MIRU-VNTR Typing of Atypical Mycobacteria Isolated from the Lymph Nodes of Slaughtered Pigs from Poland
Abstract
:1. Introduction
2. Results
2.1. Mycobacterial Analysis and Species Designation
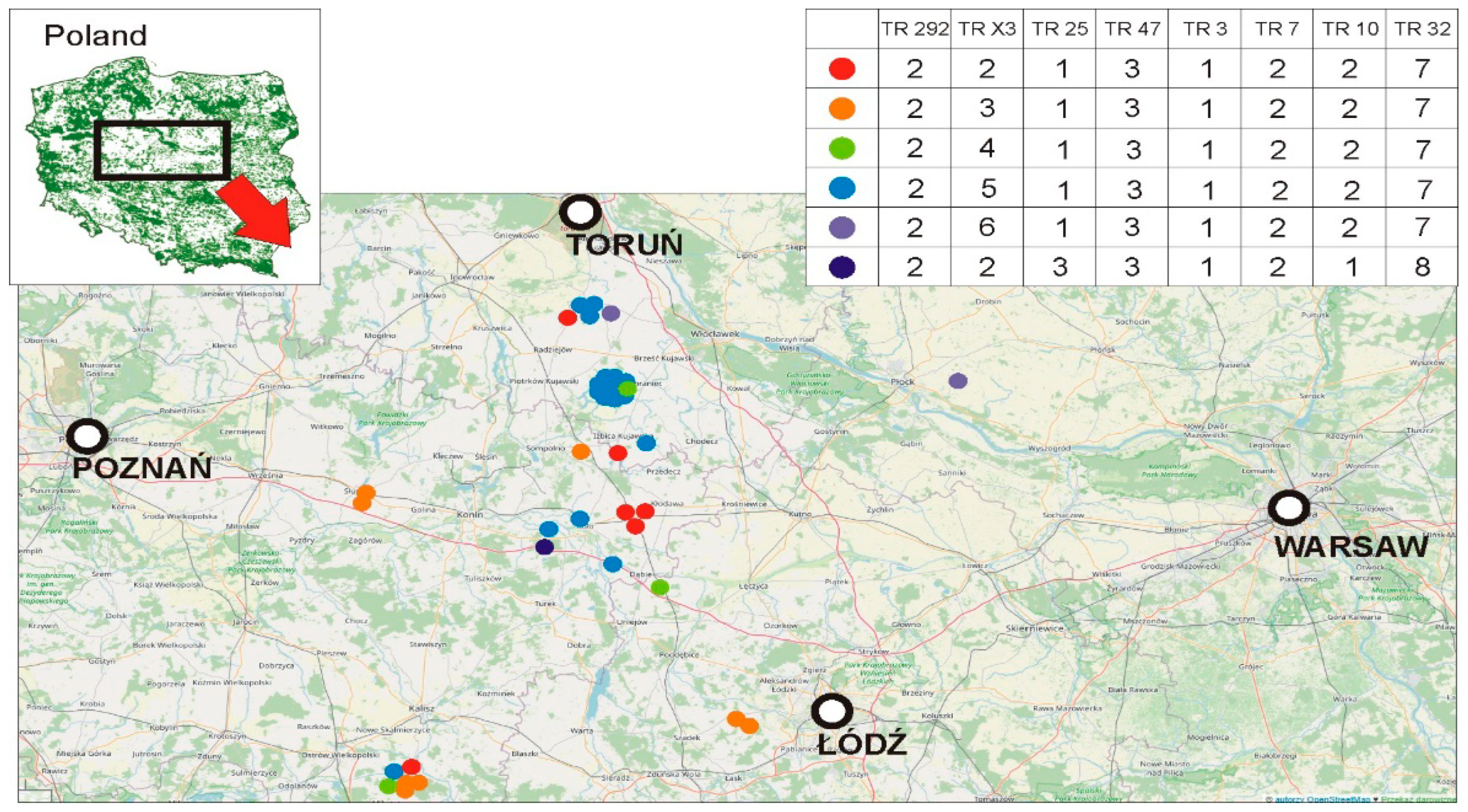
2.2. MIRU-VNTR Analysis
3. Discussion
4. Materials and Methods
4.1. The Origin of Material and Culture
4.2. DNA Isolation
4.3. Strain Identification
4.4. IS901, IS900, and IS1245 Identification
4.5. MIRU-VNTR Identification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hulinova Stromerova, N.; Faldyna, M. Mycobacterium avium complex infection in pigs: A review. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 62–68. [Google Scholar] [CrossRef]
- Rindi, L.; Garzelli, C. Genetic diversity and phylogeny of Mycobacterium avium. Infect. Genet. Evol. 2014, 21, 375–383. [Google Scholar] [CrossRef]
- Agdestein, A.; Johansen, T.B.; Kolbjørnsen, Ø.; Jørgensen, A.; Djønne, B.; Olsen, I. A comparative study of Mycobacterium avium subsp. avium and Mycobacterium avium subsp. hominissuis in experimentally infected pigs. BMC Vet. Res. 2012, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Barandiaran, S.; Perez, A.M.; Gioffre, A.K.; Vivot, M.M.; Cataldi, A.A.; Zumárraga, M.J. Tuberculosis in swine co-infected with Mycobacterium avium subsp. hominissuis and Mycobacterium bovis in a cluster from Argentina. Epidemiol. Infect. 2015, 143, 966–974. [Google Scholar] [CrossRef] [Green Version]
- Moravkova, M.; Lamka, J.; Slany, M.; Pavlik, I. Genetic IS901 RFLP diversity among Mycobacterium avium subsp. avium isolates from four pheasant flocks. J. Vet. Sci. 2013, 14, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Agdestein, A.; Olsen, I.; Jørgensen, A.; Djønne, B.; Johansen, T.B. Novel insights into transmission routes of Mycobacterium avium in pigs and possible implications for human health. Vet. Res. 2014, 45, 46. [Google Scholar] [CrossRef] [Green Version]
- Adachi, T.; Ichikawa, K.; Inagaki, T.; Moriyama, M.; Nakagawa, T.; Ogawa, K.; Hasegawa, Y.; Yagi, T. Molecular typing and genetic characterization of Mycobacterium avium subsp. hominissuis isolates from humans and swine in Japan. J. Med. Microbiol. 2016, 65, 1289–1295. [Google Scholar] [CrossRef]
- Jeong-Ih, S.; Sung Jae, S.; Min-Kyoung, S. Differential Genotyping of Mycobacterium avium Complex and Its Implications in Clinical and Environmental Epidemiology. Microorganisms 2020, 8, 98. [Google Scholar] [CrossRef] [Green Version]
- Rónai, Z.; Csivincsik, Á.; Dán, Á.; Gyuranecz, M. Molecular analysis and MIRU-VNTR typing of Mycobacterium avium subsp. avium, ‘hominissuis’ and silvaticum strains of veterinary origin. Infect. Genet. Evol. 2016, 40, 192–199. [Google Scholar] [CrossRef]
- Pate, M.; Kušar, D.; Zolnir-Dovč, M.; Ocepek, M. MIRU-VNTR typing of Mycobacterium avium in animals and humans: Heterogeneity of Mycobacterium avium subsp. hominissuis versus homogeneity of Mycobacterium avium subsp. avium strains. Rest. Vet. Sci. 2011, 91, 376–381. [Google Scholar] [CrossRef]
- Subangkit, M.; Yamamoto, T.; Ishida, M.; Nomura, A.; Yasiki, N.; Sudaryatma, P.E.; Goto, Y.; Okabayashi, T. Genotyping of swine Mycobacterium avium subsp. hominissuis isolates from Kyushu, Japan. J. Vet. Med. Sci. 2019, 81, 1074–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtasik, A.; Kubiak, A.B.; Krzyżanowska, A.; Majchrzak, M.; Augustynowicz-Kopeć, E.; Parniewski, P. Comparison of the (CCG)4-based PCR and MIRU-VNTR for molecular typing of Mycobacterium avium strains. Mol. Biol. Rep. 2012, 39, 7681–7686. [Google Scholar] [CrossRef] [PubMed]
- Motiwala, A.S.; Li, L.; Kapur, V.; Sreevatsan, S. Current understanding of the genetic diversity of Mycobacterium avium subsp. paratuberculosis. Microbes Infect. 2006, 8, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Radomski, N.; Thibault, V.C.; Karoui, C.; de Cruz, K.; Cochard, T.; Gutiérrez, C.; Supply, P.; Biet, F.; Boschiroli, M.L. Determination of Genotypic Diversity of Mycobacterium avium Subspecies from Human and Animal Origins by Mycobacterial Interspersed Repetitive-Unit-Variable-Number Tandem-Repeat and IS1311 Restriction Fragment Length Polymorphism Typing Methods. J. Clin. Microbiol. 2010, 48, 1026–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseiniporgham, S.; Biet, F.; Ganneau, C.; Bannantine, J.P.; Bay, S.; Sechi, L.A. A Comparative Study on the Efficiency of Two Mycobacterium avium subsp. paratuberculosis (MAP)-Derived Lipopeptides of L3P and L5P as Capture Antigens in an In-House Milk ELISA Test. Vaccines 2021, 9, 997. [Google Scholar] [CrossRef]
- Iakhiaeva, E.; McNulty, S.; Brown Elliott, B.A.; Falkinham, J.O.; Williams, M.D.; Vasireddy, R.; Wilson, R.W.; Turenne, C.; Wallace, R.J., Jr. Mycobacterial interspersed repetitiveunit-variable-number tandem-repeat (MIRU-VNTR) genotyping of Mycobacterium intracellulare for strain comparison with establishment of a PCR-based database. J. Clin. Microbiol. 2013, 51, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Imperiale, B.; Moyano, R.; Di Giulio, A.; Romero, M.; Pinedo, A.; Santangelo, M.P.; Travería, G.E.; Morcillo, N.S.; Romano, M.I. Genetic diversity of Mycobacterium avium complex strains isolated in Argentina by MIRU-VNTR. Epidemiol. Infect. 2017, 145, 1382–1391. [Google Scholar] [CrossRef] [Green Version]
- Thibault, V.C.; Grayon, M.; Boschiroli, M.L.; Hubbans, C.; Overduin, P.; Stevenson, K.; Gutierrez, M.C.; Supply, P.; Biet, F. New variable-number tandem-repeat markers for typing Mycobacterium avium subsp. paratuberculosis and M. avium strains: Comparison with IS900 and IS1245 restriction fragment length polymorphism typing. J. Clin. Microbiol. 2007, 45, 2404–2410. [Google Scholar] [CrossRef] [Green Version]
- Gioffré, A.; Correa Muñoz, M.; Alvarado Pinedo, M.F.; Vaca, R.; Morsella, C.; Fiorentino, M.A.; Paolicchi, F.; Ruybal, P.; Zumárraga, M.; Travería, G.E.; et al. Molecular typing of Argentinian Mycobacterium avium subsp. paratuberculosis isolates by multiple-locus variable number-tandem repeat analysis. Braz. J. Microbiol. 2015, 46, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Beumer, A.; King, D.; Donohue, M.; Mistry, J.; Covert, T.; Pfaller, S. Detection of Mycobacterium avium subsp. paratuberculosis in drinking water and biofilms by quantitative PCR. Appl. Environ. Microbiol. 2010, 76, 7367–7370. [Google Scholar] [CrossRef] [Green Version]
- Johansen, T.B.; Djønne, B.; Jensen, M.R.; Olsen, I. Distribution of IS1311 and IS1245 in Mycobacterium avium subspecies revisited. J. Clin. Microbiol. 2005, 43, 2500–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klanicova-Zalewska, B.; Slana, I. Presence and persistence of Mycobacterium avium and other nontuberculous mycobacteria in animal tissues and derived foods: A review. Meat Sci. 2014, 98, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Bacterial resistance to disinfectants: Present knowledge and future problems. J. Hosp. Infect. 1999, 43, S57–S68. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol Rev. 1999, 12, 147–179, Published correction appears in Clin. Microbiol. Rev. 2001, 14, 227. [Google Scholar] [CrossRef] [Green Version]
- Sevilla, I.A.; Molina, E.; Tello, M.; Elguezabal, N.; Juste, R.A.; Garrido, J.M. Detection of Mycobacteria by Culture and DNA-Based Methods in Animal-Derived Food Products Purchased at Spanish Supermarkets. Front. Microbiol. 2017, 8, 1030. [Google Scholar] [CrossRef]
- Kendall, B.A.; Winthrop, K.L. Update on the epidemiology of pulmonary non-tuberculous mycobacterial infections. Semin. Respir. Crit. Care Med. 2013, 34, 87–94. [Google Scholar] [CrossRef]
- Acharya, K.R.; Dhand, N.K.; Whittington, R.J.; Plain, K.M. Detection of Mycobacterium avium subspecies paratuberculosis in powdered infant formula using IS900 quantitative PCR and liquid culture media. Int. J. Food Microbiol. 2017, 257, 1–9. [Google Scholar] [CrossRef]
- Agrawal, G.; Aitken, J.; Hamblina, H.; Collins, M.; Borody, T.J. Putting Crohn’s on the MAP: Five Common Questions on the Contribution of Mycobacterium avium subspecies paratuberculosis to the Pathophysiology of Crohn’s Disease. Dig. Dis. Sci. 2021, 66, 348–358. [Google Scholar] [CrossRef]
- Tirkkonen, T.; Pakarinen, J.; Rintala, E.; Ali-Vehmas, T.; Marttila, H.; Peltoniemi, O.A.; Mäkinen, J. Comparison of Variable-Number Tandem-Repeat Markers typing and IS1245 Restriction Fragment Length Polymorphism fingerprinting of Mycobacterium avium subsp. hominissuis from human and porcine origins. Acta Vet. Scand. 2010, 52, 21. [Google Scholar] [CrossRef]
- Ahlstrom, C.; Barkema, H.; Stevenson, K.; Sadok, R.N.; Biek, R.; Kao, R.; Trewby, H.; Haupstein, D.; Kelton, D.F.; Fecteau, G.; et al. Limitations of variable number of tandem repeat typing identified through whole genome sequencing of Mycobacterium avium subsp. paratuberculosis on a national and herd level. BMC Genom. 2015, 16, 161. [Google Scholar] [CrossRef] [Green Version]
- Bryant, J.M.; Thibault, V.C.; Smith, D.G.; McLuckie, J.; Heron, I.; Sevilla, I.A.; Biet, F.; Harris, S.R.; Maskell, D.J.; Bentley, S.D.; et al. Phylogenomic exploration of the relationships between strains of Mycobacterium avium subspecies paratuberculosis. BMC Genom. 2016, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Busatto, C.; Vianna, J.S.; Scholante Silva, A.B.; Basso, R.; Silveira, J.; Groll, A.V.; Ramis, I.B.; Silva, P.E.A.D. Non-tuberculous mycobacteria in patients with suspected tuberculosis and the genetic diversity of Mycobacterium avium in the extreme south of Brazil. J. Bras. Pneumol. 2020, 46, e20190184. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, P.; Steen, B.; Giese, S.B.; Klausen, J.; Inglis, N.F. Two Markers, IS901-IS902 and p40, Identified by PCR and by Using Monoclonal Antibodies in Mycobacterium avium Strains. J. Clin. Microbiol. 1995, 33, 1049–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, C.; Bernasconi, C.; Burki, D.; Bodmer, T.; Telenti, A. A novel insertion element from Mycobacterium avium, IS1245, is a specific target for analysis of strain relatedness. J. Clin. Microbiol. 1995, 33, 304–307. [Google Scholar] [CrossRef] [Green Version]
- Hines, M.E., II; Frazier, K.S.; Baldwin, C.A.; Cole, J.R., Jr.; Sangster, L.T. Efficacy of vaccination for Mycobacterium avium with whole cell and subunit vaccines in experimentally infected swine. Vet. Microbiol. 1998, 63, 49–59. [Google Scholar] [CrossRef]
- Hussain, S.M.; Javed, M.T.; Rizvi, F.; Qamar, M. Prevalence of paratuberculosis in cattle and buffaloes in Punjab Pakistan. Pak. J. Agric. Sci. 2018, 55, 427–432. [Google Scholar]
- van Soolingen, D.; Bauer, J.; Ritacco, V.; Leão, S.C.; Pavlik, I.; Vincent, V.; Rastogi, N.; Gori, A.; Bodmer, T.; Garzelli, C.; et al. IS1245 restriction fragment length polymorphism typing of Mycobacterium avium isolates: Proposal for standardization. J. Clin. Microbiol. 1998, 36, 3051–3054. [Google Scholar] [CrossRef] [Green Version]
- Cochard, T.; Branger, M.; Supply, P.; Sreevatsan, S.; Biet, F. MAC-INMV-SSR: A web application dedicated to genotyping members of Mycobacterium avium complex (MAC) including Mycobacterium avium subsp. paratuberculosis strains. Infect. Genet. Evol. 2020, 77, 104075. [Google Scholar] [CrossRef]
| Isolate | Number of Copies MIRU-VNTR Region | Sub-Species Assignment | IS901 | IS900 | IS1245 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TR 292 | TR x3 | TR 25 | TR 47 | TR 3 | TR 7 | TR 10 | TR 32 | |||||
| 5 | 2 | 2 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 7 | 2 | 2 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 9 | 2 | 2 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 16 | 2 | 2 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 17 | 2 | 2 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 23 | 2 | 2 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 41 | 2 | 3 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 42 | 2 | 3 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 43 | 2 | 3 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 75 | 2 | 3 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 89 | 2 | 3 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 109 | 2 | 3 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 111 | 2 | 3 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 112 | 2 | 3 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 79 | 2 | 4 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 80 | 2 | 4 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 20ab | 2 | 4 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 2 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 13ab | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 14 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 27 * | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 30 ** | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 35 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 44 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 47 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 48 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 66 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 82 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 86 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 87 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 88 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 91 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 96 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 99 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 102 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 104 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 108 | 2 | 5 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 90 | 2 | 6 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 100 | 2 | 6 | 1 | 3 | 1 | 2 | 2 | 7 | M. avium spp. | + | − | + |
| 6 | 2 | 2 | 3 | 3 | 1 | 2 | 1 | 8 | M.a. paratuberculosis | − | − | − |
| 3 | not M. avium spp. | − | − | − | ||||||||
| 73 | not M. avium spp. | − | − | − | ||||||||
| 78 | not M. avium spp. | − | − | − | ||||||||
| 85 | not M. avium spp. | − | − | − | ||||||||
| 92 | not M. avium spp. | − | − | − | ||||||||
| 95 | not M. avium spp. | − | − | − | ||||||||
| 97 | not M. avium spp. | − | − | − | ||||||||
| 107 | not M. avium spp. | − | − | − | ||||||||
| 113 | not M. avium spp. | − | − | − | ||||||||
| 124 *** | not M. avium spp. | − | − | − | ||||||||
| MIRU-VNRT | Repeated Motif (bp) | Flanking Sequences (bp) |
|---|---|---|
| TR292 | 53 | 141 |
| TRX3 | 53 | 90 |
| TR25 | 58 | 193 |
| TR47 | 35 | 112 |
| TR3 | 27 | 146 |
| TR7 | 22 | 159 |
| TR10 | 55 | 198 |
| TR32 | 18 | 143 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majchrzak, M.; Kaczmarkowska, A.; Didkowska, A.; Brzezińska, S.; Orłowska, B.; Klich, D.; Augustynowicz-Kopeć, E.; Anusz, K.; Parniewski, P. MIRU-VNTR Typing of Atypical Mycobacteria Isolated from the Lymph Nodes of Slaughtered Pigs from Poland. Pathogens 2022, 11, 495. https://doi.org/10.3390/pathogens11050495
Majchrzak M, Kaczmarkowska A, Didkowska A, Brzezińska S, Orłowska B, Klich D, Augustynowicz-Kopeć E, Anusz K, Parniewski P. MIRU-VNTR Typing of Atypical Mycobacteria Isolated from the Lymph Nodes of Slaughtered Pigs from Poland. Pathogens. 2022; 11(5):495. https://doi.org/10.3390/pathogens11050495
Chicago/Turabian StyleMajchrzak, Marta, Aleksandra Kaczmarkowska, Anna Didkowska, Sylwia Brzezińska, Blanka Orłowska, Daniel Klich, Ewa Augustynowicz-Kopeć, Krzysztof Anusz, and Paweł Parniewski. 2022. "MIRU-VNTR Typing of Atypical Mycobacteria Isolated from the Lymph Nodes of Slaughtered Pigs from Poland" Pathogens 11, no. 5: 495. https://doi.org/10.3390/pathogens11050495
APA StyleMajchrzak, M., Kaczmarkowska, A., Didkowska, A., Brzezińska, S., Orłowska, B., Klich, D., Augustynowicz-Kopeć, E., Anusz, K., & Parniewski, P. (2022). MIRU-VNTR Typing of Atypical Mycobacteria Isolated from the Lymph Nodes of Slaughtered Pigs from Poland. Pathogens, 11(5), 495. https://doi.org/10.3390/pathogens11050495







