Abstract
Ticks are important vectors of numerous pathogens of medical and veterinary significance. The aim of the current study was to determine the prevalence of Babesia spp. and Borrelia burgdorferi s.l. in sled and pet dogs from Central and North-Eastern Europe. Neither Babesia spp. nor Borrelia burgdorferi s.l. infections were detected in sled dogs from seven countries (Poland, Lithuania, Latvia, Estonia, Belarus, Russia and Finland). The DNA of Babesia spp. was detected in 100% of symptomatic and 5.4% of asymptomatic pet dogs from Poland. Similarly, the DNA of Babesia spp. was identified in 82% of symptomatic and 3.8% of asymptomatic pet dogs from Ukraine. The DNA of Borrelia burgdorferi s.l. was detected in 4.4% of pet dogs. Molecular typing confirmed the presence of Babesia canis and Borrelia burgdorferi sensu stricto (s.s.) in selected samples. Four dogs were co-infected by B. canis and Borrelia burgdorferi s.l. Tick-borne pathogens constitute a serious health threat to pet dogs in Central and South-Eastern Europe, but were not observed among sled dogs from the same region of Europe nor in the Baltic countries.
Keywords:
Babesia canis; Borrelia burgdorferi; paralogs; sled dog; Lithuania; Latvia; Estonia; Poland; Ukraine; PCR 1. Introduction
Ticks are important vectors of numerous microorganisms of medical and veterinary significance [1,2]. Among tick-borne pathogens (TBP) with the greatest impact on their hosts are spirochaetes of the Borrelia burgdorferi sensu lato (s.l.) complex, responsible for development of the multisystemic disease, borreliosis (Lyme disease, Lyme borreliosis; LB). Globally, borreliosis affects between 0.3–0.5 million people/year in the Northern Hemisphere and in the next decade the incidence of Lyme disease may increase by 20% in the USA only as a consequence of climate change [3,4]. In Poland in 2019, 20,630 cases of Lyme disease were registered, and 1701 people were hospitalized. The highest incidence of 107.7/100,000 population was recorded in the Podlaskie voivodeship, for many years the province in the country with the highest incidence of borreliosis [5]. Such a high incidence in the human population is associated with the high prevalence of spirochaetes (10–25%) in its main vector, Ixodes ricinus ticks [4]. Borreliosis also affects dogs [6,7]. The most common clinical manifestation of Lyme disease in dogs is Lyme arthritis. The classical presentation is an acute monoarticular or polyarticular lameness with joint swelling, fever, lethargy, and mild local lymphadenopathy, usually in young, often large-breed dogs with an active outdoor lifestyle [8]. Rare manifestation is Lyme carditis, Lyme nephritis and Lyme neuritis [7,8,9,10]. Clinical LB cases are more common in the US than in Europe [8]. Subclinical infections are the most common [8,10,11].
Babesia spp. are protozoan parasites of red blood cells responsible for the development of babesiosis, a potentially life-threatening disease of humans and animals [12,13]. Although cases of babesiosis are rare in humans in Europe [14], the symptoms may resemble malaria infection, with hemolytic anemia, hemoglobinuria, and Severe Acute Respiratory Syndrome (SARS) development, and babesiosis can be fatal especially in immunocompromised patients [12].
Frequent clinical signs associated with canine babesiosis are apathy, weakness, anorexia, pale mucous membranes, and a poor general condition [13]. Infection can cause fever, enlarged lymph nodes and spleen, anaemia, thrombocytopenia, jaundice, and pigmenturia. Babesiosis due to B. canis can manifest with a range of clinical signs and laboratory abnormalities: petechiae, epistaxis, vomiting, lymphadenomegaly, hypotension, low T3 syndrome, mild to moderate nonregenerative, normochromic and normocytic anaemia, regenerative anaemia (less common), leukopenia with neutropenia and/or lymphopenia, hypoalbuminemia, elevation of liver enzymes (ALT, AST, ALP), hypokalemia, hyponatremia, hyperchloremia, hyperlactatemia, hyperphosphatemia [13]. Babesiosis due to Babesia canis infection is an emerging tick-borne disease in dogs in Central Europe [13,15,16,17,18,19]. The main, if not the only, vector of B. canis is the ornate dog tick, Dermacentor reticulatus [13,20,21].
We have focused here on sled dogs because they are particularly prone to vector-borne infections through their participation in training sessions and racing events, the majority of which take place in forest areas where ticks and mosquitoes are abundant. These dogs are usually housed in kennels where there may be an additional high risk of vector challenge. Infections with TBP may have negative impacts on the performance of sled dogs in competitive events and are therefore of considerable concern for sled dog owners and the organizers of race meetings [22,23]. Although reports on tick-borne infections in dogs from NE Europe, including the Baltic states, are still rare [24,25,26], we have reported recently infections with several TBP in sled dogs, including Anaplasma phagocytophilum, B. canis, and tick-borne encephalitis virus (TBEV) [22,23,27,28]. Moreover, high prevalence of Dirofilaria repens, a zoonotic mosquito-transmitted nematode, has been found recently in sled dogs from central and NE Europe [29], suggesting an increasing significance of vector-borne infections in these regions of Europe.
The aim of the current study was to determine the prevalence of Babesia spp. and Borrelia burgdorferi s.l. in sled and pet dogs from Central and North-Eastern (NE) Europe.
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
Since the study was carried out on blood samples provided for veterinary diagnostic purpose [29], no ethical approval/license was required for our study (as per resolution on the protection of animals used for scientific or educational purposes, 15th January 2015 [Dz. U. 2015 position 266] Chapter 1, Paragraph 1.2.1).
2.2. Collection of Samples
Blood samples from dogs were collected in 2015–2021, with most samples collected between 2017–2019. Five hundred and fifty-three dog blood samples were collected into EDTA-covered vials from eight countries, including 256 samples from various breeds of sled dogs (mainly Alaskan huskies, Siberian huskies, and European sled dogs [ESD]), and 297 samples were from pet dogs (Table 1). The main study was focused on sled dogs from Poland and the Baltic countries (Lithuania, Latvia, and Estonia) and on companion dogs from Poland and Ukraine (Table 1). Additionally, several sled dogs were available for sampling from Russia, Belarus, and Finland (Table 1).

Table 1.
Prevalence of pathogens in groups of sampled dogs.
All sled dogs were healthy animals, comprising racing dogs and a small group of elderly individuals, living in the same kennels. Sled dogs were sampled in their kennels or during national or international sled dog races in 2017–2021, encompassing dogs from Poland, Lithuania, Latvia, Estonia, Belarus, Russia, and Finland. Basic data were noted, including breed, age, sex, and health status (i.e., activity level, appetite, and any health or stamina problems, as reported by the owners).
Blood samples from pet dogs originating from Central Poland and Western Ukraine were from animals with laboratory-confirmed babesiosis (68 from Poland and 50 from Ukraine) and also from a diverse group of dogs attending veterinary clinics for different non-TBD-related concerns. Additionally, we sampled a diverse group of healthy pet dogs presenting no signs of TBDs (owned pedigree dogs and mixed breed dogs from a shelter) from a single location (Błędowo near Nowy Dwór Mazowiecki, Central Poland) (Table 1).
2.3. Molecular Detection of Babesia spp. and Borrelia burgdorferi Sensu Lato
Genomic DNA was extracted from EDTA-preserved blood samples using the DNAeasy Blood & Tissue kit (Qiagen, Hilden, Germany) and stored at a temperature of −20 °C, no longer than six months before PCR testing.
Molecular detection of Babesia spp. was performed by amplification of a 550 bp fragment of 18S rDNA, as described previously [30,31,32]. For molecular screening of spirochaetes (Borrelia burgdorferi s.l.) genus-specific primers: 132f/905r and 220f/824r were used to amplify the flaB gene fragments (774 and 605 bp), respectively [33], in a nested-PCR protocol using modified reaction conditions [4]. Positive (sequenced isolates of Babesia microti or Borrelia burgdorferi s.l.) and negative (sterile water) controls were incorporated in each set of PCRs. Amplicons were visualized with Midori Green stain (Nippon Genetics Europe GmbH, Düren, Germany) following electrophoresis in 1.5% agarose gels. For the identification of pathogen species, selected amplicons were purified and sequenced in both directions by a private company (Genomed S.A., Warsaw, Poland). DNA sequence alignments were conducted using MEGA X (https://www.megasoftware.net/ (accessed on 5 March 2022)) and CodonCode Aligner. The resulting consensus sequences were compared with sequences deposited in GenBank NCBI.
For the statistical evaluation of differences in pathogen prevalence (% infected), we applied maximum likelihood techniques based on log linear analysis of contingency tables in the IBM SPSS Statistics: PS IMAGO PRO Academic v.7 (institutional license purchased by the University of Warsaw, Warsaw, Poland), as described in detail in our previous papers [15,23]. Country of dog origin (eight levels), sex of dogs (males and females), sled dog status (0, 1) and Babesia infection status (veterinary laboratory result) (0, 1) were used as the factors in models with the presence or absence of Babesia DNA considered as a binary factor (0, 1). For each level of analysis in turn, beginning with the most complex model, involving all possible main effects and interactions, those combinations that did not contribute significantly (p > 0.05) to explaining variation in the data were eliminated in a stepwise fashion beginning with the highest-level interaction (backward selection procedure). A minimum sufficient model was then obtained, for which the likelihood ratio of chi-square was not significant, indicating that the model was sufficient in explaining the data [15,23].
Phylogenetic analysis was performed using the Maximum Likelihood method and Tamura 3-parameter model. The evolutionary model was chosen in accordance with the data following implementation of a model test in MEGA X. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.2939)). Borrelia miyamotoi was selected as outgroup.
Selected B. canis and Borrelia burgdorferi sequences originating from Poland and Ukraine have been deposited in the GenBank database under the accession numbers: OM350211- OM350213 for B. canis from Poland, OM362458-OM362460 for B. canis from Ukraine and OM387038-OM387040 for Borrelia burgdorferi s.s. from Poland.
3. Results
Neither Babesia spp. nor Borrelia burgdorferi s.l. infections were detected in any of the sled dogs sampled from seven countries (Poland, Lithuania, Latvia, Estonia, Belarus, Russia and Finland) (Babesia-positive x sled dog status: χ21 = 94.2, p < 0.001; Borrelia-positive x sled dog status: χ21 = 16.4, p < 0.001).
The DNA of Babesia spp. was detected in 100% of symptomatic and 5.4% of asymptomatic pet dogs from Poland (Table 1). Similarly, Babesia DNA was identified in 82% of symptomatic and 3.8% of asymptomatic pet dogs from Ukraine (Babesia-positive x Babesia-laboratory result: χ21 = 427.3, p < 0.001; Babesia-positive x country of origin: χ27 = 14.7, p = 0.04). The most common clinical signs observed in symptomatic dogs were lethargy, inappetence and fever (>80% of dogs).
Sequencing of 14 PCR products from dogs from Poland (11 from symptomatic and 3 from asymptomatic dogs) revealed B. canis infection (99.6–100% identity with B. canis isolates from dogs and red foxes; accession numbers MN173223 and MN134074). Similarly, sequencing of 19 amplicons from dogs from Ukraine confirmed B. canis infection (99.4–100% identity with B. canis isolates from dogs and red foxes; accession numbers MN134074, MN173223 and MN704759).
Inspection of 18S rDNA chromatograms of B. canis sequences from dogs from Poland and Ukraine revealed the occurrence of paralogs as defined by Hrazdilova et al. [34]. Double peaks in core positions (positions 609–610 of full-length 18S rRNA gene of the sequence AY072926) were present in 11 Polish and 9 Ukrainian B. canis sequences (Figure S1).
Among the 14 Polish samples, in 3 cases clean chromatograms were obtained, including 2 sequences with GA nucleotides in core position (previous genotype A; [35], Figure S1) and 1 sequence with AG nucleotides (previous genotype B; [35]). Eleven other sequences showed double peaks (presence of different paralogs) in these positions (Figure S1).
Among 19 Ukrainian sequences, in 2 samples a clean chromatogram with GA nucleotides (previous genotype A; [35]) was observed; in 8 samples AG nucleotides (previous genotype B; [35]) were observed, while 9 other sequences showed double peaks (presence of different paralogs) in these positions (Figure S1). Only sequences presenting ‘clean’ chromatograms were deposited in the GenBank database.
The DNA of Borrelia burgdorferi s.l. was detected in 13/297 pet dogs (4.4%) (Borrelia-positive x sled dog status: χ21 = 16.4, p < 0.001) (Table 1). In Poland, Borrelia burgdorferi s.l. was found in 4.2% of pet dogs, including 4.4% of dogs with babesiosis and 4.1% of healthy pet dogs. In Ukraine, the DNA of Borrelia burgdorferi s.l. was detected in only seven ‘healthy’ pet dogs (6.7%) (Borrelia-positive x Babesia-laboratory result x country of origin: χ21 = 3.94, p = 0.047). Interestingly, Borrelia burgdorferi DNA was detected in four dogs with B. canis infection, confirmed by molecular typing (one asymptomatic from Ukraine, three with babesiosis from Poland) (Babesia-positive x Borrelia-positive: not significant; NS). Furthermore, the DNA of Di. repens was identified in these three pet dogs from Poland co-infected by B. canis and Borrelia burgdorferi s.l. resulting in triple concurrent infections (results of testing for Dirofilaria spp. from [29]).
The sequencing of three Borrelia burgdorferi s.l. products resulted in 99.81–100% identity to Borrelia burgdorferi sensu strico (s.s.) (MH807139) isolated from an Ixodes ariadnae nymph from Poland. Identification of Borrelia burgdorferi s.s. was confirmed by the topology of the phylogenetic tree, with our three sequences grouped with other Borrelia burgdorferi s.s. sequences (Figure 1).
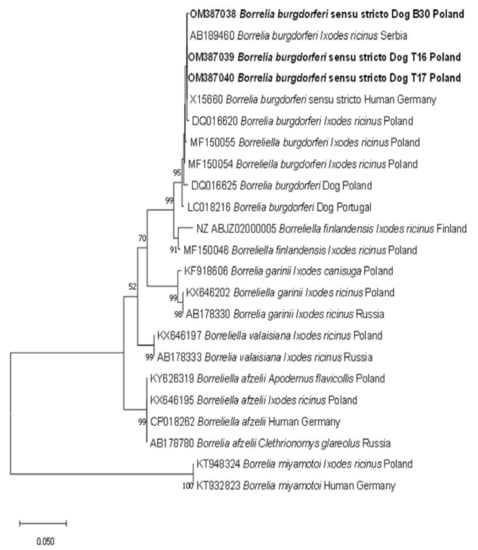
Figure 1.
Molecular phylogenetic analysis of flaB gene fragment (605 bp) of Borrelia burgdorferi s.l. The evolutionary history was inferred by using the Maximum Likelihood method and Tamura 3-parameter model. The tree with the highest log likelihood (−1385.02) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. This analysis involved 23 nucleotide sequences. There was a total of 476 positions in the final dataset.
4. Discussion
The main finding of the current study is that tick-borne pathogens, especially Babesia, and to lesser extent Borrelia burgdorferi s.l., constitute a serious health threat to pet dogs in Central (Poland) and South-Eastern (Ukraine) Europe, but neither pathogen was observed among sled dogs from the same region of Europe nor in those from the Baltic countries.
In the current study, 33 of 117 Babesia-positive samples (28%) were sequenced (14 from Poland, 19 from Ukraine); all resulted with identification of B. canis. It is in agreement with previous studies, because B. canis is reported almost exclusively as the etiological agent of canine babesiosis in Poland [15,17,22,23,27,28], with only four cases of B. gibsoni identified recently in the South-Eastern region reviewed in [17]. Our sequencing results are also in agreement with the results of commercial laboratory results. We selected for genotyping samples both with laboratory-confirmed B. canis and asymptomatic infections from Poland and Ukraine to determine the exact species involved. We also genotyped positive samples from American Pit Bull Terriers from Ukraine as this breed seems predisposed to B. gibsoni infection [17]. All sequenced samples were identified as B. canis; thus, it is likely this species is the most common in pet dogs from Central Poland and Western Ukraine, but further studies are needed to genotype more Babesia-positive samples.
Our failure to detect Babesia and Borrelia in sled dogs contrasts with the expected/predicted increased risk of exposure of these animals to vectors, compared to that of pet dogs. However, the majority of sled dogs involved in the current study were strict racing dogs, participating actively in races and training sessions. In such dogs, any health problems are likely to be easily noticed (for example by comparison with the attitudes/performance of other team members), especially regarding exercise intolerance (babesiosis and borreliosis) or joint problems (borreliosis). Furthermore, practically all the reviewed sled dog owners reported the use of prophylactic measures against ticks and tick-borne diseases, as reported in our previous studies [23,27,28].
Interestingly, even sled dogs originating from countries/regions endemic for babesiosis and borreliosis (Poland, Ukraine, Lithuania, and Latvia) were less infected than pet dogs from Poland and Ukraine. This is actually evidence for the good quality of health care that sled dogs receive these days. Interestingly, in military working dogs in the USA, seroprevalence of anti-Borrelia burgdorferi antibodies was much lower (0.9%, [36]) than in the general population (1.2–16%, [37,38]).
Our study was based on the molecular detection of microorganisms’ DNA in canine blood samples. The vast majority of published studies, however, are based on the detection of antibodies against TBPs, and hence reflect overall exposure risk, rather than currently present infections [36,37,38]. Serological tests are widely used for the diagnostics of Borrelia burgdorferi infection in dogs [37,38,39]. Seroprevalence of anti-Borrelia burgdorferi s.l antibodies differs profoundly in dogs from different countries and regions. In two large epidemiological studies from the USA, encompassing 2010–2017 and 2013–2019, seroprevalence ranged between 1.2–16% depending on the year and the state [37,38]. Interestingly, although prevalence decreased in old endemic regions, borreliosis was shown to be expanding in new regions with increasing prevalence in dog populations [37,38]. In a recent study in Canada, seroprevalence was much lower, about 2–3% [40].
In Europe, epidemiological studies have revealed a similar discrepancy in seroprevalence of borreliosis between countries/regions and dog breeds/groups [39,41,42,43]. The highest seroprevalence was noted in two groups of Bernese Mountain Dogs from Austria, including healthy dogs and dogs with renal disease (43 and 54%, respectively) [43]. Generally, in dogs in European countries, seroprevalence appears to maintain a similar range to that observed in North America, at 1–12% [39,41,42,43]. It is worth remembering that seroprevalence in dogs is positively associated with incidence of borreliosis in humans [38]. Our values for prevalence in pet dogs from Poland and Ukraine (4–7%) are within the range, but higher than values obtained for stray dogs in Sofia, Bulgaria (0.6%; [42]) and lower than in military working dogs from Austria (11%; [44]). In previous studies in Poland, (sero)prevalence ranged likewise 4–11% [39,45].
Phylogenetic and molecular analyses allowed the identification of Borrelia burgdorferi s.s. in three positive dogs in the current study. This species is responsible for canine borreliosis in North America, in Europe [11] and has also been identified previously in dogs in North-Western Poland [7,33,46,47]. It is pathogenic for humans and responsible for development of Lyme arthritis. In three other studies from Poland, Bo. afzelii DNA has been detected in dogs, including a fatal case of myocarditis [9,45,48]. Additionally, Bo. garinii has been reported as the cause of canine borreliosis in Europe [7,11,49]. Interestingly, both Bo. afzelii and Bo. garinii appear to be more common in ticks in Poland than Borrelia burgdorferi s.s., in both natural and peri-urban areas [4], so the dominance of Borrelia burgdorferi s.s. in dogs may reflect its higher pathogenicity for these hosts. However, not all Borrelia-positive samples were successfully sequenced in the present study, so other species from Borrelia burgdorferi s.l. complex could have been also involved.
Our study confirmed that babesiosis due to B. canis is endemic in companion dogs in both Poland and Ukraine. Although there is a significant amount of published data on the occurrence of B. canis in dogs and De. reticulatus ticks from Poland [15,20,21], data on B. canis in ticks from Ukraine are scarce [50,51] but nevertheless confirm endemicity of this pathogen in the country. In the current study, the DNA of B. canis was identified in symptomatic dogs from Poland and Ukraine and our study is likely the first to provide data on occurrence of B. canis paralogs in Ukrainian dogs. Analysis of chromatograms revealed the occurrence of identical paralogs in dogs from Central Poland and Western Ukraine, providing evidence that geographical differences in their distribution are unlikely. The identified paralogs were in agreement with the results of an earlier initial study deciphering their occurrence in the 18S rDNA of B. canis in dogs from Poland [34].
The DNA of B. canis was also identified in a small percentage of ‘healthy’ dogs, both from Poland and Ukraine. Subclinical infections of B. canis have been previously observed in dogs, including military working dogs from Austria and in sled dogs from Poland [28,44]. However, in the latter study a much higher percentage of healthy dogs was found to be infected (25%) in comparison to the current study (4–5.4%). A lower prevalence of B. canis in healthy dogs may reflect better current awareness of the owners and veterinary practitioners and better prophylaxis against ticks [23,27].
Interestingly, we found several dogs with co-infections of B. canis, Borrelia burgdorferi s.l. and Di. repens. Co-infections with B. canis and Di. repens have been identified previously in numerous dogs from Central Poland [52], in dogs from Slovakia [53] and also in numerous dogs from Lithuania [24]. A triple co-infection of B. canis, Borrelia burgdorferi s.l. and Di. repens was also recently identified in a Malinois military dog from Austria [44] and co-infection with four pathogens, Di. repens, A. phagocytophilum, Borrelia spp. and B. canis, was reported in two dogs from Lithuania [24]. Co-infections may be the result of higher exposure of certain dogs to vectors, ticks and mosquitoes, but may also be associated with higher individual susceptibility of some individuals. In our previous study dogs with Di. repens infection appeared to be more susceptible to B. canis infection based on prevalence [52]. Co-infections, including with Di. repens, may become more common in dogs in central, eastern and NE Europe due to the continued spread of this nematode and B. canis in these regions of Europe [17,54].
5. Conclusions
Babesia spp. and Borrelia burgdorferi s.l. infections were not detected in any of the racing sled dogs sampled from seven countries (Poland, Lithuania, Latvia, Estonia, Belarus, Russia and Finland). In contrast, these pathogens were detected in pet dogs from Poland and Ukraine, including co-infections with Di. repens. Sequencing of PCR products enabled identification of B. canis and Borrelia burgdorferi s.s., the etiological agent of Lyme arthritis, in dogs. Furthermore, a wide occurrence of B. canis 18S rDNA paralogs was identified both in dogs from Poland and Ukraine. The study revealed endemic occurrence of vector-borne infections in dogs from Central Poland and Western Ukraine.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11050499/s1, Figure S1: Chromatograms representing different 18S rDNA paralogs.
Author Contributions
A.B. conceived and planned the manuscript. A.B., V.A.L., V.P., A.R., E.J.M. and M.A. collected samples, A.B., V.A.L., M.K., M.A., D.W. and D.D.-S. conducted laboratory work and analyses of data and results. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financially supported by the National Science Centre (NCN) grant OPUS 2017/27/B/NZ6/01691 (AB).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available from the corresponding author on reasonable request.
Acknowledgments
We are very grateful to the owners of all the dogs involved in the study for their willingness to participate in the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129 (Suppl. S1), S3–S14. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.; Léger, E.; Dietrich, M. Host specialization in ticks and transmission of tick-borne diseases: A review. Front. Cell Infect. Microbiol. 2013, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L. Vector competence studies with hard ticks and Borrelia burgdorferi. Ticks Tick Borne Dis. 2020, 11, 101359. [Google Scholar] [CrossRef] [PubMed]
- Kowalec, M.; Szewczyk, T.; Welc-Falęciak, R.; Siński, E.; Karbowiak, G.; Bajer, A. Ticks and the city—Are there any differences between city parks and natural forests in terms of tick abundance and prevalence of spirochaetes? Parasit. Vectors 2017, 10, 573. [Google Scholar] [CrossRef]
- Zbrzeźniak, J.; Rosolak, A.; Paradowska-Stankiewicz, I. Lyme disease in Poland in 2019. Przegląd Epidemiol. 2021, 75, 210–214. [Google Scholar] [CrossRef]
- Skotarczak, B. Canine borreliosis-epidemiology and diagnostics. Ann. Agric. Environ. Med. 2002, 9, 137–140. [Google Scholar]
- Skotarczak, B. Why are there several species of Borrelia burgdorferi sensu lato detected in dogs and humans? Infect. Gen. Evol. 2014, 23, 182–188. [Google Scholar] [CrossRef]
- Littman, M.P.; Gerber, B.; Goldstein, R.E.; Labato, M.A.; Lappin, M.R.; Moore, G.E. ACVIM consensus update on Lyme borreliosis in dogs and cats. J. Vet. Intern. Med. 2018, 32, 887–903. [Google Scholar] [CrossRef]
- Adaszek, L.; Gatellet, M.; Mazurek, L.; Debiak, P.; Skrzypczak, M.; Winiarczyk, S. Myocarditis secondary to Borrelia infection in a dog: A case report. Ann. Parasitol. 2020, 66, 255–257. [Google Scholar]
- Pantchev, N.; Pluta, S.; Huisinga, E.; Nather, S.; Scheufelen, M.; Vrhovec, M.G.; Schweinitz, A.; Hampel, H.; Straubinger, R.K. Tick-borne diseases (borreliosis, anaplasmosis, babesiosis) in German and Austrian dogs: Status quo and review of distribution, transmission, clinical findings, diagnostics and prophylaxis. Parasitol. Res. 2015, 114 (Suppl. S1), S19–S54. [Google Scholar] [CrossRef]
- Hovius, K.E.; Stark, L.A.; Bleumink-Pluym, N.M.; van de Pol, I.; Verbeek-de Kruif, N.; Rijpkema, S.G.; Schouls, L.M.; Houwers, D.J. Presence and distribution of Borrelia burgdorferi sensu lato species in internal organs and skin of naturally infected symptomatic and asymptomatic dogs, as detected by polymerase chain reaction. Vet. Q. 1999, 21, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J. Human babesiosis. Int. J. Parasitol. 2019, 49, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Sainz, Á.; Roura, X.; Estrada-Peña, A.; Miró, G. A review of canine babesiosis: The European perspective. Parasit. Vectors 2016, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, A.; Zintl, A.; Montero, E.; Hunfeld, K.-P.; Gray, J. Human babesiosis in Europe. Pathogens 2021, 10, 1165. [Google Scholar] [CrossRef]
- Dwużnik-Szarek, D.; Mierzejewska, E.J.; Rodo, A.; Goździk, K.; Behnke-Borowczyk, J.; Kiewra, D.; Kartawik, N.; Bajer, A. Monitoring the expansion of Dermacentor reticulatus and occurrence of canine babesiosis in Poland in 2016-2018. Parasit. Vectors 2021, 14, 267. [Google Scholar] [CrossRef]
- Danek, O.; Hrazdilová, K.; Kozderková, D.; Jirků, D.D.; Modrý, D. The distribution of Dermacentor reticulatus in the Czech Republic re-assessed: Citizen Science approach to understanding current distribution of vector of Babesia canis. Parasit. Vectors 2022, 15, 132. [Google Scholar] [CrossRef]
- Bajer, A.; Fuehrer, H.-P.; Dwużnik-Szarek, D.; Beck, R.; Beck, A.; Farkas, R.; Heddergott, M.; Jokelainen, P.; Leschnik, M.; Oborina, V.; et al. Babesiosis in South-Eastern, Central and North-Eastern Europe: Emerging and re-emerging tick-borne disease of humans and animals. Microorganisms, 2022; in press. [Google Scholar]
- Halos, L.; Lebert, I.; Chao, I.; Vourc’h, G.; Ducrot, C.; Abrial, D.; Ravier, J.F.; Guillot, J. Questionnaire-based survey on distribution and clinical incidence of canine babesiosis in France. BMC Vet. Res. 2013, 9, 41. [Google Scholar] [CrossRef]
- Adaszek, L.; Winiarczyk, S.; Skrzypczak, M. The clinical course of babesiosis in 76 dogs infected with protozoan parasites Babesia canis canis. Pol. J. Vet. Sci. 2009, 12, 81–87. [Google Scholar]
- Bajer, A.; Dwużnik-Szarek, D. The specificity of Babesia-tick vector interactions: Recent advances and pitfalls in molecular and field studies. Parasit. Vectors 2021, 14, 507. [Google Scholar] [CrossRef]
- Mierzejewska, E.J.; Pawełczyk, A.; Radkowski, M.; Welc-Falęciak, R.; Bajer, A. Pathogens vectored by the tick, Dermacentor reticulatus, in endemic regions and zones of expansion in Poland. Parasit. Vectors 2015, 8, 490. [Google Scholar] [CrossRef] [PubMed]
- Bajer, A.; Rodo, A.; Bednarska, M.; Mierzejewska, E.; Welc-Falęciak, R. Babesia canis and tick-borne encephalitis virus (TBEV) co-infection in a sled dog. Ann. Agric. Environ. Med. 2013, 20, 426–430. [Google Scholar] [PubMed]
- Bajer, A.; Mierzejewska, E.J.; Rodo, A.; Bednarska, M.; Kowalec, M.; Welc-Falęciak, R. The risk of vector-borne infections in sled dogs associated with existing and new endemic areas in Poland: Part 1: A population study on sled dogs during the racing season. Vet. Parasitol. 2014, 202, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Radzijevskaja, J.; Tamoliūnaitė, D.; Sabūnas, V.; Aleksandravičienė, A.; Paulauskas, A. Prevalence and co-infection of mosquito and tick-borne pathogens in domestic dogs suspected for canine babesiosis in Lithuania. Biologija 2020, 66, 94–102. [Google Scholar] [CrossRef]
- Sabūnas, V. Prevalence of Vector-Borne Pathogens in Dogs. Ph.D. Thesis, Vytautas Magnus University, Kaunas, Lithuania, 2019. [Google Scholar]
- Tiškina, V.; Jokelainen, P. Vector-borne parasitic infections in dogs in the Baltic and Nordic countries: A questionnaire study to veterinarians on canine babesiosis and infections with Dirofilaria immitis and Dirofilaria repens. Vet. Parasitol. 2017, 244, 7–11. [Google Scholar] [CrossRef]
- Bajer, A.; Mierzejewska, E.J.; Rodo, A.; Welc-Falęciak, R. The risk of vector-borne infections in sled dogs associated with existing and new endemic areas in Poland. Part 2: Occurrence and control of babesiosis in a sled dog kennel during a 13-year-long period. Vet. Parasitol. 2014, 202, 234–240. [Google Scholar] [CrossRef]
- Welc-Faleciak, R.; Rodo, A.; Siński, E.; Bajer, A. Babesia canis and other tick-borne infections in dogs in Central Poland. Vet. Parasitol. 2009, 166, 191–198. [Google Scholar] [CrossRef]
- Alsarraf, M.; Levytska, V.; Mierzejewska, E.J.; Poliukhovych, V.; Rodo, A.; Alsarraf, M.; Kavalevich, D.; Dwużnik-Szarek, D.; Behnke, J.M.; Bajer, A. Emerging risk of Dirofilaria spp. infection in Northeastern Europe: High prevalence of Dirofilaria repens in sled dog kennels from the Baltic countries. Sci. Rep. 2021, 11, 1068. [Google Scholar] [CrossRef]
- Bonnet, S.; Jouglin, M.; L’Hostis, M.; Chauvin, A. Babesia sp. EU1 from roe deer and transmission within Ixodes ricinus. Emerg. Infect. Dis. 2007, 13, 1208–1210. [Google Scholar] [CrossRef]
- Bonnet, S.; Jouglin, M.; Malandrin, L.; Becker, C.; Agoulon, A.; L’Hostis, M.; Chauvin, A. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology 2007, 134, 197–207. [Google Scholar] [CrossRef]
- Tołkacz, K.; Bednarska, M.; Alsarraf, M.; Dwużnik, D.; Grzybek, M.; Welc-Falęciak, R.; Behnke, J.M.; Bajer, A. Prevalence, genetic identity and vertical transmission of Babesia microti in three naturally infected species of vole, Microtus spp. (Cricetidae). Parasit. Vectors 2017, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Wodecka, B.; Rymaszewska, A.; Sawczuk, M.; Skotarczak, B. Detectability of tick-borne agents DNA in the blood of dogs, undergoing treatment for borreliosis. Ann. Agric. Environ. Med. 2009, 16, 33–38. [Google Scholar]
- Hrazdilová, K.; Myśliwy, I.; Hildebrand, J.; Buńkowska-Gawlik, K.; Janaczyk, B.; Perec-Matysiak, A.; Modrý, D. Paralogs vs. genotypes? Variability of Babesia canis assessed by 18S rDNA and two mitochondrial markers. Vet. Parasitol. 2019, 266, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Adaszek, L.; Winiarczyk, S. Molecular characterization of Babesia canis canis isolates from naturally infected dogs in Poland. Vet. Parasitol. 2008, 152, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.C.; Auckland, L.; Meyers, H.F.; Rodriguez, C.A.; Kontowicz, E.; Petersen, C.A.; Travi, B.L.; Sanders, J.P.; Hamer, S.A. Epidemiology of vector-borne pathogens among U.S. government working dogs. Vector Borne Zoonotic Dis. 2021, 21, 358–368. [Google Scholar] [CrossRef]
- Dewage, B.G.; Little, S.; Payton, M.; Beall, M.; Braf, J.; Szlosek, D.; Buch, J.; Knupp, A. Trends in canine seroprevalence to Borrelia burgdorferi and Anaplasma spp. in the eastern USA, 2010–2017. Parasit. Vectors 2019, 12, 476. [Google Scholar] [CrossRef]
- Little, S.; Braf, J.; Place, J.; Buch, J.; Dewage, B.G.; Knupp, A.; Beall, M. Canine infection with Diroflaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2013–2019. Parasit. Vectors 2021, 14, 10. [Google Scholar] [CrossRef]
- Krämer, F.; Schaper, R.; Schunack, B.; Połozowski, A.; Piekarska, J.; Szwedko, A.; Jodies, R.; Kowalska, D.; Schüpbach, D.; Pantchev, N. Serological detection of Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Ehrlichia canis antibodies and Dirofilaria immitis antigen in a countrywide survey in dogs in Poland. Parasitol. Res. 2014, 113, 3229–3239. [Google Scholar] [CrossRef]
- Evason, M.; Stull, J.W.; Pearl, D.L.; Peregrine, A.S.; Jardine, C.; Buch, J.S.; Lailer, Z.; O’Connor, T.; Chandrashekar, R.; Weese, J.S. Prevalence of Borrelia burgdorferi, Anaplasma spp., Ehrlichia spp. and Dirofilaria immitis in Canadian dogs, 2008 to 2015: A repeat cross-sectional study. Parasit. Vectors 2019, 12, 64. [Google Scholar] [CrossRef]
- Barth, C.; Straubinger, R.K.; Sauter-Louis, C.; Hartmann, K. Prevalence of antibodies against Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum and their clinical relevance in dogs in Munich, Germany. Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 337–344. [Google Scholar]
- Manev, I. Serological survey of vector-borne pathogens in stray dogs from Sofia area, Bulgaria. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100441. [Google Scholar] [CrossRef] [PubMed]
- Preyss-Jägeler, C.; Hartmann, K.; Dorsch, R. Changes in renal parameters and their association with subclinical vector-borne infections in Bernese Mountain dogs. BMC Vet. Res. 2020, 16, 285. [Google Scholar] [CrossRef]
- Sonnberger, B.W.; Graf, B.; Straubinger, R.K.; Rackl, D.; Obwaller, A.G.; Peschke, R.; Barogh, B.S.; Joachim, A.; Fuehrer, H.P. Vector-borne pathogens in clinically healthy military working dogs in eastern Austria. Parasitol. Int. 2021, 84, 102410. [Google Scholar] [CrossRef] [PubMed]
- Dzięgiel, B.; Adaszek, L.; Carbonero, A.; Łyp, P.; Winiarczyk, M.; Debiak, P.; Winiarczyk, S. Detection of canine vector-borne diseases in eastern Poland by ELISA and PCR. Parasitol. Res. 2016, 115, 1039–1044. [Google Scholar] [CrossRef][Green Version]
- Skotarczak, B.; Wodecka, B. Identification of Borrelia burgdorferi genospecies inducing Lyme disease in dogs from Poland. Acta Vet. Hung. 2005, 53, 12–21. [Google Scholar] [CrossRef]
- Skotarczak, B.; Wodecka, B.; Rymaszewska, A.; Sawczuk, M.; Maciejewska, A.; Adamska, M.; Hermanowska-Szpakowicz, T.; Swierzbinska, R. Prevalence of DNA and antibodies to Borrelia burgdorferi sensu lato in dogs suspected of borreliosis. Ann. Agric. Environ. Med. 2005, 12, 199–205. [Google Scholar] [PubMed]
- Zygner, W.; Górski, P.; Wedrychowicz, H. Detection of the DNA of Borrelia afzelii, Anaplasma phagocytophilum and Babesia canis in blood samples from dogs in Warsaw. Vet. Rec. 2009, 164, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Kybicowa, K.; Schanilec, P.; Hulinska, D.; Uherkova, L.; Kurzova, Z.; Spejchalova, S. Detection of Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in dogs in the Czech Republic. Vector Borne Zoonotic Dis. 2009, 9, 655–661. [Google Scholar] [CrossRef]
- Levytska, V.A.; Mushinsky, A.B.; Zubrikova, D.; Blanarova, L.; Długosz, E.; Vichova, B.; Slivinska, K.A.; Gajewski, Z.; Gizinski, S.; Liu, S.; et al. Detection of pathogens in ixodid ticks collected from animals and vegetation in five regions of Ukraine. Ticks Tick Borne Dis. 2021, 12, 101586. [Google Scholar] [CrossRef]
- Rogovskyy, A.; Batool, M.; Gillis, D.C.; Holman, P.J.; Nebogatkin, I.V.; Rogovska, Y.V.; Rogovskyy, M.S. Diversity of Borrelia spirochetes and other zoonotic agents in ticks from Kyiv, Ukraine. Ticks Tick Borne Dis. 2018, 9, 404–409. [Google Scholar] [CrossRef]
- Bajer, A.; Rodo, A.; Mierzejewska, E.J.; Tołkacz, K.; Welc-Faleciak, R. The prevalence of Dirofilaria repens in cats, healthy dogs and dogs with concurrent babesiosis in an expansion zone in central Europe. BMC Vet. Res. 2016, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Vichova, B.; Miterpakova, M.; Iglodyova, A. Molecular detection of co-infections with Anaplasma phagocytophilum and/or Babesia canis canis in Dirofilaria-positive dogs from Slovakia. Vet. Parasitol. 2014, 203, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Fuehrer, H.P.; Morelli, S.; Unterköfler, M.S.; Bajer, A.; Bakran-Lebl, K.; Dwużnik-Szarek, D.; Farkas, R.; Grandi, G.; Heddergott, M.; Jokelainen, P.; et al. Dirofilaria spp. and Angiostrongylus vasorum: Current risk of spreading in central and Northern Europe. Pathogens 2021, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).