Antimicrobial Activity of Pinus wallachiana Leaf Extracts against Fusarium oxysporum f. sp. cubense and Analysis of Its Fractions by HPLC
Abstract
:1. Introduction
2. Results
2.1. Fungicidal Analysis
2.2. Effects on Biomass Production
2.3. Fractions of P. wallachiana
2.3.1. The Percentage Yield of Fractions
2.3.2. Antifungal Assays of Fractions
2.3.3. Greenhouse Experiment of Fractions
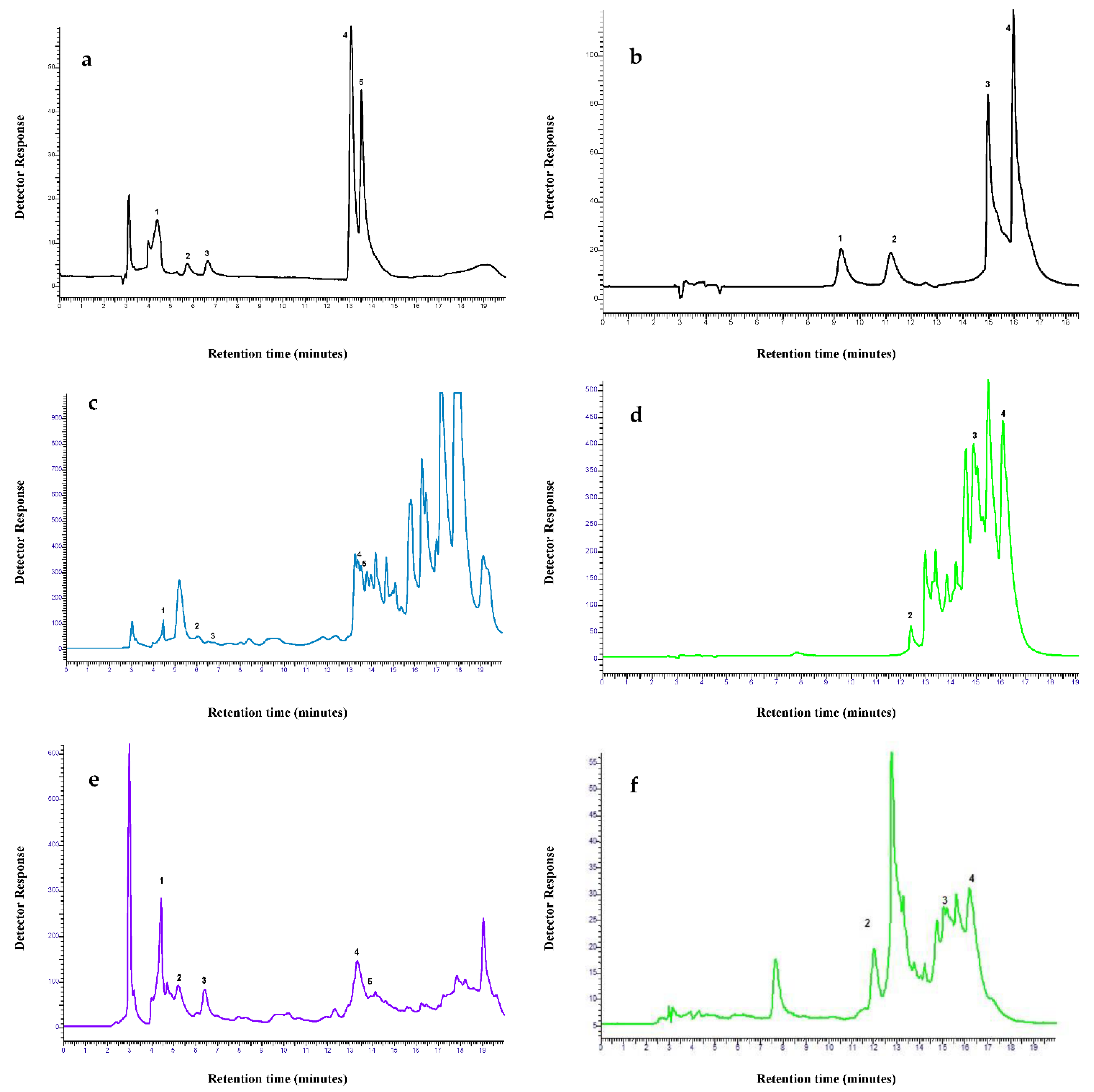
2.4. HPLC of Fractions
3. Discussion
4. Materials and Methods
4.1. Acquisition, Revival, and Confirmation of Fungal Culture
4.2. Plant Sample and Extraction
4.3. Fungicidal Analysis
4.4. Effect on Foc Biomass Production
4.5. Fractionation
4.6. Antifungal Assay of Fractions
4.6.1. Food Poisoning Assay
4.6.2. Well Diffusion Assay
4.7. Greenhouse Experiment
4.8. HPLC Analysis of Fractions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimm, D. Plant genomics. A bunch of trouble. Science 2008, 322, 1046–1047. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C. Fusarium wilt of banana is caused by several pathogens referred to as Fusarium oxysporum f. sp. cubense. Phytopathology 2006, 96, 653–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ploetz, R.C. Management of Fusarium wilt of banana: A review with special reference to tropical race 4. Crop Prot. 2015, 73, 7–15. [Google Scholar] [CrossRef]
- Wen, T.; Huang, X.; Zhang, J.; Zhu, T.; Meng, L.; Cai, Z. Effects of water regime, crop residues, and application rates on control of Fusarium oxysporum f. sp. cubense. J. Environ. Sci. 2015, 31, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Maymon, M.; Sela, N.; Shpatz, U.; Galpaz, N.; Freeman, S. The origin and current situation of Fusarium oxysporum f. sp. cubense tropical race 4 in Israel and the Middle East. Sci. Rep. 2020, 10, 1590. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Ma, L.-J.; Molina, A.B. Fusarium wilt (Panama disease) and monoculture in banana production: Resurrgence of a century-old disease. In Emerging Plant Diseases and Global Food Security; Ristaino, J.B., Records, A., Eds.; American Phytopathological Society: Saint Paul, MN, USA, 2020. [Google Scholar]
- Zhang, H.; Mallik, A.; Zeng, R.S. Control of Panama disease of banana by rotating and intercropping with chineese chive (Allium tuberosum Rottler): Role of plant volatiles. J. Chem. Ecol. 2013, 39, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, P.; Salique, S.M.; Panneerselvam, A.; Umamagheswari, K. In vitro biological control of Fusarium oxysporum f. sp. cubense by using some Indian medicinal plants. Int. J. Curr. Res. Acad. Rev. 2015, 3, 107–116. [Google Scholar]
- Katan, J. Diseases caused by soil-borne pathogens: Biology, management and challenges. J. Plant Pathol. 2017, 99, 305–315. [Google Scholar]
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Cabanas, C.G.-L.; Mercado-Blanco, J. Biological control agents against Fusarium wilt of banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef] [Green Version]
- Doughari, J.H.; Human, I.S.; Bennade, S.; Ndakidemi, P.A. Phytochemicals as chemotherapeutic agents and antioxidants: Possible solution to the control of antibiotic resistant verocytotoxin producing bacteria. J. Med. Plants Res. 2009, 3, 839–848. [Google Scholar]
- Saravanakumar, D.; Karthiba, L.; Ramjegathesh, R.; Prabakar, K.; Raguchander, T. Characterization of bioactive compounds from botanicals for the management of plant diseases. In Sustainable Crop Disease Management Using Natural Products; Ganesan, S., Vadivel, K., Jayaraman, J., Eds.; CAB International: Wallingford, UK, 2015. [Google Scholar]
- Thorat, P.; Kshirsagar, R.; Sawate, A.; Patil, B. Effect of lemongrass powder on proximate and phytochemical content of herbal cookies. J. Pharmacogn. Phytochem. 2017, 6, 155–159. [Google Scholar]
- Masarirambi, M.T.; Nxumalo, K.A.; Kunene, E.N.; Dlamini, D.V.; Mpofu, M.; Manwa, L.; Earnshaw, D.M.; Bwembya, G.C. Traditional/indigenous vegetables of the kingdom of eswatini: Biodiversity and their importance: A review. J. Exp. Agric. Int. 2020, 42, 204–215. [Google Scholar] [CrossRef]
- Nxumalo, K.A.; Aremu, A.O.; Fawole, O.A. Potentials of medicinal plant extracts as an alternative to synthetic chemicals in postharvest protection and preservation of horticultural crops: A review. Sustainability 2021, 13, 5897. [Google Scholar] [CrossRef]
- Stefanovic, O.; Comic, L. Synergistic antibacterial interaction between Melissa officinalis extracts and antibiotics. J. Appl. Pharm. Sci. 2012, 2, 1–5. [Google Scholar]
- Wink, M.; Ashour, M.L.; El-Readi, M.Z. Secondary metabolites from plants inhibiting ABC transporters and reversing resistance of cancer cells and microbes to cytotoxic and antimicrobial agents. Front. Microbiol. 2012, 3, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Pereira, D.M.; Valentao, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Marchand, L.L.; Murphy, S.P.; Hankin, J.H.; Wilkens, L.R.; Kolonel, L.N. Intake of flavonoids and lung cancer. J. Natl. Cancer Inst. 2000, 92, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Cozier, A.; Cifford, M.N.; Ashihara, H. Plant Secondary Metabolites–Occurrence, Structure and Role in the Human Diet; Blackwell Publishing: Hoboken, NJ, USA, 2006; pp. 1–13. [Google Scholar]
- Jalaj, A.V.; Radhamany, P.M. Identification and quantification of phenolic compounds from Operculina turpethum (L.) Silva manso leaf by HPLC method. Int. J. Pharm. Sci. Res. 2016, 7, 1656–1661. [Google Scholar]
- Harborne, J.B.; Baxter, H. The Chemical Dictionary of Economic Plants; Wiley and Sons: Chichester, UK, 2001; p. 582. [Google Scholar]
- Watanabe, K.; Fukao, T. Antibacterial effects of unripe Cephalotaxus harringtonia fruit extract on gram-positive bacteria. J. Jpn. Soc. Food Sci. Tech. 2009, 56, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Sati, S.C. Antifungal potential of gymnosperms: A review. In Contribution to the Mycological Progress; Sati, S.C., Belwal, M., Eds.; Daya Publishing House: New Delhi, India, 2012; pp. 333–345. [Google Scholar] [CrossRef]
- Joshi, S.; Sati, S.C.; Kumar, P. Antibacterial potential and ethnomedical relevance of Kumaun himalayan gymnosperms. J. Phytopharm. 2016, 5, 190–200. [Google Scholar] [CrossRef]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Agroforestree Database: A Tree Reference and Selection Guide Version 4.0. 2009. Available online: http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp (accessed on 4 August 2018).
- Khan, N.; Khan, I.; Nadhman, A.; Azam, S.; Ullah, I.; Ahmad, F.; Khan, H.A. Pinus wallichiana-synthesized silver nanoparticles as biomedical agents: In-Vitro and in-vivo approach. Green Chem. Lett. Rev. 2020, 13, 69–82. [Google Scholar] [CrossRef]
- Rahman, I.U.; Khan, N.; Ali, K. Variability assessment of some morphological traits among blue pine (Pinus wallichiana) communities in Hindukush ranges of SWAT, Pakistan. Pak. J. Bot. 2017, 49, 1351–1357. [Google Scholar]
- Sharma, A.; Sharma, L.; Goyal, R. A review on Himalayan pine species: Ethnopharmacological, phytochemical and pharmacological aspects. Pharmacogn. J. 2018, 10, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Sinha, D. A review on ethnobotanical, phytochemical and pharmacological profile of Pinus wallichiana A.B. Jacks. Pharmacogn. J. 2019, 11, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Khan, I.; Azam, S.; Ahmad, F.; Khan, H.A.; Shah, A.; Ullah, M. Potential cytotoxic and mutagenic effect of Pinus wallichiana, Daphne oleiodes and Bidens chinensis. Saudi J. Biol. Sci. 2021, 28, 4793–4799. [Google Scholar] [CrossRef]
- Emami, S.A.; Shahani, A.; Khayyat, M.H. Antioxidant activity of leaves and fruits of cultivated conifers in Iran. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Painuli, S.; Rai, N.; Meena, R.C.; Misra, K.; Kumar, N. Aqueous extract of Pinus wallichiana inhibits proliferation of cervical cancer cell line HeLa and represses the transcription of angiogenic factors HIF1α and VEGF. Ecol. Environ. Conserv. 2020, 26, S12–S19. [Google Scholar]
- Qadir, M.; Shah, W.A. Comparative GC-MS analysis, antioxidant, antibacterial and anticancer activity of essential oil of Pinus wallichaina from Kashmir, India. Elixir Appl. Chem. 2014, 72, 25819–25823. [Google Scholar]
- Dambolena, J.S.; Gallucci, M.N.; Luna, A.; Gonzalez, S.B.; Guerra, P.E.; Zunino, M.P. Composition, antifungal and antifumonisin activity of Pinus wallichiana, Pinus monticola and Pinus strobus essential oils from Patagonia Argentina. J. Essent. Oil Bear. Plants 2016, 19, 1769–1775. [Google Scholar] [CrossRef]
- Sharma, A.; Goyal, R.; Sharma, L. Potential biological efficacy of Pinus plant species against oxidative, inflammatory and microbial disorders. BMC Complementary Altern. Med. 2016, 16, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Gupta, S.; Bhatt, N.; Ahanger, S.H.; Gupta, D.; Singh, P.; Lochan, R.; Bhagat, M. Antioxidant and phytochemical analysis of volatile oil and extracts of Pinus wallichiana. MOJ Biol. Med. 2019, 4, 37–40. [Google Scholar] [CrossRef]
- Agrios, G.N. How plants defend themselves against pathogens. In Plant Pathology, 5th ed.; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Naczk, M.; Shahidi, F. Review: Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 2005, 68, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Khateeb, A.Y.; Elsherbiny, E.A.; Tadros, L.K.; Ali, S.M.; Hamed, H.B. Phytochemical analysis and antifungal activity of fruit leaves extracts on the mycelial growth of fungal plant pathogens. J. Plant Pathol. Microbiol. 2013, 4, 1–6. [Google Scholar]
- Dua, A.; Garg, G.; Mahajan, R. Polyphenols, flavonoids and antimicrobial properties of methanolic extract of fennel (Foeniculum vulgare Miller). Eur. J. Exp. Biol. 2013, 3, 203–208. [Google Scholar]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [Green Version]
- Adekunle, A.S.; Adekunle, O.C. Preliminary assessment of antimicrobial properties of aqueous extract of plants against infectious diseases. Biol. Med. 2009, 1, 20–24. [Google Scholar]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Sahraoui, A.L.-H. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.; Singh, V.P.; Kumar, R.; Srivastava, M.; Sinha, A.; Simon, S. In vitro evaluation of carbendazim 50% WP, antagonists and botanicals against Fusarium oxysporum f.sp. psidii associated with rhizosphere soil of guava. Asian J. Plant Pathol. 2011, 5, 46–53. [Google Scholar]
- Yuliar, N.Y.A.; Toyota, K. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ. 2015, 30, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Joshi, S.; Kumar, N. Antioxidant and antibacterial properties of leaves of Elaeocarpus sphaericus Roxb. and Pinus wallichiana from Uttarakhand region of India. Int. J. Green Pharm. 2015, 9, 246. [Google Scholar]
- Rahman, T.U.; Uddin, G.; Khattak, K.F.; Liaqat, W.; Choudhary, M.I. Antibacterial, antifungal, insecticidal and phytotoxic activities of leaves of Pinus wallachiana. J. Chem. Pharm Res. 2016, 8, 420–424. [Google Scholar]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Nel, B.; Steinberg, C.; Labuschagne, N.; Viljoen, A. Evaluation of fungicides and sterilants for potential application in the management of fusarium wilt if banana. Crop Prot. 2007, 26, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Dziedzinski, M.; Kobus-Cisowska, J.; Stachowiak, B. Pinus species as prospective reserves of bioactive compounds with potential use in functional food—Current state of knowledge. Plants 2021, 10, 1306. [Google Scholar] [CrossRef]
- Willför, S.; Ali, M.; Karonen, M.; Reunanen, M.; Arfan, M.; Harlamow, R. Extractives in bark of different conifer species growing in Pakistan. Holzforschung 2009, 63, 551–558. [Google Scholar] [CrossRef]
- Karapandzova, M.; Stefkov, G.; Cvetkovikj, I.; Stanoeva, J.P.; Stefova, M.; Kulevanova, S. Flavonoids and other phenolic compounds in needles of Pinus peuce and other pine species from the Macedonian flora. Nat. Prod. Commun. 2015, 10, 987–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naeem, I.; Taskeen, A.; Mubeen, H.; Maimoona, A. Characterization of flavonols present in barks and needles of Pinus wallichiana and Pinus roxburghii. Asian J. Chem. 2010, 22, 41–44. [Google Scholar]
- Maimoona, A.; Naeem, I.; Saddiqe, Z.; Ali, N.; Ahmed, G.; Shah, I. Analysis of total flavonoids and phenolics in different fractions of bark and needle extracts of Pinus roxburghii and Pinus wallachiana. J. Med. Plants Res. 2011, 5, 2724–2728. [Google Scholar]
- Yesil-Celiktas, O.; Ganzera, M.; Akgun, I.; Sevimli, C.; KS, K.; Erdal, B. Determination of polyphenolic constituents and biological activities of bark extracts from different Pinus species. J. Sci. Food Agric. 2009, 89, 1339–1345. [Google Scholar] [CrossRef]
- Zuo, C.; Li, C.; Li, B.; Wei, Y.; Hu, C.; Yang, Q.; Yang, J.; Sheng, O.; Kuang, R.; Deng, G.; et al. The toxic mechanism and bioactive components of chinese leek root exudates acting against Fusarium oxysporum f. sp. cubense tropical race 4. Eur. J. Plant Pathol. 2015, 143, 447–460. [Google Scholar] [CrossRef]
- Mackesy, D.; Sullivan, M. CPHST Pest Datasheet for Phytophthora kernoviae; 2015. Available online: http://download.ceris.purdue.edu/file/2780 (accessed on 24 February 2018).
- Moses, A.O. Diversity of Fusarium oxysporum f. sp. cubense in Mozambique and Associated In Vitro Response to Fungicides, Biocontrol-Agents and Phenolic Compounds. Master’s Thesis, Eduardo Mondlane University, Maputo, Mozambique, 2016. [Google Scholar]
- Muhammad, A.; Hussain, I.; Khanzada, K.A.; Kumar, L.; Ali, M.; Yasmin, T.; Hyder, M.Z. Molecular characterization of Fusarium oxysporum f. sp. cubense (FOC) tropical race 4 causing panama disease in cavendish banana in Pakistan. Pak. J. Agri. Sci. 2017, 54, 1–8. [Google Scholar] [CrossRef]
- Sati, S.C.; Joshi, S. Antibacterial potential of leaf extracts of Juniperus communis L. from Kumaun Himalaya. Afr. J. Microbiol. Res. 2010, 4, 1291–1294. [Google Scholar]
- Bajpai, V.K.; Kang, S.C. Antifungal activity of leaf essential oil and extracts of Metasequoia glyptostroboides Miki ex Hu. J. Am. Oil Chem. Soc. 2010, 87, 327–336. [Google Scholar] [CrossRef]
- Al-Rahmah, A.N.; Mostafa, A.A.; Abdel-Megeed, A.; Yakout, S.M.; Hussein, S.A. Fungicidal activities of certain methanolic plant extracts against tomato phytopathogenic fungi. Afr. J. Microbiol. Res. 2013, 7, 517–524. [Google Scholar] [CrossRef]
- Georgopoulos, S.G.; Dekker, J. Detection and measurement of fungicide resistance general principles. FAO Plant Prot. Bull. 1982, 30, 39–42. [Google Scholar]
- Siripornvisal, S. Antifungal activity of ajowan oil against Fusarium oxysporum. Curr. Appl. Sci. Technol. 2010, 10, 45–51. [Google Scholar]
- Egua, M.O.; Etuk, E.U.; Bello, S.O.; Hassan, S.W. Antidiabetic potential of liquid-liquid partition fractions of ethanolic seed extract of Corchorus olitorious. J. Pharmacogn. Phytother. 2014, 6, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Bouson, S.; Krittayavathananon, A.; Phattharasupakun, N.; Siwayaprahm, P.; Sawangphruk, M. Antifungal activity of water-stable copper-containing metal-organic frameworks. R. Soc. Open Sci. 2017, 4, 170654. [Google Scholar] [CrossRef] [Green Version]
- Magaldi, S.; Mata-Essayag, S.; Capriles, C.H.D.; Perez, C.; Colella, M.T.; Olaizola, C.; Ontiveros, Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.J.; Smith, M.K.; Tree, D.; Keefe, D.O.; Galea, V.J. Development of small-plant bioassay to assess banana grown from tissue culture for consistent infection by Fusarium oxysporum f. sp. cubense. Australas. Plant Pathol. 2008, 37, 171–179. [Google Scholar] [CrossRef]
- Vicente, L.P.; Dita, M.A.; Martínez de la Parte, E. Prevention and diagnostic of fusarium Wilt (Panama disease) of banana caused by Fusarium oxysporum f. sp. cubense tropical race 4 (TR4). Technical Manual Prepared for the Regional Training Workshop on the Diagnosis of Fusarium Wilt Organized by FAO Re-Gional Office of the Caribbean and CARDI on 5–9 May in St. Augustine, Trinidad and Tobago. 2014, p. 74. Available online: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/caribbeantr4/13ManualFusarium.pdf (accessed on 24 February 2018).
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef] [Green Version]
| P. wallachiana Leaf Extract (Concentration in mg/mL) | Percent Inhibition | IC50 | R2 | Regression Equation |
|---|---|---|---|---|
| 1.25 | 25 ± 1.20 | 6.09 | 0.9435 | y = 3.7999x + 26.853 |
| 2.5 | 32.2 ± 0.7 | |||
| 5 | 54.5 ± 0.5 | |||
| 10 | 71.6 ± 0.3 | |||
| 20 | 98.3 ± 0.4 |
| Treatments | Biomass Production | |
|---|---|---|
| Dry Weight (mg) | Percent Inhibition | |
| Control (0) | 158 | 0.00 |
| IC50 (6.09) | 58.7 | 62.9 |
| MIC (20) | 2.4 | 98. 5 |
| MFC (40) | 0 | 100 |
| Fractions | Percentage Yield (%) |
|---|---|
| n-Hexane fraction | 21.8 |
| Dichloromethane fraction | 27.8 |
| Ethyl acetate fraction | 24.68 |
| n-Butanol fraction | 25.12 |
| Treatments | Percent Inhibition | Zone of Inhibition (ZOI) |
|---|---|---|
| n-Hexane control | 0.00 ± 0.00 E | 0.00 ± 0.00 D |
| n-Hexane fraction | 68.93 ± 0.47 C | 21.0 ± 0.92 B |
| Dichloromethane control | 0.00 ± 0.00 E | 0.00 ± 0.00 D |
| Dichloromethane fraction | 75.96 ±0.30 B | 23.80 ± 1.12 A |
| Ethyl acetate control | 0.00 ± 0.00 E | 0.00 ± 0.00 D |
| Ethyl acetate fraction | 57.26 ± 0.39 D | 18.60 ± 0.51 C |
| n-butanol control | 0.00 ± 0.00 E | 0.00 ± 0.00 D |
| n-butanol fraction | 100 ± 0.00 A | 24.40 ± 0.43 A |
| Treatments | First Drenching | Second Drenching | Third Drenching | |||
|---|---|---|---|---|---|---|
| 1st Severity Score | DSI | 2nd Severity Score | DSI | 3rd Severity Score | DSI | |
| Simple Control | 4.286 ± 0.29 BC | 85.71 | 5.000 ± 0.00 A | 100 | 5.000 ± 0.00 A | 100 |
| Fungicide (100 µg/mL) Conc. 1 | 3.429 ± 0.20 DE | 68.57 | 4.000 ± 0.31 BC | 80 | 3.857 ± 0.34 B | 77.14 |
| Fungicide (200 µg/mL) Conc. 2 | 4.286 ± 0.29 BC | 85.7 | 5.000 ± 0.00 A | 100 | 5.000 ± 0.00 A | 100 |
| Hexane Control | 3.714 ± 0.29 CDE | 74.28 | 5.000 ± 0.00 A | 100 | 5.000 ± 0.00 A | 100 |
| Hexane (20 mg/mL) Conc. 1 | 2.286 ± 0.18 G | 45.71 | 2.571 ± 0.37 F | 51.43 | 3.571 ± 0.37 BC | 71.43 |
| Hexane (40 mg/mL) Conc. 2 | 2.571 ± 0.20 G | 51.43 | 2.571 ± 0.30 F | 51.43 | 3.000 ± 0.22 C | 60 |
| Dichloromethane Control | 4.286 ± 0.36 BC | 85.71 | 5.000 ± 0.00 A | 100 | 5.000 ± 0.00 A | 100 |
| Dichloromethane (20 mg/mL) Conc. 1 | 2.286 ± 0.29 G | 42.85 | 2.857 ± 0.26 EF | 57.14 | 3.000 ± 0.38 C | 60 |
| Dichloromethane (40 mg/mL) Conc. 2 | 3.571 ± 0.20 DE | 71.43 | 3.714 ± 0.29 CD | 74.28 | 3.571 ± 0.53 BC | 71.43 |
| Ethyl acetate Control | 4.000 ± 0.22 BCD | 80 | 5.000 ± 0.00 A | 100 | 5.000 ± 0.00 A | 100 |
| Ethyl acetate (20 mg/mL) Conc. 1 | 2.714 ± 0.18 FG | 54.28 | 4.571 ± 0.30 AB | 60 | 4.286 ± 0.29 AB | 71.43 |
| Ethyl acetate (40 mg/mL) Conc. 2 | 3.286 ± 0.29 EF | 65.71 | 5.000 ± 0.00 A | 65.71 | 5.000 ± 0.00 A | 74.28 |
| n-butanol Control | 4.571 ± 0.20 AB | 91.43 | 5.000 ± 0.00 A | 100 | 5.000 ± 0.00 A | 100 |
| n-butanol (20 mg/mL) Conc. 1 | 4.429 ± 0.20 AB | 88.57 | 3.000 ± 0.31 EF | 91.43 | 3.571 ± 0.37 BC | 85.71 |
| n-butanol (40 mg/mL) Conc. 2 | 5.000 ± 0.00 A | 100 | 3.286 ± 0.29 DE | 100 | 3.714 ± 0.36 BC | 100 |
| Phenolic Compounds (mg/g of Extract) | n-Hexane Fraction | Dichloromethane Fraction | Ethyl Acetate Fraction | n-Butanol Fraction |
|---|---|---|---|---|
| Gallic acid | N.D. | 0.10 ± 0.0033 | 3.57 ± 0.016 | 11.57 ± 0.0089 |
| Catechin | N.D. | N.D. | 13.46 ± 0.007 | 33.44 ± 0.0087 |
| Epicatechin | N.D. | 1.19 ± 0.0053 | 3.23 ± 0.0090 | 16.74 ± 0.0074 |
| Coumeric acid | N.D. | 0.61 ± 0.0043 | 2.94 ± 0.0068 | 4.33 ± 0.0034 |
| Trans-Ferulic acid | 0.13 ± 0.0004 | 0.61 ± 0.0037 | 2.84 ± 0.0039 | 0.52 ± 0.0018 |
| Rutin | N.D. | N.D. | N.D. | N.D. |
| Myrecitin | N.D. | N.D. | 2.15 ± 0.0044 | 0.74 ± 0.0064 |
| Quercitin | 0.04 ± 0.00001 | 0.06 ± 0.0005 | 7.9 ± 0.0056 | 0.52 ± 0.0041 |
| Kaempferol | N.D. | 0.09 ± 0.0034 | 7.81 ± 0.011 | 0.66 ± 0.0058 |
| Total Polyphenolic Content | 0.17 mg/g | 2.66 mg/g | 43.90 mg/g | 68.52 mg/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ain, Q.U.; Asad, S.; Ahad, K.; Safdar, M.N.; Jamal, A. Antimicrobial Activity of Pinus wallachiana Leaf Extracts against Fusarium oxysporum f. sp. cubense and Analysis of Its Fractions by HPLC. Pathogens 2022, 11, 347. https://doi.org/10.3390/pathogens11030347
Ain QU, Asad S, Ahad K, Safdar MN, Jamal A. Antimicrobial Activity of Pinus wallachiana Leaf Extracts against Fusarium oxysporum f. sp. cubense and Analysis of Its Fractions by HPLC. Pathogens. 2022; 11(3):347. https://doi.org/10.3390/pathogens11030347
Chicago/Turabian StyleAin, Qurat Ul, Shahzad Asad, Karam Ahad, Muhammad Naeem Safdar, and Atif Jamal. 2022. "Antimicrobial Activity of Pinus wallachiana Leaf Extracts against Fusarium oxysporum f. sp. cubense and Analysis of Its Fractions by HPLC" Pathogens 11, no. 3: 347. https://doi.org/10.3390/pathogens11030347
APA StyleAin, Q. U., Asad, S., Ahad, K., Safdar, M. N., & Jamal, A. (2022). Antimicrobial Activity of Pinus wallachiana Leaf Extracts against Fusarium oxysporum f. sp. cubense and Analysis of Its Fractions by HPLC. Pathogens, 11(3), 347. https://doi.org/10.3390/pathogens11030347






