Dynamics of Theileria equi Infection in Rhipicephalus (Boophilus) microplus during the Parasitic Phase in a Chronically Infected Horse
Abstract
:1. Introduction
2. Results
2.1. Detection Limit and Efficiency of qPCR in Whole Ticks and Tick Organ Samples
2.2. Dynamics of Theileria equi Infection in Whole Rhipicephalus (Boophilus) microplus Tick Samples
2.3. Dynamics of Infection with Theileria equi in Salivary Glands and Guts of Rhipicephalus (Boophilus) microplus
2.4. Stability of Parasitic Load of Theileria equi during the Experimental Period in the Equine Chronically Infected with Rhipicephalus (Boophilus) microplus
3. Discussion
3.1. Remarks on Rhipicephalus (Boophilus) microplus Life Cycle in Equine
3.2. Transmission of Theileria equi by Ticks in Brazil
3.3. Chronically Infected Equines as Reservoirs of Theileria equi
3.4. Trans-Stadial Transmission of Theileria equi by Rhipicephalus (Boophilus) microplus
3.5. Intrastadial Transmission: Is the Male Guilty?
3.6. Dynamics of Infection of Theileria equi in Rhipicephalus (Boophilus) microplus
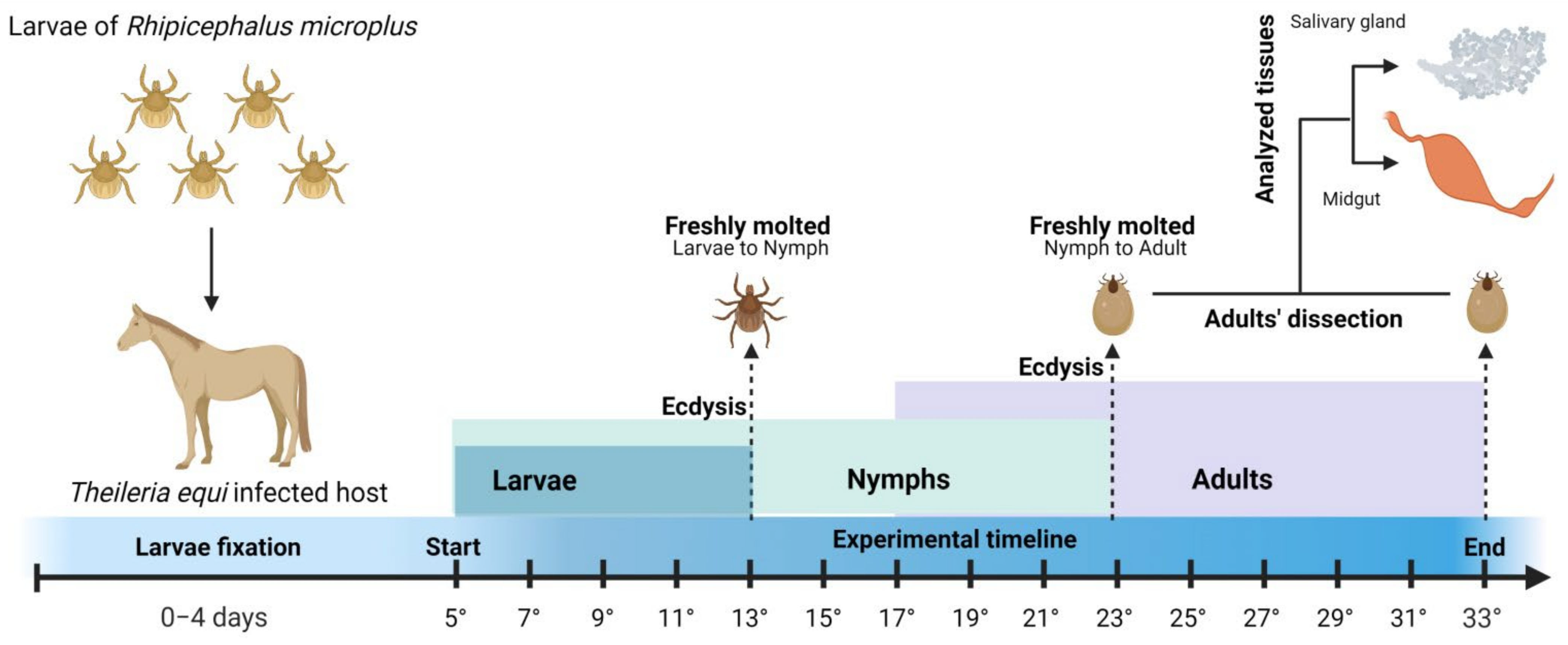
4. Materials and Methods
4.1. Hemoparasite-Free Tick Colonies
4.2. Tick Acquisition by Feeding on a Chronically Infected Horse
4.3. Tick and Blood Collection
4.4. DNA Extraction
4.5. Real-Time PCR Reaction (qPCR)
4.6. Determination of Theileria equi Levels in the Peripheral Blood, Ticks, and Tick Tissues Using Real-Time PCR
4.7. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- De Waal, D.T. The Transovarial Transmission of Babesia caballi by Hyalomma truncatum. Onderstepoort J. Vet. Res. 1990, 57, 99–100. [Google Scholar]
- De Waal, D.T. Equine Piroplasmosis: A Review. Br. Vet. J. 1992, 148, 6–14. [Google Scholar] [CrossRef]
- Knowles, D.P.; Kappmeyer, L.S.; Haney, D.; Herndon, D.R.; Fry, L.M.; Munro, J.B.; Sears, K.; Ueti, M.W.; Wise, L.N.; Silva, M.; et al. Discovery of a Novel Species, Theileria haneyi n. sp., Infective to Equids, Highlights Exceptional Genomic Diversity within the Genus Theileria: Implications for Apicomplexan Parasite Surveillance. Int. J. Parasitol. 2018, 48, 679–690. [Google Scholar] [CrossRef]
- Ueti, M.W.; Palmer, G.H.; Kappmeyer, L.S.; Statdfield, M.; Scoles, G.A.; Knowles, D.P. Ability of the Vector Tick Boophilus microplus To Acquire and Transmit Babesia equi Following Feeding on Chronically Infected Horses with Low-Level Parasitemia. J. Clin. Microbiol. 2005, 43, 3755–3759. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P.H. Ticks as Vectors of Equine Piroplasmosis. J. Am. Vet. Med. Assoc. 1969, 155, 454–457. [Google Scholar]
- Scoles, G.A.; Ueti, M.W. Vector Ecology of Equine Piroplasmosis. Annu. Rev. Entomol. 2015, 60, 561–580. [Google Scholar] [CrossRef]
- Guimarães, A.M.; Lima, J.D.; Ribeiro, M.F.B. Sporogony and Experimental Transmission of Babesia equi by Boophilus microplus. Parasitol. Res. 1998, 84, 323–327. [Google Scholar] [CrossRef]
- Mujica, F.F.; Massard, C.L.; Franque, M.P.; Coronado, A.; Forlano, M.; Suarez, C. Grado de infección y mortalidad en la garrapata del caballo Anocentor nitens (Acari: Ixodidae) naturalmente infectada por el protozoa Babesia caballi (Apicomplexa: Babesiidae). Rev. Cient. FCV-LUZ 2004, 5, 440–443. [Google Scholar]
- Daemon, E.; Prata, M.C.A.; Faccini, J.L.H. Goats as alternative host of Boophilus microplus (Acari: Ixodidae). Rev. Braz. Parasitol. Vet. 1998, 7, 123–128. [Google Scholar]
- Franque, M.P.; Santos, H.A.; Da Silva, G.V.O.; Tajiri, J.T.; Massard, C.L. Caracteríticas Biológicas de Boophilus microplus (Acari: Ixodidae) a Partir de Infestação Experimental em Cão. Rev. Brasz. Parasitol. Vet. 2007, 16, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Heuchert, C.M.S.; De Giulli, V., Jr.; de Athaide, D.F.; Böse, R.; Friedhoff, K.T. Seroepidemiologic Studies on Babesia equi and Babesia caballi Infections in Brazil. Vet. Parasitol. 1999, 85, 1–11. [Google Scholar] [CrossRef]
- Labruna, M.B.; Kerber, C.E.; Ferreira, F.; Faccini, J.L.H.; De Waal, D.T.; Gennari, S.M. Risk Factors to Tick Infestations and Their Occurrence on Horses in the State of São Paulo, Brazil. Vet. Parasitol. 2001, 97, 1–14. [Google Scholar] [CrossRef]
- Guimarães, A.M.; Lima, J.D.; Ribeiro, M.F.B.; Camargos, E.R.S.; Bozzi, I.A. Ultrastructure of Sporogony in Babesia equi in Salivary Glands of Adult Female Boophilus microplus Ticks. Parasitol. Res. 1997, 84, 69–74. [Google Scholar] [CrossRef]
- Battsetseg, B.; Lucero, S.; Xuan, X.; Claveria, F.G.; Inoue, N.; Alhassan, A.; Kanno, T.; Igarashi, I.; Nagasawa, H.; Mikami, T.; et al. Detection of Natural Infection of Boophilus microplus with Babesia equi and Babesia caballi in Brazilian Horses Using Nested Polymerase Chain Reaction. Vet. Parasitol. 2002, 107, 351–357. [Google Scholar] [CrossRef]
- Ueti, M.W.; Palmer, G.H.; Scoles, G.A.; Kappmeyer, L.S.; Knowles, D.P. Persistently Infected Horses Are Reservoirs for Intrastadial Tick-Borne Transmission of the Apicomplexan Parasite Babesia equi. Infect. Immun. 2008, 76, 3525–3529. [Google Scholar] [CrossRef] [Green Version]
- Peckle, M.; Pires, M.S.; dos Santos, T.M.; Roier, E.C.R.; da Silva, C.B.; Vilela, J.A.R.; Santos, H.A.; Massard, C.L. Molecular Epidemiology of Theileria equi in Horses and Their Association with Possible Tick Vectors in the State of Rio de Janeiro, Brazil. Parasitol. Res. 2013, 112, 2017–2025. [Google Scholar] [CrossRef] [Green Version]
- Labruna, M.B.; Kasai, N.; Ferreira, F.; Faccini, J.L.H.; Gennari, S.M. Seasonal Dynamics of Ticks (Acari: Ixodidae) on Horses in the State of São Paulo, Brazil. Vet. Parasitol. 2002, 105, 65–77. [Google Scholar] [CrossRef]
- Schein, E. Equine Babesiosis. In Babesiosis of Domestic Animals and Man; Ristic, M., Ed.; CRS Press: Boca Raton, FL, USA, 1988; pp. 197–208. [Google Scholar]
- Aragão, H. Ixodidas Brasileiros e de Alguns Paizes Limitrophes. Mem. Inst. Oswaldo Cruz 1936, 31, 759–843. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.C. Boophilus microplus (Canestrini, 1887): Revisão Taxonômica e Morfo-Biológica; Tese, Universidade de São Paulo: São Paulo, Brazil, 1980. [Google Scholar]
- Szabo, M.P.J.; Labruna, M.B.; Pereira, M.C.; Duarte, J.M.B. Ticks (Acari: Ixodidae) on Wild Marsh-Deer (Blastocerus dichotomus) from Southeast Brazil: Infestations Before and After Habitat Loss. J. Med. Entomol. 2003, 40, 7. [Google Scholar] [CrossRef]
- Franque, M.P.; Santos, H.A.; Linarez, F.F.M.; Massard, C.L. Infestação experimental de equinos por Rhipicephalus (Boophilus) microplus. Cienc. Rural 2009, 39, 2117–2122. [Google Scholar] [CrossRef] [Green Version]
- Guglielmone, A.A.; Mangold, A.J.; Viñabal, A.E. Ticks (Ixodidae) Parasitizing Humans in Four Provinces of North-Western Argentina. Ann. Trop. Med. Parasitol. 1991, 85, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Freire, J.J. Revisão das espécies da familia Ixodidae. Rev. Med. Vet. 1972, 8, 1–16. [Google Scholar]
- Bittencourt, A.J. Boophilus Microplus (Canestrini, 1887): Infestações Artificiais, Biologia da Fase Não Parasitária e Prevalência em Equinos e Caprinos; Universidade Federal Rural do Rio de Janeiro: Seropédica, Brazil, 1990. [Google Scholar]
- Davey, R.B.; Garza, J.; Thompson, G.D. Seasonal Observations on the Development and Ovipositional Capability of Boophilus annulatus and B. microplus (Acari: Ixodidae) Reared on Bovines. J. Med. Entomol. 1982, 19, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.P.; Serra-Freire, N.M. Variação Sazonal Dos Estádios Adultos de Amblyomma cajennense e Anocentor nitens, Como Parasitas de Cavalos, No Município de Itaguai, RJ, Brasil. Rev. Bras. Parasitol. Vet. 1992, 1, 31–34. [Google Scholar]
- Stiller, D.; Goff, W.L.; Johnson, L.W.; Knowles, D.P. Dermacentor variabilis and Boophilus microplus (Acari: Ixodidae): Experimental Vectors of Babesia Equi to Equids. J. Med. Entomol. 2002, 39, 667–670. [Google Scholar] [CrossRef]
- Kerber, C.E.; Labruna, M.B.; Ferreira, F.; De Waal, D.T.; Knowles, D.P.; Gennari, S.M. Prevalence of Equine Piroplasmosis and Its Association with Tick Infestation in the State of São Paulo, Brazil. Rev. Bras. Parasitol. Vet. 2009, 18, 1–8. [Google Scholar] [CrossRef]
- Dos Santos, T.M.; Roier, E.C.R.; Santos, H.A.; Pires, M.S.; Vilela, J.A.R.; de Moraes, L.M.B.; de Almeida, F.Q.; Baldani, C.D.; Machado, R.Z.; Massard, C.L. Factors Associated to Theileria equi in Equids of Two Microregions from Rio de Janeiro, Brazil. Rev. Bras. Parasitol. Vet. 2011, 20, 235–241. [Google Scholar] [CrossRef]
- Ribeiro, M.F.B.; da Silveira, J.A.G.; Bastos, C.V. Failure of the Amblyomma cajennense Nymph to Become Infected by Theileria equi after Feeding on Acute or Chronically Infected Horses. Exp. Parasitol. 2011, 128, 324–327. [Google Scholar] [CrossRef] [Green Version]
- Riek, R.F. The Life Cycle of Babesia bigemina (Smith and Kilborne, 1893) in the Tick Vector Boophilus microplus (Canestrini). Aust. J. Agric. Res. 1964, 15, 802–821. [Google Scholar] [CrossRef]
- Eriks, I.S.; Stiller, D.; Palmer, G.H. Impact of Persistent Anaplasma marginale Rickettsemia on Tick Infection and Transmission. J. Clin. Microbiol. 1993, 31, 2091–2096. [Google Scholar] [CrossRef] [Green Version]
- Little, S.E.; Hostetler, J.; Kocan, K.M. Movement of Rhipicephalus sanguineus Adults between Co-Housed Dogs during Active Feeding. Vet. Parasitol. 2007, 150, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Battsetseg, B.; Boldbaatar, D.; Miyoshi, T.; Xuan, X.; Oliver, J.H.; Fujisaki, K. Babesial Vector Tick Defensin against Babesia sp. Parasites. Infect. Immun. 2007, 75, 3633–3640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, N.; Miyoshi, T.; Battsetseg, B.; Matsuo, T.; Xuan, X.; Fujisaki, K. A Cysteine Protease is Critical for Babesia spp. Transmission in Haemaphysalis Ticks. PLoS Pathog. 2008, 4, e1000062. [Google Scholar] [CrossRef] [Green Version]
- Ueti, M.W.; Palmer, G.H.; Kappmeyer, L.S.; Scoles, G.A.; Knowles, D.P. Expression of Equi Merozoite Antigen 2 during Development of Babesia equi in the Midgut and Salivary Gland of the Vector Tick Boophilus microplus. J. Clin. Microbiol. 2003, 41, 5803–5809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehlhorn, H.; Schein, E. Redescription of Babesia equi Laveran, 1901 as Theileria equi Mehlhorn, Schein 1998. Parasitol. Res. 1998, 84, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Bechara, G.H.; Szabo, M.P.J.; Ferreira, B.R.; Garcia, M.V. Rhipicephalus sanguineus Tick in Brazil: Feeding and Reproductive Aspects under Laboratorial Conditions. Rev. Bras. Parasitol. Vet. 1995, 4, 61–66. [Google Scholar]
- Davey, R.B.; Osburn, R.L.; Miller, J.A. Ovipositional and Morphological Comparisons of Boophilus microplus (Acari: Ixodidae) Collected from Different Geographic Areas. Ann. Entomol. Soc. Am. 1984, 77, 1–5. [Google Scholar] [CrossRef]
- Gloria, M.A.; Faccini, J.L.H.; Daemon, E.; Grisi, L. Biologia Comparativa da Fase não Parasitária de Estirpes de Boophilus microplus (Can., 1887) Resistente e Sensível a Carrapaticidas em Condições de Laboratório. Rev. Bras. Parasitol. Vet. 1993, 2, 79–84. [Google Scholar]
- Do Amaral, M.A.Z.; de Prata, M.C.A.; Daemon, E.; Furlong, J. Biological Parameters of Cattle Ticks Fed on Rabbits. Rev. Bras. Parasitol. Vet. 2012, 21, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Edwards, K.T.; Goddard, J.; Varela-Stokes, A.S. Examination of the Internal Morphology of the Ixodid Tick, Amblyomma maculatum Koch, (Acari: Ixodidae); a “How-to” Pictorial Dissection Guide. Midsouth Entomol. 2009, 2, 28–39. [Google Scholar]
- Kim, C.; Blanco, L.B.C.; Alhassan, A.; Iseki, H.; Yokoyama, N.; Xuan, X.; Igarashi, I. Diagnostic Real-Time PCR Assay for the Quantitative Detection of Theileria equi from Equine Blood Samples. Vet. Parasitol. 2008, 151, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Ayres, M.; Aures Junior, M.; Ayres, D.L.; Santos, A.A. BIOESTAT–Aplicações Estatísticas Nas Áreas Das Ciências Bio-Médicas; BioEstat 5.0; Ong Mamiraua: Belem, PA, USA, 2007. [Google Scholar]



| DPI | n | Md | SD | SE | Min | Max | |
|---|---|---|---|---|---|---|---|
| 5–9 | 10 | 102.699 c | 102.412 | 102.825 | 102.326 | 11.7 | 103.309 |
| 11–15 | 12 | 104.750 bc | 103.284 | 105.173 | 104.634 | 45.5 | 105.715 |
| 17–21 | 21 | 105.651 ab | 104.640 | 106.041 | 105.382 | 51.6 | 106.636 |
| 23–27 | 33 | 106.204 a | 105.603 | 106.454 | 105.695 | 13.4 | 107.117 |
| 29–33 | 13 | 105.953 a | 104.686 | 106.220 | 105.664 | 103.418 | 106.742 |
| DPI | n | Md | SD | SE | Min | Max | |
|---|---|---|---|---|---|---|---|
| Nymphs | |||||||
| 5–9 | 4 | 102.555a | 102.032 | 102.758 | 102.457 | 11.7 | 103.083 |
| 11–15 | 8 | 104.235ab | 103.184 | 104.590 | 104.139 | 45.5 | 105.053 |
| 17–21 | 4 | 105.400b | 105.201 | 105.416 | 105.115 | 104.746 | 105.800 |
| Male | |||||||
| 17–21 | 6 | 106.094a | 104.746 | 106.285 | 105.896 | 51.6 | 106.636 |
| 23–27 | 11 | 106.307a | 105.634 | 106.586 | 106.065 | 102.260 | 107.117 |
| 29–33 | 7 | 106.188a | 105.472 | 106.323 | 105.900 | 103.418 | 106.742 |
| Female | |||||||
| 17–21 | 11 | 104.937a | 104.526 | 105.220 | 104.699 | 102.215 | 105.758 |
| 23–27 | 20 | 106.167a | 105.498 | 106.374 | 105.724 | 13.4 | 106.844 |
| 29–33 | 6 | 105.155a | 104.512 | 105.433 | 105.044 | 104.187 | 105.842 |
| DPI | n | Md | SD | SE | Min | Max | |
|---|---|---|---|---|---|---|---|
| Salivary Gland | |||||||
| 17–21 | 8 | 103.666a | 102.928 | 104.034 | 103.582 | 101.223 | 104.495 |
| 23–27 | 15 | 105.832a | 104.657 | 106.254 | 105.666 | 100.995 | 106.846 |
| 29–33 | 12 | 104.694a | 104.515 | 104.736 | 104.196 | 102.833 | 105.239 |
| Gut | |||||||
| 17–21 | 7 | 104.593a | 102.412 | 104.911 | 104.489 | 101.270 | 105.338 |
| 23–27 | 13 | 104.205a | 103.468 | 104.366 | 103.809 | 100.816 | 104.814 |
| 29–33 | 11 | 105.142a | 102.526 | 105.620 | 105.099 | 100.916 | 106.143 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peckle, M.; Santos, H.; Pires, M.; Silva, C.; Costa, R.; Vitari, G.; Camilo, T.; Meireles, N.; Paulino, P.; Massard, C. Dynamics of Theileria equi Infection in Rhipicephalus (Boophilus) microplus during the Parasitic Phase in a Chronically Infected Horse. Pathogens 2022, 11, 525. https://doi.org/10.3390/pathogens11050525
Peckle M, Santos H, Pires M, Silva C, Costa R, Vitari G, Camilo T, Meireles N, Paulino P, Massard C. Dynamics of Theileria equi Infection in Rhipicephalus (Boophilus) microplus during the Parasitic Phase in a Chronically Infected Horse. Pathogens. 2022; 11(5):525. https://doi.org/10.3390/pathogens11050525
Chicago/Turabian StylePeckle, Maristela, Huarrisson Santos, Marcus Pires, Claudia Silva, Renata Costa, Gabriela Vitari, Tays Camilo, Nelson Meireles, Patrícia Paulino, and Carlos Massard. 2022. "Dynamics of Theileria equi Infection in Rhipicephalus (Boophilus) microplus during the Parasitic Phase in a Chronically Infected Horse" Pathogens 11, no. 5: 525. https://doi.org/10.3390/pathogens11050525






