Niacin Limitation Promotes Candida glabrata Adhesion to Abiotic Surfaces
Abstract
:1. Introduction
2. Results
2.1. C. glabrata Growth under Various NA Concentrations
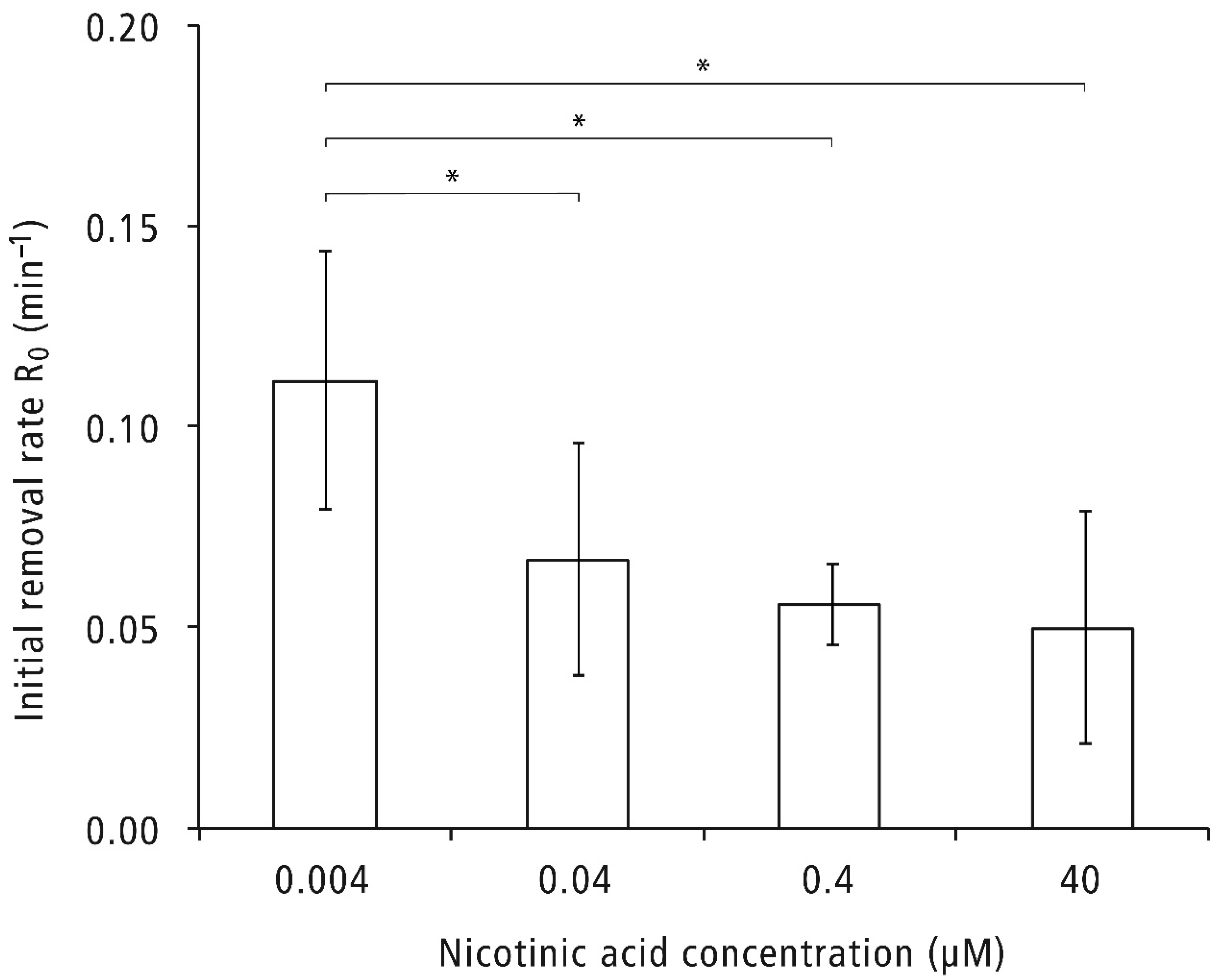
2.2. Adherence of C. glabrata to Abiotic Surfaces
2.3. Cell Surface Hydrophobicity
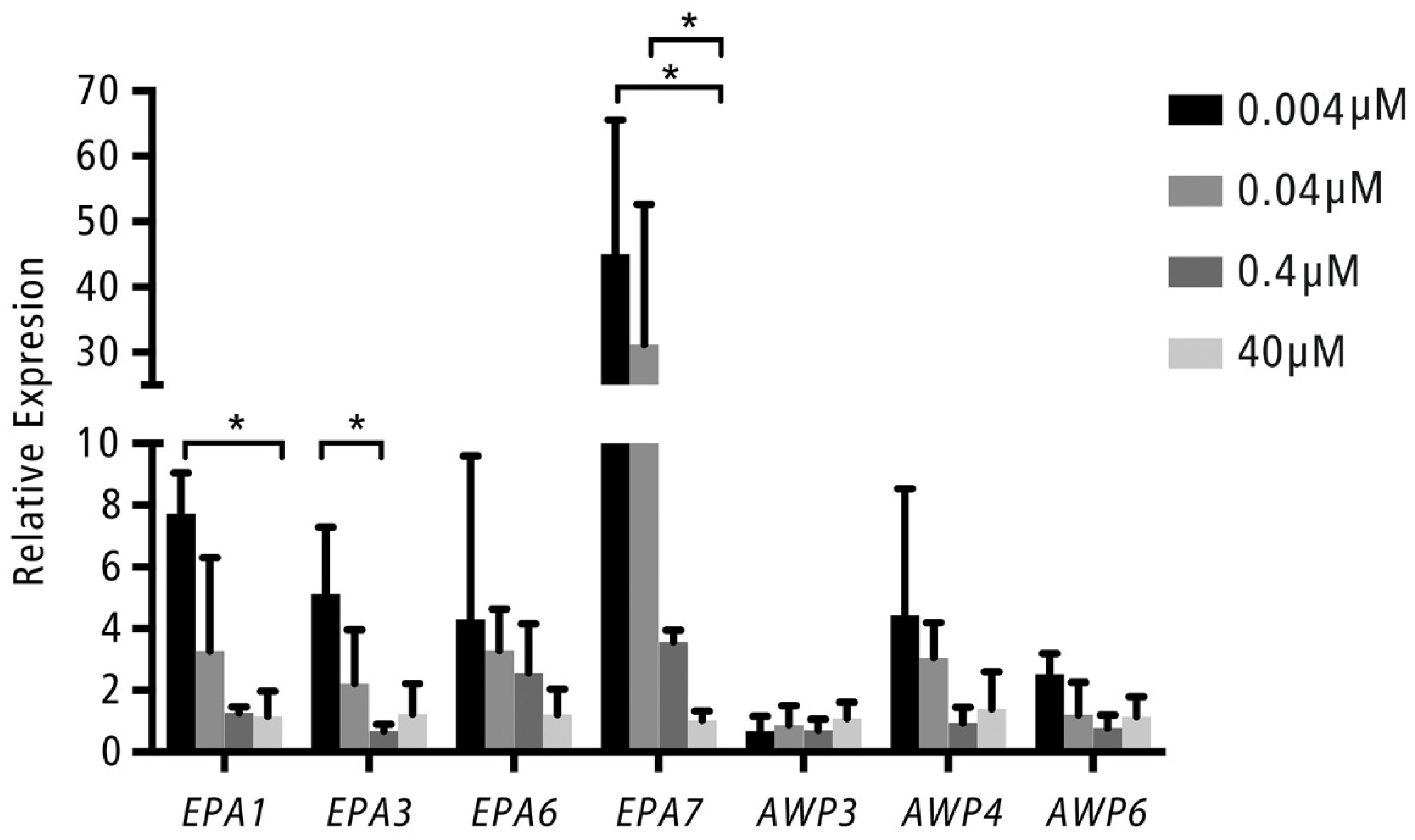
2.4. Adhesion Gene Expression
3. Discussion
4. Materials and Methods
4.1. Strain and Growth Medium
4.2. Substrata
4.3. Growth under Nicotinic Acid Limitation
4.4. Adherence Assay
4.5. Microbial Adhesion to Hexadecane (MATH)
4.6. Adhesion Gene Expression by Quantitative PCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, C.F.; Silva, S.; Henriques, M. Candida glabrata: A review of its features and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 673–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whaley, S.G.; Rogers, P.D. Azole Resistance in Candida glabrata. Curr. Infect. Dis. Rep. 2016, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Healey, K.R.; Perlin, D.S. Fungal resistance to echinocandins and the MDR phenomenon in Candida glabrata. J. Fungi 2018, 4, 105. [Google Scholar] [CrossRef]
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20 (Suppl. S6), 5–10. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Samaranayake, L.P. Candida glabrata, an emerging fungal pathogen, exhibits superior relative cell surface hydrophobicity and adhesion to denture acrylic surfaces compared with Candida albicans. APMIS 2002, 110, 601–610. [Google Scholar] [CrossRef]
- Malani, A.N.; Psarros, G.; Malani, P.N.; Kauffman, C.A. Is age a risk factor for Candida glabrata colonisation? Mycoses 2011, 54, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Hesstvedt, L.; Gaustad, P.; Andersen, C.T.; Haarr, E.; Hannula, R.; Haukland, H.H.; Hermansen, N.-O.; Larssen, K.W.; Mylvaganam, H.; Ranheim, T.E.; et al. Twenty-two years of candidaemia surveillance: Results from a Norwegian national study. Clin. Microbiol. Infect. 2015, 21, 938–945. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.A.; Domingues, N.; Araújo, M.I.D.S.A.; Junqueira, J.C.; Back-Brito, G.N.; Jorge, A.O.C. Production of virulence factors in Candida strains isolated from patients with denture stomatitis and control individuals. Diagn. Microbiol. Infect. Dis. 2016, 85, 66–72. [Google Scholar] [CrossRef]
- Parvinen, T.; Larmas, M. The relation of stimulated salivary flow rate and pH to Lactobacillus and yeast concentrations in saliva. J. Dent. Res. 1981, 60, 1929–1935. [Google Scholar] [CrossRef]
- Timmermans, B.; De Las Peñas, A.; Castaño, I.; Van Dijck, P. Adhesins in Candida glabrata. J. Fungi 2018, 4, 60. [Google Scholar] [CrossRef] [Green Version]
- Diderrich, R.; Kock, M.; Maestre-Reyna, M.; Keller, P.; Steuber, H.; Rupp, S.; Essen, L.-O.; Mösch, H.-U. Structural hot spots determine functional diversity of the Candida glabrata epithelial adhesin family. J. Biol. Chem. 2015, 290, 19597–19613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, Y.; Chew, S.Y.; Than, L.T.L. Candida glabrata: Pathogenicity and Resistance Mechanisms for Adaptation and Survival. J. Fungi 2021, 7, 667. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.W.; Kraneveld, E.A.; Yin, Q.Y.; Dekker, H.L.; Gross, U.; Crielaard, W.; de Koster, C.G.; Bader, O.; Klis, F.M.; Weig, M. The cell wall of the human pathogen Candida glabrata: Differential incorporation of novel adhesin-like wall proteins. Eukaryot. Cell 2008, 7, 1951–1964. [Google Scholar] [CrossRef] [Green Version]
- Pathak, A.K.; Sharma, S.; Shrivastva, P. Multi-species biofilm of Candida albicans and non-Candida albicans Candida species on acrylic substrate. J. Appl. Oral Sci. 2012, 20, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Vargas, L.O.; Estrada-Barraza, D.; Pozos-Guillen, A.J.; Rivas-Caceres, R. Biofilm formation by oral clinical isolates of Candida species. Arch. Oral Biol. 2013, 58, 1318–1326. [Google Scholar] [CrossRef]
- Pereira-Cenci, T.; da Silva, W.J.; Cenci, M.S.; Cury, A.A.D.B. Temporal changes of denture plaque microbiologic composition evaluated in situ. Int. J. Prosthodont. 2010, 23, 239–242. [Google Scholar]
- El-Kirat-Chatel, S.; Beaussart, A.; Derclaye, S.; Alsteens, D.; Kucharíková, S.; Van Dijck, P.; Dufrêne, Y.F. Force nanoscopy of hydrophobic interactions in the fungal pathogen Candida glabrata. ACS Nano 2015, 9, 1648–1655. [Google Scholar] [CrossRef]
- Valotteau, C.; Prystopiuk, V.; Cormack, B.P.; Dufrêne, Y.F. Atomic force microscopy demonstrates that Candida glabrata uses three Epa proteins to mediate adhesion to abiotic surfaces. mSphere 2019, 4, e00277-19. [Google Scholar] [CrossRef] [Green Version]
- Domergue, R.; Castaño, I.; De Las Peñas, A.; Zupancic, M.; Lockatell, V.; Hebel, J.R.; Johnson, D.; Cormack, B.P. Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science 2005, 308, 866–870. [Google Scholar] [CrossRef]
- Kauffman, S.L.; Kasai, G.J.; Koser, S.A. The amounts of folic acid and vitamin B6 in saliva. J. Dent. Res. 1953, 32, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Evered, D.F.; Sadoogh-Abasian, F.; Patel, P.D. Absorption of nicotinic acid and nicotinamide across human buccal mucosa in vivo. Life Sci. 1980, 27, 1649–1651. [Google Scholar] [CrossRef]
- Minagi, S.; Miyake, Y.; Inagaki, K.; Tsuru, H.; Suginaka, H. Hydrophobic interaction in Candida albicans and Candida tropicalis adherence to various denture base resin materials. Infect. Immun. 1985, 47, 11–14. [Google Scholar] [CrossRef] [Green Version]
- Geertsema-Doornbusch, G.I.; Vandermei, H.C.; Busscher, H.J. Microbial cell surface hydrophobicity the involvement of electrostatic interactions in microbial adhesion to hydrocarbons (MATH). J. Microbiol. Methods 1993, 18, 61–68. [Google Scholar] [CrossRef]
- Linde, J.; Duggan, S.; Weber, M.; Horn, F.; Sieber, P.; Hellwig, D.; Riege, K.; Marz, M.; Martin, R.; Guthke, R.; et al. Defining the transcriptomic landscape of Candida glabrata by RNA-Seq. Nucleic Acids Res. 2015, 43, 1392–1406. [Google Scholar] [CrossRef] [PubMed]
- López-Fuentes, E.; Gutiérrez-Escobedo, G.; Timmermans, B.; Van Dijck, P.; De Las Peñas, A.; Castaño, I. Candida glabrata’s genome plasticity confers a unique pattern of expressed cell wall proteins. J. Fungi 2018, 4, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Las Peñas, A.; Pan, S.J.; Castaño, I.; Alder, J.; Cregg, R.; Cormack, B.P. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev. 2003, 17, 2245–2258. [Google Scholar] [CrossRef] [Green Version]
- Iraqui, I.; Garcia-Sanchez, S.; Aubert, S.; Dromer, F.; Ghigo, J.M.; D’Enfert, C.; Janbon, G. The Yak1p kinase controls expression of adhesins and biofilm formation in Candida glabrata in a Sir4p-dependent pathway. Mol. Microbiol. 2005, 55, 1259–1271. [Google Scholar] [CrossRef]
- Halliwell, S.C.; Smith, M.C.; Muston, P.; Holland, S.L.; Avery, S.V. Heterogeneous expression of the virulence-related adhesin Epa1 between individual cells and strains of the pathogen Candida glabrata. Eukaryot. Cell 2012, 11, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Kraneveld, E.A.; de Soet, J.J.; Deng, D.M.; Dekker, H.L.; de Koster, C.G.; Klis, F.M.; Crielaard, W.; de Groot, P.W.J. Identification and differential gene expression of adhesin-like wall proteins in Candida glabrata biofilms. Mycopathologia 2011, 172, 415–427. [Google Scholar] [CrossRef]
- Gómez-Molero, E.; de Boer, A.D.; Dekker, H.L.; Moreno-Martinez, A.; Kraneveld, E.A.; Ichsan; Chauhan, N.; Weig, M.; de Soet, J.J.; de Koster, C.G.; et al. Proteomic analysis of hyperadhesive Candida glabrata clinical isolates reveals a core wall proteome and differential incorporation of adhesins. FEMS Yeast Res. 2015, 15, fov098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucharíková, S.; Neirinck, B.; Sharma, N.; Vleugels, J.; Lagrou, K.; Van Dijck, P. In vivo Candida glabrata biofilm development on foreign bodies in a rat subcutaneous model. J. Antimicrob. Chemother. 2015, 70, 846–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, D.M.; Hoogenkamp, M.A.; Exterkate, R.A.M.; Jiang, L.M.; van der Sluis, L.W.M.; Ten Cate, J.M.; Crielaard, W. Influence of Streptococcus mutans on Enterococcus faecalis biofilm formation. J. Endod. 2009, 35, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Skinner, J.; Bennett, J.E. Evaluation of reference genes for real-time quantitative PCR studies in Candida glabrata following azole treatment. BMC Mol. Biol. 2012, 13, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sheiham, A.; Steele, J.G.; Marcenes, W.; Lowe, C.; Finch, S.; Bates, C.J.; Prentice, A.; Walls, A.W.G. The relationship among dental status, nutrient intake, and nutritional status in older people. J. Dent. Res. 2001, 80, 408–413. [Google Scholar] [CrossRef]



| Gene | Primer Sequences (5′–3′) | Annealing Temperature (°C) | |
|---|---|---|---|
| EPA1 | F | TTCAGACCAAAAGTAACTGGCTTC | 57 |
| R | CCTAATAGGGTAATATACGCCCG | ||
| EPA3 | F | TGGATGTTCTCCTCAGGATGTTG | 55 |
| R | TGTAGACCAGTTGTTTGAGCCTTG | ||
| EPA6 | F | TGATTATTTGAAATCAGGATCGAATC | 55 |
| R | TGTCATTGTCAATGGTGTACGATAG | ||
| EPA7 | F | GATTTACGGAAGAATGGTTCGTAC | 55 |
| R | GGTAAATGATCTATTTCGGGTGTG | ||
| AWP3 | F | GCCCAGATCAATGGAGCGG | 57 |
| R | CACAGCGATTGACGTAACACCAG | ||
| AWP4 | F | CAATTACGATGTCCTGGATAATCCGR | 59 |
| R | CTTGAAAGGCTAAGTAAACACCTCC | ||
| AWP6 | F | CGCATTGGCTGCGGTAGC | 59 |
| R | TGGCCTTTGATATCAGCCAAG | ||
| 18S rRNA | F | CCGAGGACTGCGATACTTGT | 57 |
| R | CACCCAAACACTCGCATAGA | ||
| PGK1 | F | ACGAAGTTGTCAAGTCCTCCA | 57 |
| R | TTACCTTCCAACAATTCCAAGGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; He, L.; Krom, B.P.; Cheng, L.; de Soet, J.J.; Deng, D.M. Niacin Limitation Promotes Candida glabrata Adhesion to Abiotic Surfaces. Pathogens 2022, 11, 387. https://doi.org/10.3390/pathogens11040387
Li X, He L, Krom BP, Cheng L, de Soet JJ, Deng DM. Niacin Limitation Promotes Candida glabrata Adhesion to Abiotic Surfaces. Pathogens. 2022; 11(4):387. https://doi.org/10.3390/pathogens11040387
Chicago/Turabian StyleLi, Xiaolan, Libang He, Bastiaan P. Krom, Lei Cheng, Johannes J. de Soet, and Dong M. Deng. 2022. "Niacin Limitation Promotes Candida glabrata Adhesion to Abiotic Surfaces" Pathogens 11, no. 4: 387. https://doi.org/10.3390/pathogens11040387








