In Vitro Comparative Study of Platelets Treated with Two Pathogen-Inactivation Methods to Extend Shelf Life to 7 Days
Abstract
:1. Introduction
2. Results
2.1. PLT Product Quality Attributes
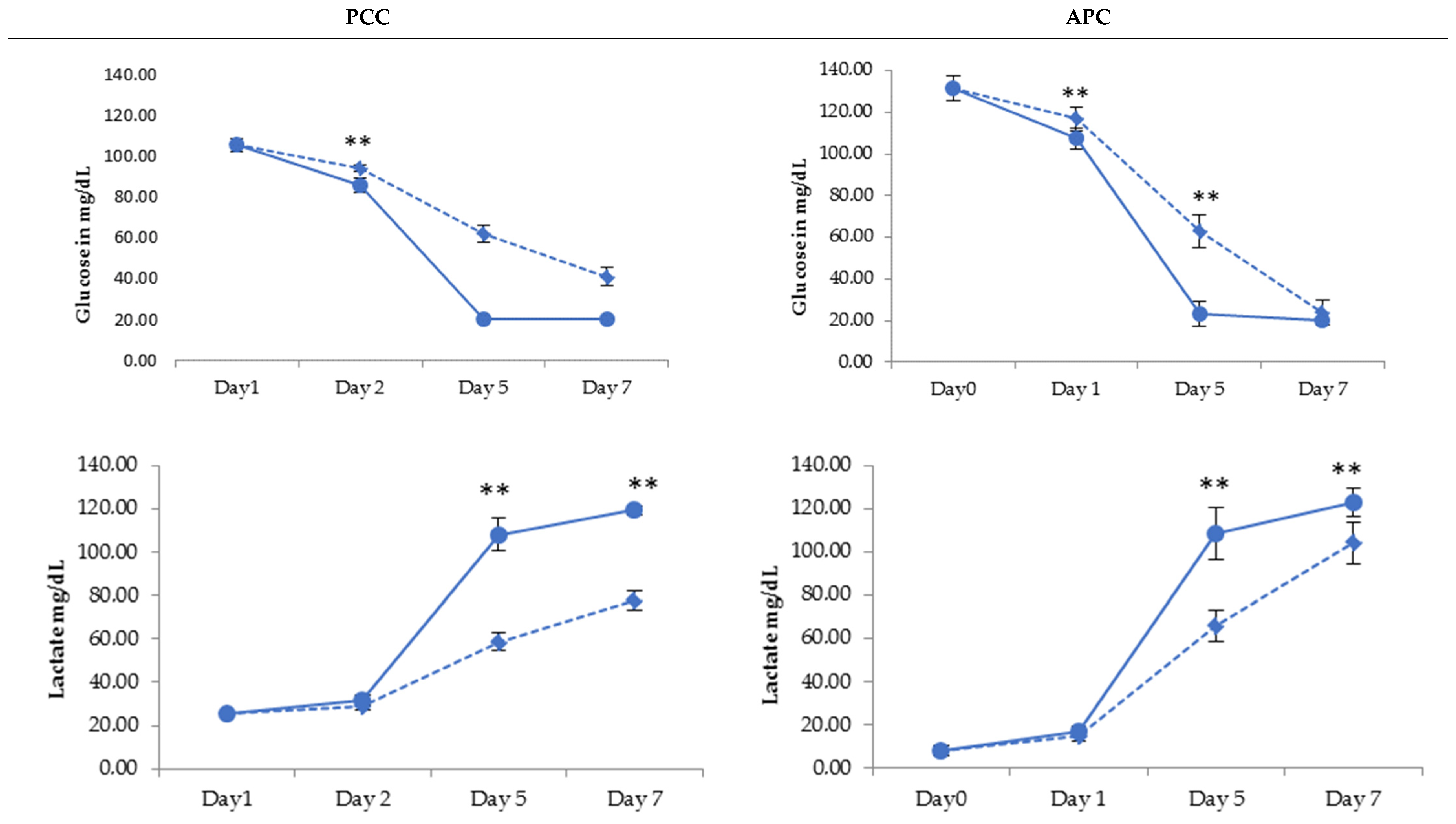
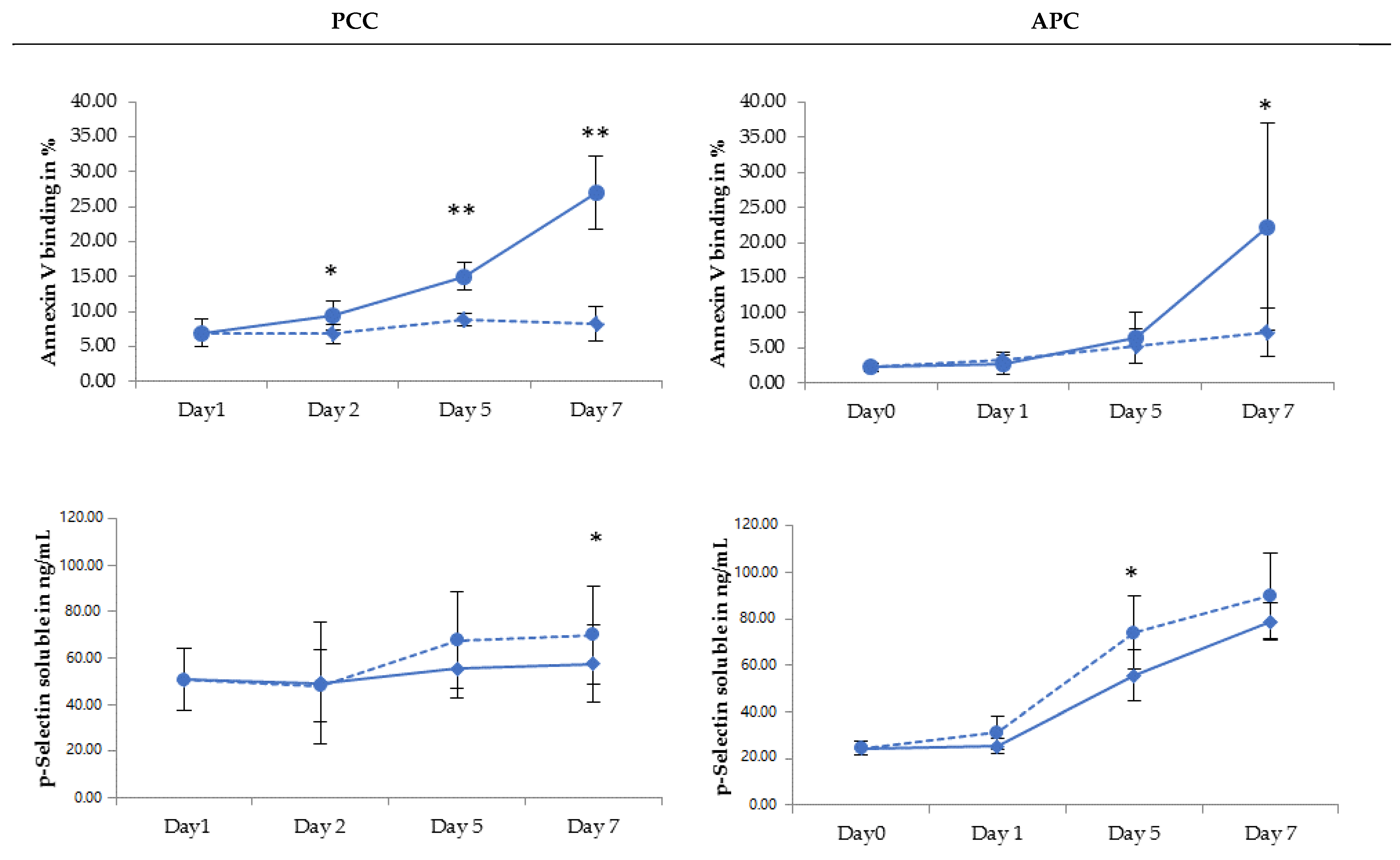
2.2. PLT Storage Study
3. Discussion
4. Materials and Methods
4.1. Platelet Preparation
4.2. Study Design
4.3. Pathogen-Inactivation Treatment
4.4. Sampling of Platelet Concentrates
4.5. Analytical Methods
4.6. Statistics
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escolar, G.; Diaz-Ricart, M.; McCullough, J. Impact of different pathogen reduction technologies on the biochemistry, function, and clinical effectiveness of platelet concentrates: An updated view during a pandemic. Transfusion 2022, 62, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Fast, L.D.; DiLeone, G.; Marschner, S. Inactivation of human white blood cells in platelet products after pathogen reduction technology treatment in comparison to gamma irradiation. Transfusion 2011, 51, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Cid, J. Prevention of transfusion-associated graft-versus-host disease with pathogen-reduced platelets with amotosalen and ultraviolet A light: A review. Vox Sang. 2017, 112, 607–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, P.; Johnson, L.; Marks, D.C.; Devine, D.V. Ultraviolet-based pathogen inactivation systems: Untangling the molecular targets activated in platelets. Front. Med. 2018, 5, 129. [Google Scholar] [CrossRef] [PubMed]
- Feys, H.B.; Van Aelst, B.; Compernolle, V. Biomolecular consequences of platelet pathogen inactivation methods. Transfus. Med. Rev. 2019, 33, 29–34. [Google Scholar] [CrossRef]
- Leitner, G.C.; Ho, M.; Tolios, A.; Hopfinger, G.; Rabitsch, W.; Wohlfarth, P. The assessment of platelet function by thromboelastometry as a point-of-care test to guide Intercept-treated platelet support in hemato-oncological patients and hematopoietic stem cell transplantation recipients. Transfusion 2020, 60, 1391–1399. [Google Scholar] [CrossRef] [Green Version]
- Cid, J.; Escolar, G.; Lozano, M. Therapeutic efficacy of platelet components treated with amotosalen and ultraviolet A pathogen inactivation method: Results of a meta-analysis of randomized controlled trials. Vox Sang. 2012, 103, 322–330. [Google Scholar] [CrossRef]
- Schlenke, P. Pathogen inactivation technologies for cellular blood components: An update. Transfus. Med. Hemotherapy 2014, 41, 309–325. [Google Scholar] [CrossRef] [Green Version]
- McDonald, C.P.; Bearne, J.; Aplin, K.; Sawicka, D. Assessing the inactivation capabilities of two commercially available platelet component pathogen inactivation systems: Effectiveness at end of shelf life. Vox Sang. 2021, 116, 416–424. [Google Scholar] [CrossRef]
- McCullough, J.; Vesole, D.H.; Benjamin, R.J.; Slichter, S.J.; Pineda, A.; Snyder, E.; Stadtmauer, E.A.; Lopez-Plaza, I.; Coutre, S.; Strauss, R.G.; et al. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: The SPRINT Trial. Blood 2004, 104, 1534–1541. [Google Scholar] [CrossRef] [Green Version]
- Garban, F.; Guyard, A.; Labussiere, H.; Bulabois, C.E.; Marchand, T.; Mounier, C.; Caillot, D.; Bay, J.O.; Coiteux, V.; Schmidt-Tanguy, A.; et al. Comparison of the Hemostatic Efficacy of Pathogen-Reduced Platelets vs Untreated Platelets in Patients With Thrombocytopenia and Malignant Hematologic Diseases: A Randomized Clinical Trial. JAMA Oncol. 2018, 4, 468–475. [Google Scholar] [CrossRef]
- Lozano, M.; Knutson, F.; Tardivel, R.; Cid, J.; Maymo, R.M.; Lof, H.; Roddie, H.; Pelly, J.; Docherty, A.; Sherman, C.; et al. A multi-centre study of therapeutic efficacy and safety of platelet components treated with amotosalen and ultraviolet A pathogen inactivation stored for 6 or 7 d prior to transfusion. Br. J. Haematol. 2011, 153, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Mirasol Clinical Evaluation Study Group. A randomized controlled clinical trial evaluating the performance and safety of platelets treated with MIRASOL pathogen reduction technology. Transfusion 2010, 50, 2362–2375. [Google Scholar] [CrossRef]
- Rebulla, P.; Vaglio, S.; Beccaria, F.; Bonfichi, M.; Carella, A.; Chiurazzi, F.; Coluzzi, S.; Cortelezzi, A.; Gandini, G.; Girelli, G.; et al. Clinical effectiveness of platelets in additive solution treated with two commercial pathogen-reduction technologies. Transfusion 2017, 57, 1171–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Meer, P.F.; Ypma, P.F.; van Geloven, N.; van Hilten, J.A.; van Wordragen-Vlaswinkel, R.J.; Eissen, O.; Zwaginga, J.J.; Trus, M.; Beckers, E.A.M.; Te Boekhorst, P.; et al. Hemostatic efficacy of pathogen-inactivated vs untreated platelets: A randomized controlled trial. Blood 2018, 132, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilarino, M.D.; Castrillo, A.; Campos, A.; Kilian, R.; Villamayor, M.; Cardoso, M. Assessment of the Clinical Performance of Platelet Concentrates Treated by Pathogen Reduction Technology in Santiago de Compostela. Transfus. Med. Hemotherapy 2017, 44, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Infanti, L.; Holbro, A.; Passweg, J.; Bolliger, D.; Tsakiris, D.A.; Merki, R.; Plattner, A.; Tappe, D.; Irsch, J.; Lin, J.S.; et al. Clinical impact of amotosalen-ultraviolet A pathogen-inactivated platelets stored for up to 7 days. Transfusion 2019, 59, 3350–3361. [Google Scholar] [CrossRef] [Green Version]
- Amato, M.; Schennach, H.; Astl, M.; Chen, C.Y.; Lin, J.S.; Benjamin, R.J.; Nussbaumer, W. Impact of platelet pathogen inactivation on blood component utilization and patient safety in a large Austrian Regional Medical Centre. Vox Sang. 2017, 112, 47–55. [Google Scholar] [CrossRef]
- Jimenez-Marco, T.; Garcia-Recio, M.; Girona-Llobera, E. Our experience in riboflavin and ultraviolet light pathogen reduction technology for platelets: From platelet production to patient care. Transfusion 2018, 58, 1881–1889. [Google Scholar] [CrossRef] [Green Version]
- Picker, S.M.; Schneider, V.; Oustianskaia, L.; Gathof, B.S. Cell viability during platelet storage in correlation to cellular metabolism after different pathogen reduction technologies. Transfusion 2009, 49, 2311–2318. [Google Scholar] [CrossRef]
- Abonnenc, M.; Sonego, G.; Crettaz, D.; Aliotta, A.; Prudent, M.; Tissot, J.D.; Lion, N. In vitro study of platelet function confirms the contribution of the ultraviolet B (UVB) radiation in the lesions observed in riboflavin/UVB-treated platelet concentrates. Transfusion 2015, 55, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Picker, S.M.; Oustianskaia, L.; Schneider, V.; Gathof, B.S. Functional characteristics of apheresis-derived platelets treated with ultraviolet light combined with either amotosalen-HCl (S-59) or riboflavin (vitamin B2) for pathogen-reduction. Vox Sang. 2009, 97, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Guide to the preparation, use and quality assurance of BLOOD COMPONENTS. In Council of Europe, 20th ed.; EDQM: Strasbourg, France, 2020.
- Gorria, C.; Labata, G.; Lezaun, M.; Lopez, F.J.; Perez Aliaga, A.I.; Perez Vaquero, M.A. Impact of implementing pathogen reduction technologies for platelets on reducing outdates. Vox Sang. 2020, 115, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Hitzler, W.E.; Provost, P. The platelets’ perspective to pathogen reduction technologies. Platelets 2018, 29, 140–147. [Google Scholar] [CrossRef]
- Johnson, L.; Loh, Y.S.; Kwok, M.; Marks, D.C. In vitro assessment of buffy-coat derived platelet components suspended in SSP+ treated with the INTERCEPT Blood system. Transfus. Med. 2013, 23, 121–129. [Google Scholar] [CrossRef]
- Castrillo, A.; Cardoso, M.; Rouse, L. Treatment of buffy coat platelets in platelet additive solution with the mirasol((R)) pathogen reduction technology system. Transfus. Med. Hemotherapy 2013, 40, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Schubert, P.; Culibrk, B.; Devine, D.V. p38MAPK is involved in apoptosis development in apheresis platelet concentrates after riboflavin and ultraviolet light treatment. Transfusion 2015, 55, 848–857. [Google Scholar] [CrossRef]
- Stivala, S.; Gobbato, S.; Infanti, L.; Reiner, M.F.; Bonetti, N.; Meyer, S.C.; Camici, G.G.; Luscher, T.F.; Buser, A.; Beer, J.H. Amotosalen/ultraviolet A pathogen inactivation technology reduces platelet activatability, induces apoptosis and accelerates clearance. Haematologica 2017, 102, 1650–1660. [Google Scholar] [CrossRef]
- Prudent, M.; D’Alessandro, A.; Cazenave, J.P.; Devine, D.V.; Gachet, C.; Greinacher, A.; Lion, N.; Schubert, P.; Steil, L.; Thiele, T.; et al. Proteome changes in platelets after pathogen inactivation--an interlaboratory consensus. Transfus. Med. Rev. 2014, 28, 72–83. [Google Scholar] [CrossRef]
- Martınez, N.; Valdivia, E.; Fernandez, A.; Casamitjana, N.; Puig, L.; Gomez, S. In vitro assessment of pools of pools of intermediate platelet units photochemically treated to deliver two pathogen inactivated platelet concentrates. Vox Sang. 2019, 114, 131–132. [Google Scholar]
- Picker, S.M.; Speer, R.; Gathof, B.S. Functional characteristics of buffy-coat PLTs photochemically treated with amotosalen-HCl for pathogen inactivation. Transfusion 2004, 44, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Galan, A.M.; Lozano, M.; Molina, P.; Navalon, F.; Marschner, S.; Goodrich, R.; Escolar, G. Impact of pathogen reduction technology and storage in platelet additive solutions on platelet function. Transfusion 2011, 51, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S. Metabolic patterns of platelets--impact on storage for transfusion. Vox Sang. 1994, 67 (Suppl. 3), 271–273. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.; Rowe, G.; Wilkins, K.; Collins, P. Impact of glucose and acetate on the characteristics of the platelet storage lesion in platelets suspended in additive solutions with minimal plasma. Vox Sang. 2013, 105, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Holme, S. Storage and quality assessment of platelets. Vox Sang. 1998, 74 (Suppl. 2), 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gulliksson, H. Defining the optimal storage conditions for the long-term storage of platelets. Transfus. Med. Rev. 2003, 17, 209–215. [Google Scholar] [CrossRef]
- Leytin, V.; Freedman, J. Platelet apoptosis in stored platelet concentrates and other models. Transfus. Apher. Sci. 2003, 28, 285–295. [Google Scholar] [CrossRef]
- Reid, S.; Johnson, L.; Woodland, N.; Marks, D.C. Pathogen reduction treatment of buffy coat platelet concentrates in additive solution induces proapoptotic signaling. Transfusion 2012, 52, 2094–2103. [Google Scholar] [CrossRef]
- Hechler, B.; Ravanat, C.; Gachet, C. Amotosalen/UVA pathogen inactivation technology reduces platelet activability, induces apoptosis and accelerates clearance. Haematologica 2017, 102, e502–e503. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, S.K.; Argaiz, E.R.; Mercado, J.E.; Maul, H.O.; Garza, J.; Enriquez, A.B.; Abdel-Monem, H.; Prakasam, A.; Andreeff, M.; Thiagarajan, P. Platelet senescence and phosphatidylserine exposure. Transfusion 2010, 50, 2167–2175. [Google Scholar] [CrossRef] [Green Version]
- Abonnenc, M.; Sonego, G.; Kaiser-Guignard, J.; Crettaz, D.; Prudent, M.; Tissot, J.D.; Lion, N. In vitro evaluation of pathogen-inactivated buffy coat-derived platelet concentrates during storage: Psoralen-based photochemical treatment step-by-step. Blood Transfus. 2015, 13, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Hechler, B.; Ohlmann, P.; Chafey, P.; Ravanat, C.; Eckly, A.; Maurer, E.; Mangin, P.; Isola, H.; Cazenave, J.P.; Gachet, C. Preserved functional and biochemical characteristics of platelet components prepared with amotosalen and ultraviolet A for pathogen inactivation. Transfusion 2013, 53, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, S.; Diedrich, B.; Uhlin, M.; Sandgren, P. Optimized processing for pathogen inactivation of double-dose buffy-coat platelet concentrates: Maintained in vitro quality over 7-day storage. Vox Sang. 2018, 113, 611–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolini, F.; Murphy, S. A multicenter inspection of the swirling phenomenon in platelet concentrates prepared in routine practice. Biomedical Excellence for Safer Transfusion (BEST) Working Party of the International Society of Blood Transfusion. Transfusion 1996, 36, 128–132. [Google Scholar] [CrossRef]
- Mathai, J.; Resmi, K.R.; Sulochana, P.V.; Sathyabhama, S.; Baby Saritha, G.; Krishnan, L.K. Suitability of measurement of swirling as a marker of platelet shape change in concentrates stored for transfusion. Platelets 2006, 17, 393–396. [Google Scholar] [CrossRef]
- Bertolini, F.; Agazzi, A.; Peccatori, F.; Martinelli, G.; Sandri, M.T. The absence of swirling in platelet concentrates is highly predictive of poor posttransfusion platelet count increments and increased risk of a transfusion reaction. Transfusion 2000, 40, 121–122. [Google Scholar] [CrossRef]
- Cardigan, R.; Turner, C.; Harrison, P. Current methods of assessing platelet function: Relevance to transfusion medicine. Vox Sang. 2005, 88, 153–163. [Google Scholar] [CrossRef]

| PPC | APC | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 Before PI | Day 2 | Day 5 | Day 7 | Day 0 Before PI | Day 1 | Day 5 | Day 7 | |
| Volume (mL) | ||||||||
| RF-PI | 370 ± 3 | 397 ± 4 | 381 ± 5 | 369 ± 5 | 282 ± 5 | 305 ± 5 | 291 ± 5 | 282 ± 3 |
| AM-PI | 373 ± 6 | 360 ± 6 | 345 ± 6 | 335 ± 5 | 281 ± 5 | 260 ± 6 | 246 ± 7 | 237 ± 7 |
| Platelet Concentration (109/L) | ||||||||
| RF-PI | 890 ± 54 | 818 ± 35 | 815 ± 48 | 771 ± 49 | 1107 ± 48 | 964 ± 33 | 988 ± 44 | 953 ± 62 |
| AM-PI | 874 ± 43 | 838 ± 35 | 814 ± 47 | 1052 ± 76 | 1016 ± 43 | 993 ± 54 | ||
| t-Test | p = 0.001 | ns | p = 0.03 | p = 0.012 | ns | p = 0.013 | ||
| Platelet Dose (1011 per unit) | ||||||||
| RF-PI | 3.30 ± 0.19 | 3.24 ± 0.12 | 3.10 ± 0.16 | 2.84 ± 0.17 | 3.13 ± 0.18 | 2.95 ± 0.13 | 2.87 ± 0.17 | 2.69 ± 0.20 |
| AM-PI | 3.32 ± 0.20 | 3.14 ± 0.19 | 2.89 ± 0.11 | 2.73 ± 0.15 | 3.11 ± 0.19 | 2.74 ± 0.25 | 2.50 ± 0.17 | 2.36 ± 0.17 |
| t-Test | p = 0.043 | p = 0.03 | ns | p = 0.017 | p < 0.001 | p < 0.001 | ||
| PPC | APC | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 Before PI | Day 2 | Day 5 | Day 7 | Day 0 Before PI | Day 1 | Day 5 | Day 7 | |
| LDH Release (%) | ||||||||
| RF-PI | 4.74 ± 1.37 | 3.84 ± 0.38 | 3.96 ± 0.29 | 4.29 ± 0.43 | 2.34 ± 0.92 | 2.90 ± 1.15 | 2.84 ± 0.69 | 3.13 ± 0.43 |
| AM-PI | 3.80 ± 0.21 | 5.29 ± 1.21 | 5.43 ± 1.56 | 3.01 ± 0.61 | 3.67 ± 0.76 | 3.67 ± 0.44 | ||
| t-Test | ns | ns | ns | ns | p = 0.002 | ns | ||
| MPV | ||||||||
| RF-PI | 8.06 ± 0.37 | 8.00 ± 0.33 | 8.92 ± 0.42 | 9.84 ± 0.49 | 7.15 ± 0.55 | 6.84 ± 0.56 | 8.11 ± 0.95 | 8.85 ± 1.07 |
| AM-PI | 8.11 ± 0.30 | 8.15 ± 0.28 | 8.23 ± 0.36 | 7.10 ± 0.53 | 7.53 ± 0.68 | 7.76 ± 0.59 | ||
| t-Test | p = 0.004 | p < 0.001 | p < 0.001 | p = 0.004 | p = 0.011 | p = 0.003 | ||
| Swirl | ||||||||
| RF-PI | 2.00 | 1.92 | 1.17 | 0.17 | 2.00 | 1.83 | 1.67 | 0.00 |
| AM-PI | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malvaux, N.; Defraigne, F.; Bartziali, S.; Bellora, C.; Mommaerts, K.; Betsou, F.; Schuhmacher, A. In Vitro Comparative Study of Platelets Treated with Two Pathogen-Inactivation Methods to Extend Shelf Life to 7 Days. Pathogens 2022, 11, 343. https://doi.org/10.3390/pathogens11030343
Malvaux N, Defraigne F, Bartziali S, Bellora C, Mommaerts K, Betsou F, Schuhmacher A. In Vitro Comparative Study of Platelets Treated with Two Pathogen-Inactivation Methods to Extend Shelf Life to 7 Days. Pathogens. 2022; 11(3):343. https://doi.org/10.3390/pathogens11030343
Chicago/Turabian StyleMalvaux, Nicolas, Fanette Defraigne, Styliani Bartziali, Camille Bellora, Kathleen Mommaerts, Fay Betsou, and Anne Schuhmacher. 2022. "In Vitro Comparative Study of Platelets Treated with Two Pathogen-Inactivation Methods to Extend Shelf Life to 7 Days" Pathogens 11, no. 3: 343. https://doi.org/10.3390/pathogens11030343
APA StyleMalvaux, N., Defraigne, F., Bartziali, S., Bellora, C., Mommaerts, K., Betsou, F., & Schuhmacher, A. (2022). In Vitro Comparative Study of Platelets Treated with Two Pathogen-Inactivation Methods to Extend Shelf Life to 7 Days. Pathogens, 11(3), 343. https://doi.org/10.3390/pathogens11030343






