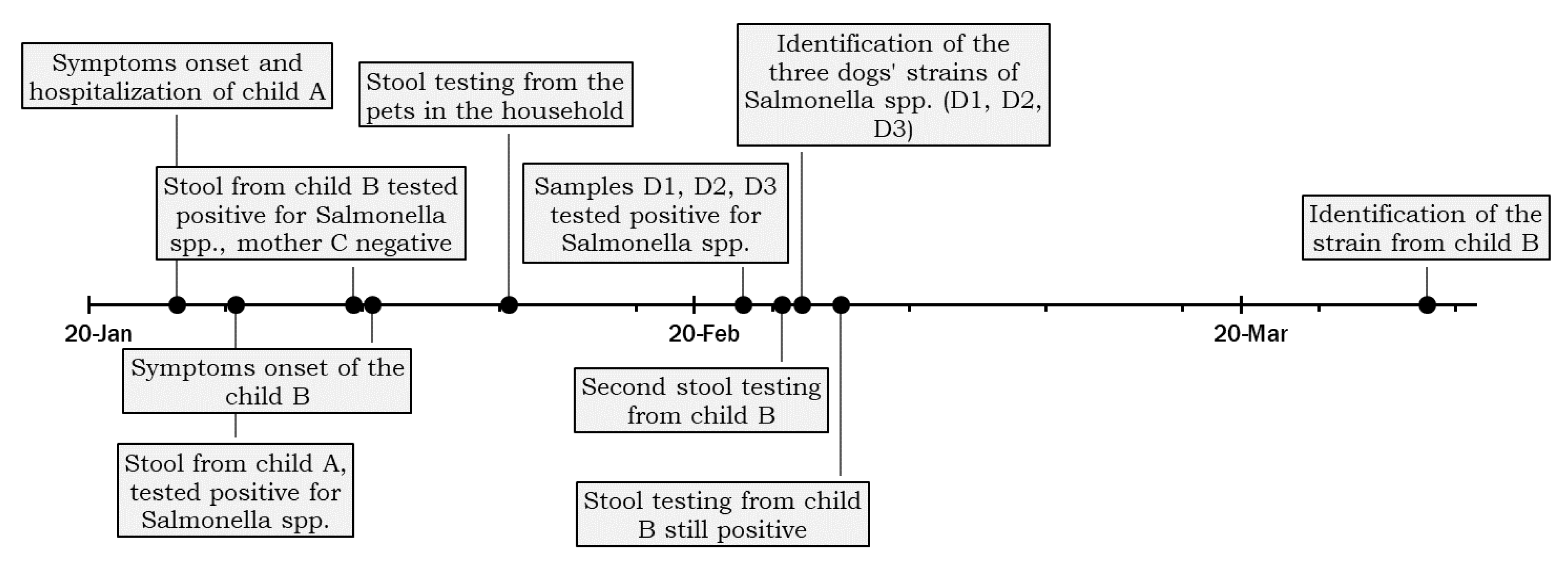
3.1. Case Presentation
3.2. Strains Characterization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, 6971. [Google Scholar] [CrossRef]
- ECDC—European Centre for Disease Prevention and Control. Salmonellosis—Annual Epidemiological Report for 2017. Annu. Epidemiol. Rep. Commun. Dis. Eur. 2020, 1–8. [Google Scholar]
- ECDC—European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases. Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 14 October 2022).
- Finley, R.; Reid-Smith, R.; Weese, J.S. Human health implications of Salmonella-contaminated natural pet treats and raw pet food. Clin. Infect. Dis. 2006, 42, 686–691. [Google Scholar] [CrossRef] [Green Version]
- Hoelzer, K.; Moreno Switt, A.I.; Wiedmann, M. Animal contact as a source of human non-typhoidal salmonellosis. Vet. Res. 2011, 42, 34. [Google Scholar] [CrossRef] [Green Version]
- Pfleger, S.; Benyr, G.; Sommer, R.; Hassl, A. Pattern of Salmonella excretion in amphibians and reptiles in a vivarium. Int. J. Hyg. Environ. Health 2003, 206, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Levings, R.S.; Lightfoot, D.; Hall, R.M.; Djordjevic, S.P. Aquariums as Reservoirs for Multidrug-resistant Salmonella Paratyphi, B. Emerg. Infect. Dis. 2006, 12, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Messenger, A.M.; Barnes, A.N.; Gray, G.C. Reverse Zoonotic Disease Transmission (Zooanthroponosis): A Systematic Review of Seldom-Documented Human Biological Threats to Animals. PLoS ONE 2014, 9, e89055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Organisation for Animal Health (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Organisation for Animal Health: Paris, France, 2022; Chapter 3.10.7. [Google Scholar]
- Russini, V.; Corradini, C.; de Marchis, M.L.; Bogdanova, T.; Lovari, S.; de Santis, P.; Migliore, G.; Bilei, S.; Bossù, T. Foodborne Toxigenic Agents Investigated in Central Italy: An Overview of a Three-Year Experience (2018–2020). Toxins 2022, 14, 40. [Google Scholar] [CrossRef]
- Andrews, S.; Babraham Bioinformatics. FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 25 January 2021).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, B. BBMap. Available online: https://sourceforge.net/projects/bbmap/ (accessed on 11 February 2022).
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; den Bakker, H.C.; Li, S.; Chen, J.; Dinsmore, B.A.; Lane, C.; Lauer, A.C.; Fields, P.I.; Deng, X. SeqSero2: Rapid and Improved Salmonella Serotype Determination Using Whole-Genome Sequencing Data. Appl. Environ. Microbiol. 2019, 85, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yin, Y.; Jones, M.B.; Zhang, Z.; Deatherage Kaiser, B.L.; Dinsmore, B.A.; Fitzgerald, C.; Fields, P.I.; Deng, X. Salmonella serotype determination utilizing high-throughput genome sequencing data. J. Clin. Microbiol. 2015, 53, 1685–1692. [Google Scholar] [CrossRef] [Green Version]
- Seemann, T. GitHub—Tseemann/mlst: Scan Contig Files Against PubMLST Typing Schemes. Available online: https://github.com/tseemann/mlst (accessed on 11 February 2022).
- Kidgell, C.; Reichard, U.; Wain, J.; Linz, B.; Torpdahl, M.; Dougan, G.; Achtman, M. Salmonella typhi, the causative agent of typhoid fever, is approximately 50,000 years old. Infect. Genet. Evol. 2002, 2, 39–45. [Google Scholar] [CrossRef]
- Silva, M.; Machado, M.P.; Silva, D.N.; Rossi, M.; Moran-Gilad, J.; Santos, S.; Ramirez, M.; Carriço, J.A. chewBBACA: A complete suite for gene-by-gene schema creation and strain identification. Microb. Genom. 2018, 4, 3. [Google Scholar] [CrossRef]
- Achtman, M.; Zhou, Z.; Alikhan, N.-F.; Tyne, W.; Parkhill, J.; Cormican, M.; Chiou, C.-S.; Torpdahl, M.; Litrup, E.; Prendergast, D.M.; et al. Genomic diversity of Salmonella enterica—The UoWUCC 10K genomes project. Wellcome Open Res. 2021, 5, 223. [Google Scholar] [CrossRef]
- Zhou, Z.; Alikhan, N.-F.; Mohamed, K.; Fan, Y.; Agama Study Group; Achtman, M. The EnteroBase user’s guide, with case studies on Salmonella transmissions, Yersinia pestis phylogeny, and Escherichia core genomic diversity. Genome Res. 2020, 30, 138–152. [Google Scholar] [CrossRef] [Green Version]
- Clausen, P.T.L.C.; Aarestrup, F.M.; Lund, O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinform. 2018, 19, 307. [Google Scholar] [CrossRef]
- Alikhan, N.-F.; Zhou, Z.; Sergeant, M.J.; Achtman, M. A genomic overview of the population structure of Salmonella. PLoS Genet. 2018, 14, e1007261. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Alikhan, N.-F.; Sergeant, M.J.; Luhmann, N.; Vaz, C.; Francisco, A.P.; Carriço, J.A.; Achtman, M. GrapeTree: Visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res. 2018, 28, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Kaas, R.S.; Leekitcharoenphon, P.; Aarestrup, F.M.; Lund, O. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS ONE 2014, 9, e104984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [Green Version]
- Sköld, O. Resistance to trimethoprim and sulfonamides. Vet. Res. 2001, 32, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-Y.; Nace, G.W.; Solow, B.; Fratamico, P. Complete nucleotide sequences of 84.5- and 3.2-kb plasmids in the multi-antibiotic resistant Salmonella enterica serovar Typhimurium U302 strain G8430. Plasmid 2007, 57, 29–43. [Google Scholar] [CrossRef]
- Nikiema, M.E.M.; Kakou-ngazoa, S.; Ky/Ba, A.; Sylla, A.; Bako, E.; Addablah, A.Y.A.; Ouoba, J.B.; Sampo, E.; Gnada, K.; Zongo, O.; et al. Characterization of virulence factors of Salmonella isolated from human stools and street food in urban areas of Burkina Faso. BMC Microbiol. 2021, 21, 338. [Google Scholar] [CrossRef]
- Jibril, A.H.; Okeke, I.N.; Dalsgaard, A.; Menéndez, V.G.; Olsen, J.E. Genomic Analysis of Antimicrobial Resistance and Resistance Plasmids in Salmonella Serovars from Poultry in Nigeria. Antibiotics 2021, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Oladeinde, A.; Cook, K.; Orlek, A.; Zock, G.; Herrington, K.; Cox, N.; Plumblee Lawrence, J.; Hall, C. Hotspot mutations and ColE1 plasmids contribute to the fitness of Salmonella Heidelberg in poultry litter. PLoS ONE 2018, 13, e0202286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadomski, A.; Scribani, M.B.; Tallman, N.; Krupa, N.; Jenkins, P.; Wissow, L.S. Impact of pet dog or cat exposure during childhood on mental illness during adolescence: A cohort study. BMC Pediatr. 2022, 22, 572. [Google Scholar] [CrossRef] [PubMed]
- Chomel, B. Emerging and Re-Emerging Zoonoses of Dogs and Cats. Animals 2014, 4, 434–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groat, E.F.; Williams, N.J.; Pinchbeck, G.; Warner, B.; Simpson, A.; Schmidt, V.M. UK dogs eating raw meat diets have higher risk of Salmonella and antimicrobial-resistant Escherichia coli faecal carriage. J. Small Anim. Pract. 2022, 63, 435–441. [Google Scholar] [CrossRef]
- Lowden, P.; Wallis, C.; Gee, N.; Hilton, A. Investigating the prevalence of Salmonella in dogs within the Midlands region of the United Kingdom. BMC Vet. Res. 2015, 11, 239. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-M.; Wang, Y.; Su, L.-H.; Chiu, C.-H. Nontyphoid Salmonella Infection: Microbiology, Clinical Features, and Antimicrobial Therapy. Pediatr. Neonatol. 2013, 54, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Darton, T.C.; Jones, C.; Blohmke, C.J.; Waddington, C.S.; Zhou, L.; Peters, A.; Haworth, K.; Sie, R.; Green, C.A.; Jeppesen, C.A.; et al. Using a Human Challenge Model of Infection to Measure Vaccine Efficacy: A Randomised, Controlled Trial Comparing the Typhoid Vaccines M01ZH09 with Placebo and Ty21a. PLoS Negl. Trop. Dis. 2016, 10, e0004926. [Google Scholar] [CrossRef] [Green Version]
- Eetemadi, A.; Rai, N.; Pereira, B.M.P.; Kim, M.; Schmitz, H.; Tagkopoulos, I. The Computational Diet: A Review of Computational Methods Across Diet, Microbiome, and Health. Front. Microbiol. 2020, 11, 393. [Google Scholar] [CrossRef] [Green Version]
- Selmi, M.; Stefanelli, S.; Bilei, S.; Tolli, R.; Bertolotti, L.; Marconi, P.; Giurlani, S.; de Lucia, P.G.; Ruggeri, G.; Pagani, A. Contaminated commercial dehydrated food as source of multiple Salmonella serotypes outbreak in a municipal kennel in Tuscany. Vet. Ital. 2011, 47, 183–190. [Google Scholar] [PubMed]
- Van Knapen, F.; Overgaauw, P. Dogs and Transmission of Infection to Man, “Respected Member of the Family?”. In Zoonoses—Infections Affecting Humans and Animals; Sing, A., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 575–585. [Google Scholar]
- Viegas, F.M.; Ramos, C.P.; Xavier, R.G.C.; Lopes, E.O.; Júnior, C.A.O.; Bagno, R.M.; Diniz, A.N.; Lobato, F.C.F.; Silva, R.O.S. Fecal shedding of Salmonella spp., Clostridium perfringens, and Clostridioides difficile in dogs fed raw meat-based diets in Brazil and their owners’ motivation. PLoS ONE 2020, 15, e0231275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dróżdż, M.; Małaszczuk, M.; Paluch, E.; Pawlak, A. Zoonotic potential and prevalence of Salmonella serovars isolated from pets. Infect. Ecol. Epidemiol. 2021, 11, 1975530. [Google Scholar] [CrossRef] [PubMed]
- Bataller, E.; García-Romero, E.; Llobat, L.; Lizana, V.; Jiménez-Trigos, E. Dogs as a source of Salmonella spp. in apparently healthy dogs in the Valencia Region. Could it be related with intestinal lactic acid bacteria? BMC Vet. Res. 2020, 16, 268. [Google Scholar] [CrossRef]
- Behravesh, C.B.; Ferraro, A.; Deasy, M.; Dato, V.; Moll, M.; Sandt, C.; Rea, N.K.; Rickert, R.; Marriott, C.; Warren, K.; et al. Human Salmonella infections linked to contaminated dry dog and cat food, 2006–2008. Pediatrics 2010, 126, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Zając, M.; Wasyl, D.; Różycki, M.; Bilska-Zając, E.; Fafiński, Z.; Iwaniak, W.; Krajewska, M.; Hoszowski, A.; Konieczna, O.; Fafińska, P.; et al. Free-living snakes as a source and possible vector of Salmonella spp. and parasites. Eur. J. Wildl. Res. 2016, 62, 161–166. [Google Scholar] [CrossRef]
- Bjelland, A.M.; Sandvik, L.M.; Skarstein, M.M.; Svendal, L.; Debenham, J.J. Prevalence of Salmonella serovars isolated from reptiles in Norwegian zoos. Acta Vet. Scand. 2020, 62, 3. [Google Scholar] [CrossRef] [Green Version]
- Wooldridge, M. Evidence for the circulation of antimicrobialresistant strains and genes in nature and especially between humans and animals. Rev. Sci. Tech. l’OIE 2012, 31, 231–247. [Google Scholar] [CrossRef] [Green Version]
- Dickson, A.; Smith, M.; Smith, F.; Park, J.; King, C.; Currie, K.; Langdridge, D.; Davis, M.; Flowers, P. Understanding the relationship between pet owners and their companion animals as a key context for antimicrobial resistance-related behaviours: An interpretative phenomenological analysis. Health Psychol. Behav. Med. 2019, 7, 45–61. [Google Scholar] [CrossRef] [Green Version]
- Achtman, M.; Wain, J.; Weill, F.-X.; Nair, S.; Zhou, Z.; Sangal, V.; Krauland, M.G.; Hale, J.L.; Harbottle, H.; Uesbeck, A.; et al. Multilocus Sequence Typing as a Replacement for Serotyping in Salmonella enterica. PLoS Pathog. 2012, 8, e1002776. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Li, Y.; Elbediwi, M.; Yue, M. Emergence and Dissemination of mcr-Carrying Clinically Relevant Salmonella Typhimurium Monophasic Clone ST34. Microorganisms 2019, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention; European Food Safety Authority. Multi-country outbreak of monophasic Salmonella Typhimurium sequence type 34 linked to chocolate products—First update—18 May 2022. EFSA Support. Publ. 2022, 19, 7352E. [Google Scholar] [CrossRef]
- Lund, S.; Tahir, M.; Vohra, L.I.; Hamdana, A.H.; Ahmad, S. Outbreak of monophasic Salmonella Typhimurium Sequence Type 34 linked to chocolate products. Ann. Med. Surg. 2022, 82, 104597. [Google Scholar] [CrossRef]
- Antunes, P.; Mourão, J.; Pestana, N.; Peixe, L. Leakage of emerging clinically relevant multidrug-resistant Salmonella clones from pig farms. J. Antimicrob. Chemother. 2011, 66, 2028–2032. [Google Scholar] [CrossRef]
- Diaconu, E.L.; Alba, P.; Feltrin, F.; di Matteo, P.; Iurescia, M.; Chelli, E.; Donati, V.; Marani, I.; Giacomi, A.; Franco, A.; et al. Emergence of IncHI2 Plasmids with Mobilized Colistin Resistance (mcr)-9 Gene in ESBL-Producing, Multidrug-Resistant Salmonella Typhimurium and Its Monophasic Variant ST34 From Food-Producing Animals in Italy. Front. Microbiol. 2021, 12, 1866. [Google Scholar] [CrossRef] [PubMed]
- Fortini, D.; Owczarek, S.; Dionisi, A.M.; Lucarelli, C.; Arena, S.; Carattoli, A.; Villa, L.; García-Fernández, A. Colistin Resistance Mechanisms in Human Salmonella enterica Strains Isolated by the National Surveillance Enter-Net Italia (2016–2018). Antibiotics 2022, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wan, F.; Yu, X.; Zheng, B.; Chen, Y.; Gong, C.; Fu, H.; Xiao, Y.; Li, L. MDR Salmonella enterica serovar Typhimurium ST34 carrying mcr-1 isolated from cases of bloodstream and intestinal infection in children in China. J. Antimicrob. Chemother. 2020, 75, 92–95. [Google Scholar] [CrossRef]
- Mancin, M.; Barco, L.; Losasso, C.; Belluco, S.; Cibin, V.; Mazzucato, M.; Bilei, S.; Carullo, M.R.; Decastelli, L.; di Giannatale, E.; et al. Salmonella serovar distribution from non-human sources in Italy; results from the IT-Enter-Vet network. Vet. Rec. 2018, 183, 69. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russini, V.; Corradini, C.; Rasile, E.; Terracciano, G.; Senese, M.; Bellagamba, F.; Amoruso, R.; Bottoni, F.; De Santis, P.; Bilei, S.; et al. A Familiar Outbreak of Monophasic Salmonella serovar Typhimurium (ST34) Involving Three Dogs and Their Owner’s Children. Pathogens 2022, 11, 1500. https://doi.org/10.3390/pathogens11121500
Russini V, Corradini C, Rasile E, Terracciano G, Senese M, Bellagamba F, Amoruso R, Bottoni F, De Santis P, Bilei S, et al. A Familiar Outbreak of Monophasic Salmonella serovar Typhimurium (ST34) Involving Three Dogs and Their Owner’s Children. Pathogens. 2022; 11(12):1500. https://doi.org/10.3390/pathogens11121500
Chicago/Turabian StyleRussini, Valeria, Carlo Corradini, Emilia Rasile, Giuliana Terracciano, Matteo Senese, Federica Bellagamba, Roberta Amoruso, Francesco Bottoni, Paola De Santis, Stefano Bilei, and et al. 2022. "A Familiar Outbreak of Monophasic Salmonella serovar Typhimurium (ST34) Involving Three Dogs and Their Owner’s Children" Pathogens 11, no. 12: 1500. https://doi.org/10.3390/pathogens11121500





