Abstract
Artemisinin (ART) is recommended as the first-line drug for P. falciparum infections combined with a long-acting partner drug. The emergence of P. falciparum resistance to ART (ARTR) is a concern for malaria. The most feared threat remains the spread of ARTR from Southeast Asia to Africa or the independent emergence of ARTR in Africa, where malaria accounts for 93% of all malaria cases and 94% of deaths. To avoid this worst-case scenario, surveillance of Pfkelch13 mutations is essential. We investigated mutations of Pfkelch13 in 78 P. falciparum samples from Huambo, Angola. Most of the parasites had a wild-type Pfkelch13 allele. We identified one synonymous mutation (R471R) in 10 isolates and one non-synonymous mutation (A578S) in two samples. No Pfkelch13 validated or candidate ARTR mutants were identified. The finding suggests that there is little polymorphism in Pfkelch13 in Huambo. Since cases of late response to ART in Africa and the emergence of ARTR mutations in Rwanda and Uganda have been reported, efforts should be made toward continuous molecular surveillance of ARTR. Our study has some limitations. Since we analyzed P. falciparum parasites from a single health facility, the study may not be representative of all Angolan endemic areas.
1. Introduction
Malaria is an endemic disease of mandatory notification, caused by Plasmodium parasites that are transmitted to humans by infected female Anopheles mosquito bites. It is estimated that among the 100 species of Plasmodium only eight of them are able to infect humans: P. falciparum, P. vivax, P. malariae, P. ovale curtisi, P. ovale wallikeri, P. simium [1], P. knowlesi [2], and P. cynomolgi [3].
This endemic disease remains a global public health problem threatening the most vulnerable populations in Africa, South and Central America, and Asia. Malaria attacks over 241 million people, with at least 627,000 deaths annually, with 77% of those deaths occurring in children under the age of five, and 94% of them occurring in Africa [4]. Notably, in Africa, these malaria figures are worsening due to the SARS-COVID 19 pandemic, which has compromised malaria treatment and control measures [5].
P. falciparum is the species responsible for the highest death rate, mainly in sub-Saharan Africa, where the disease has never been controlled [6].
Besides controlling the vector—an important measure for controlling the spread of the disease—prompt diagnosis and treatment must be implemented in endemic countries to cure patients, eliminate the sexual and asexual blood stages, and prevent the transmission of parasites to the mosquito vector [7]. The control of the disease also depends on the availability and adequate use of effective antimalarial drugs; therefore, antimalarial drug resistance is a serious obstacle [8]. P. falciparum’s resistance to previous generations of drugs, such as chloroquine and sulfadoxine-pyrimethamine (SP), became widespread in the 1950s and 1960s. Since then, resistance to other alternative drugs has been increasingly reported. Currently, artemisinin (ART) is the most effective drug available for malaria therapy. Meanwhile, in 2007, in Cambodia, cases of ART-resistant P. falciparum parasites were reported, and since then, this resistance has continued spreading across Southeast Asia [9,10]. Consequently, in 2013, an emergency proposal related to artemisinin resistance (ARTR) was launched by the WHO, whose main objective was to prevent the spread of resistant parasites, mainly to African countries, where 93% of cases of falciparum malaria occur.
In this scenario, the implementation of immediate actions aimed at the elimination of malaria by 2030, includes priority measures focused on monitoring the appearance of P. falciparum ARTR parasite populations in endemic areas of Africa, South and Central America, and the Caribbean, besides interventions targeting the Mekong areas [4]. Mutations in the Pfkelch13 helix domain, notably that identified on the Asian continent as C580Y, are associated with ARTR in vitro and in vivo [10,11]. The Pfkelch13 gene encodes a 726-amino acid protein that consists of a specific N-terminal region of the Apicomplexa with three somewhat conserved domains: one comprising codons 212–341, the other comprising codons 350–437, and another domain that corresponds to a Kelch-repeat C-terminal helix comprising codons 443–726, which harbors the vast majority of Pfkelch13 polymorphisms associated with ARTR [12].
Considering that molecular surveillance of ART resistance-associated mutations is essential for maintaining the useful life of this drug and the gains in child survival in Africa, here, we investigated mutations in the Pfkelch13 gene of P. falciparum parasites from Huambo, a state of Angola.
2. Results
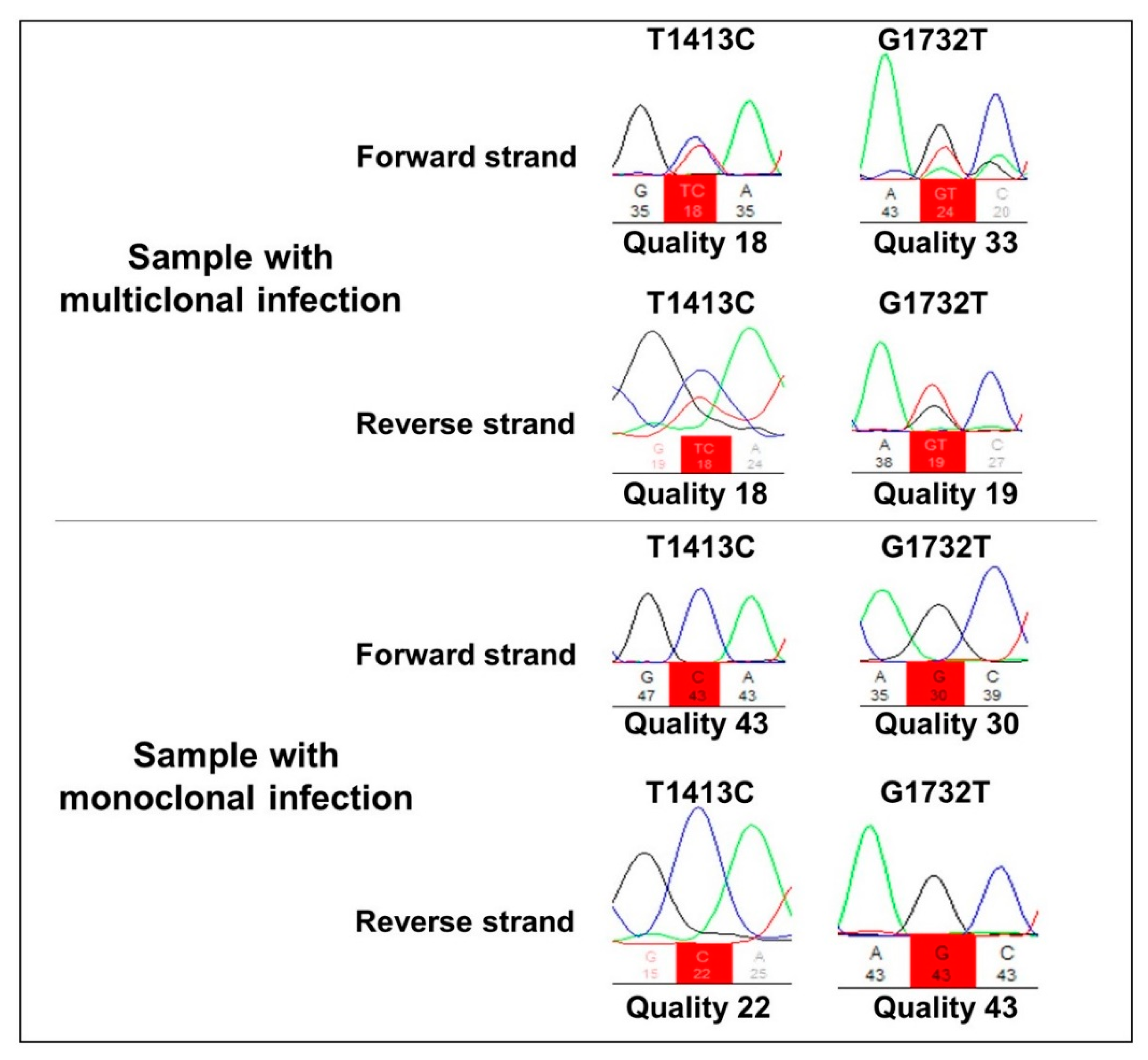
All 78 P. falciparum samples were amplified using Pfkelch13 primers. The R471R synonym SNP was detected in 10 samples (13%), whereas the non-synonymous A578S polymorphism was found in two samples (2.5%). The two samples containing A578S also presented the R471R polymorphism, showing a multiclonal infection (Figure 1). All falciparum malaria patients were aparasitemic on Day 3 according to their thick blood smears, indicating the absence of parasites with the slow clearance phenotype, a measure of ARTR in vivo [13].
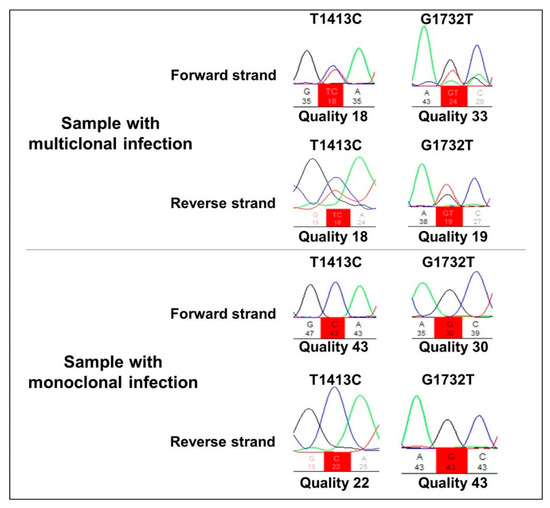
Figure 1.
Representative electropherogram of multi-clone and monoclonal infections in the T1413C and G1732T nucleotide positions, corresponding to the amino acids R471R and A578S, respectively.
Among the P. falciparum samples, 66 (85%) presented a haplotypic profile identical to the 3D7 reference strain (FGNLCRTMAYVGATVPGNRIPVERMVPRAMCDEEQSIA), which was denominated the T0 haplotype. Two other haplotypes, named T1 and T2, were also identified: T1 (R471R) was detected in 10 (13%) samples, and T2, which comprises both R471R + A578S SNPs, was detected in two (2%) samples (Table 1).

Table 1.
Haplotypes of pfk13 in 78 P. falciparum samples from Huambo, Angola.
3. Discussion
In Angola, artemisinin combined treatments (ACT) have been used nationwide since 2007 [14]. Thus, the samples here analyzed were collected 10 years after the introduction of ACT in the Angola National Treatment guidelines. Only two Pfkelch13 mutants were detected: the non-synonymous (A578S) and the synonymous (R471R) mutations. The R471R mutant had been previously detected in the Democratic Republic of Congo (DRC) [15] and Gabon [16], as well as in Angola in two (4%) of the P. falciparum samples, which were collected in Malanje (one) and Luanda (one) in 2010, although prior to the introduction of ACT in 2003, this mutation was not reported [14]. Here, 13% of the P. falciparum samples from Huambo collected in 2017 presented the R471R (synonymous) pfk13 polymorphism, showing an increase in this SNP compared with 2010 in Angola. Even though a synonymous mutation is not reflected in the protein sequence, it seems that the shift in drug policy in Africa towards ACTs has been accompanied by selection for k13 polymorphisms. In fact, three non-synonymous (A578S, M579I, and Q613E) and two synonymous (R471R and R575R) mutations were found in Angola, showing a total mutation rate of 3.8% from 2012 to 2017 [17].
We also observed the non-synonymous polymorphism A578S, a Pfkelch13 mutant frequently reported in P. falciparum samples from Sub-Saharan African countries (Comoros Islands, Kenya, Uganda [18,19], Ghana, Congo, DRC, Gabon, Kenya, Mali, and Rwanda [20,21]) as well as in samples from Asia (southern Bangladesh and Cambodia [18]). These findings reflect that this mutation in the Pfkelch13 helix of P. falciparum parasites has a global distribution and is a common polymorphism across Africa since this mutation was not related to in vivo and in vitro ARTR [22]. It remains to elucidate whether the presence of the A578S mutation in Huambo is the result of an independent appearance or if this variant came from other Angolan endemic areas. Indeed, the migration of people from the south (Huambo) to the north (Luanda, Malanje) searching for better living conditions (e.g., employment, education, goods, animals, and food transport) was very common after the civil war ended.
In response to antimalarial drug pressure, non-synonymous polymorphisms may be selected in the Pfkelch13 helix region to favor parasite survival. The survival of the mutant parasites would therefore be the result of adapting to an oxidative stress environment, such as that generated by ART. This selective advantage would be reflected, for example, in an increase in the time to eliminate parasitemia. C580Y and M579I incur substantial fitness costs, which may slow their dissemination in high-transmission settings, in contrast with R561H, which, in African 3D7 parasites, is fitness-neutral [23].
To date, wild-type K13 continues to be dominant and few cases of late response to ART treatment have been reported in Africa. However, the emergence of the artemisinin resistance-related K13 R561H mutation in Rwanda [20,21], and the A675V and C469Y mutations in Uganda were recently reported [24]; consequently, the potential for the emergence of ARTR P. falciparum parasites in the African continent cannot be disregarded. In this light, efforts should be made toward continuous molecular surveillance to detect early signs of ARTR to prolong the lifespan of ACT in Africa, including mutations outside the propeller domain such as P413A. This mutation was very recently identified in a West African strain from Mali exposed to 18 cycles of sequential artemisinin pressure [25].
4. Materials and Methods
4.1. Study Population and Sampling
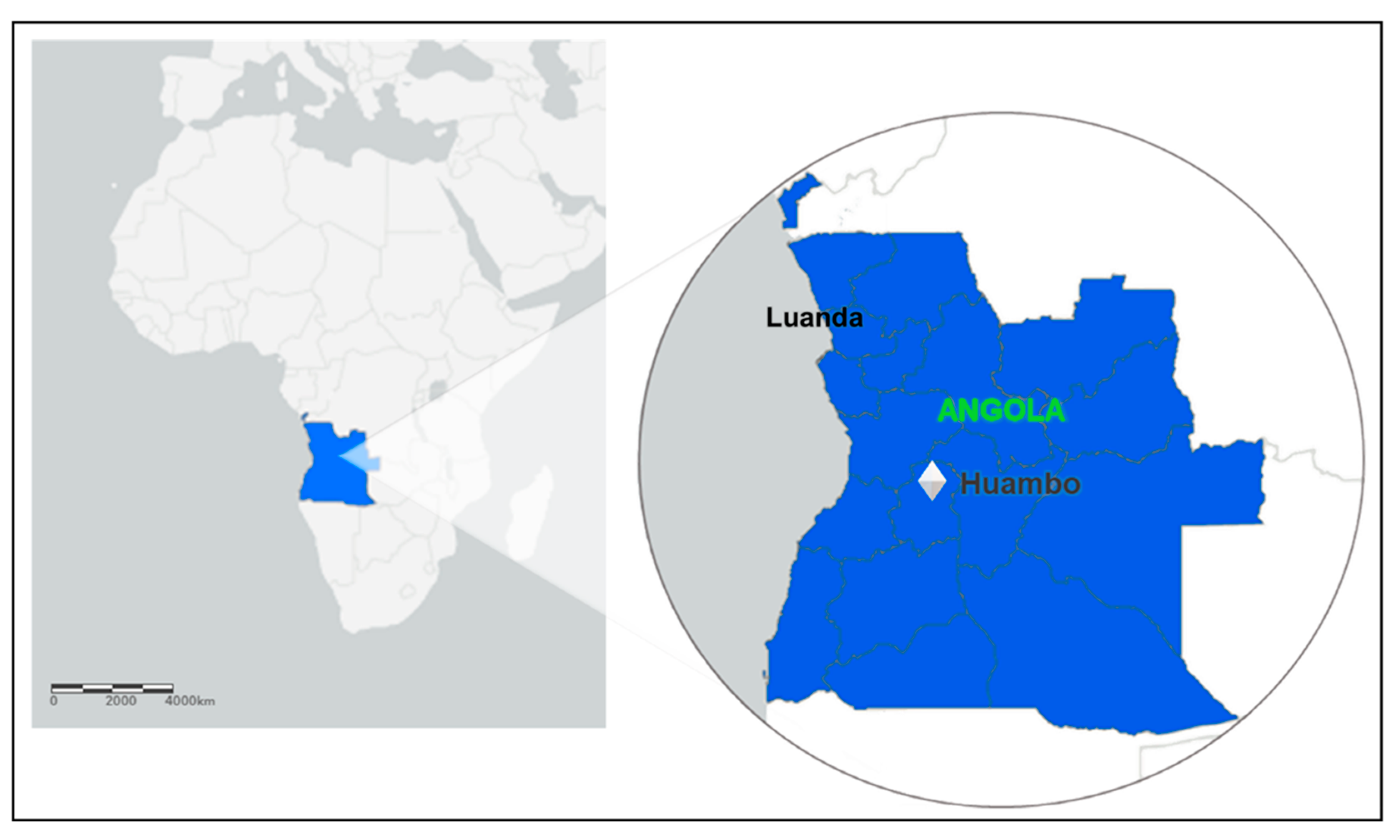
In Angola, the entire country is endemic to malaria. There is heterogeneity in malaria transmission, ranging from low, seasonal, and epidemic-prone transmission in the dry south—which is the case for Huambo—to high, year-round transmission in the wet, tropical north of the country [26]. All patients engaged in the study are living in the municipality of Huambo, the capital of the province (state) of the same name. Huambo is a low-risk malaria area and corresponds to only 1% of Angolan malaria cases. The province of Huambo is moving toward the pre-elimination phase, a fact that motivated this study. According to the population projections of 2018 prepared by the National Statistics Institute (INE), Huambo has a population of 815,685 inhabitants and a territorial area of 2609 km², being the most populous municipality in the province and the seventh most populous in the country. After having a large part of its infrastructure destroyed by the war, it rebuilt economically after peace came in 2002. Located in the Central Plateau of Angola, the municipality has an altitude above 1774 m. The climate is characterized by humid and warm summers, with mild nights and relatively hot days, and dry winters with mild days and relatively cold nights. The city of Huambo is 513 km in a straight-line distance from Luanda, the capital of Angola (Figure 2).
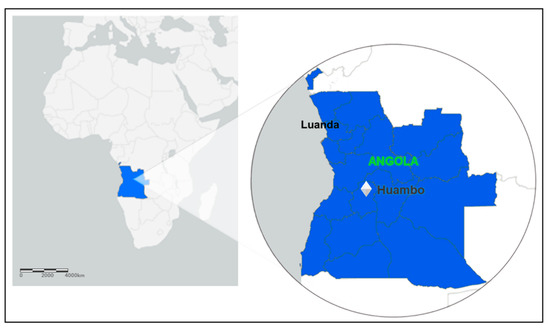
Figure 2.
Map of Africa, highlighting the Luanda and Huambo provinces (states); ArcGIS free online interactive maps https://www.arcgis.com/home/webmap/viewer.html accessed on 4 March 2022.
Paper-dotted blood samples from febrile outpatients with axillary temperature ≥37.5 °C were collected in 2017 at the Huambo Central Hospital. In total, 78 blood spots were dried overnight, placed in individual bags with a desiccant, and then transported to the Fundação Oswaldo Cruz (Fiocruz) Malaria Research Laboratory in Rio de Janeiro, Brazil. In Fiocruz, DNA was extracted using QIAamp DNA mini kits (Qiagen, Hilden, Germany).
4.2. Ethical Aspects
The project was approved by the Ministry of Health of Angola (MINSA) Research Ethics Committee, in the context of a collaboration between the National Institute of Health Research (INIS, MINSA) and the Laboratório de Pesquisa em Malária, Fiocruz. Informed consent was obtained from all subjects involved in the study.
4.3. Malaria Diagnosis
The diagnosis of P. falciparum was performed by nested-PCR [27].
4.4. DNA Extraction, Amplification, and Sequencing
DNA extraction was performed using the QIAamp DNA blood mini kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. Amplification of the Pfkelch13 gene was conducted as previously described [9,28], using the following primers: K13 F: 5’ GGG AAT CTG GTG GTA ACA GC 3’ and K13 A: 3’ CGG AGT GAC CAA ATC TGG GA 5’ for the outer PCR, and K13 N_ F: 5’ GCC TTG TTG AAA GAA GCA GA 3’ and K13 N_R: 3’ GCC AAG CTG CCA TTC ATT TG 5’ for the inner PCR. Amplicons were purified using Wizard SV Gel and a PCR Clean-Up System kit (Promega, Wisconsin, EUA), according to the manufacturer’s instructions.
The sequencing reactions were performed with the same inner PCR primers (3.2 pmol/μL), according to the Big Dye Terminator Cycle Sequencing Ready Reaction version 3.1. protocol (Applied Biosystems, Waltham, MA, USA). DNA Sanger sequencing was performed on the ABI PRISM DNA Analyzer 3730 (Applied Biosystems, Waltham, MA, USA) of the PDTIS/Fiocruz genomic platform. The polymorphisms from 427 to 709 codons were examined.
4.5. Sequence Analyses of Polymorphisms
Nucleotide sequences were aligned with the P. falciparum reference genome 3D7 strain (PF3D7_1343700) using the ClustalW multiple sequence aligner in BioEdit software [29]. The electropherogram was set to a cutoff score of 10 with NovoSNP10 [30] and Chromas version 2.6 software. DNA sequences were deposited in GenBank (the NIH’s genetic sequence database; www.ncbi/nlm/nih.gov/GenBank accessed on 4 May 2022) with the accession numbers OL456446–OL456519.
Author Contributions
Conceptualization: M.d.F.F.-d.-C., J.M. and Z.N.; supervision: M.d.F.F.-d.-C.; methodology: A.B.B.R., R.d.A.-F., Z.N., D.J., A.R.d.L.M.; formal analysis: A.B.B.R., R.d.A.-F., N.K.A.-d.-O., M.d.F.F.-d.-C., D.M.; investigation: M.d.F.F.-d.-C., C.T.D.-R.; resources: M.d.F.F.-d.-C.; original draft preparation: A.B.B.R., R.d.A.-F.; writing—review: M.d.F.F.-d.-C., D.M., J.M., Z.N., C.T.D.-R.; editing: A.B.B.R., R.d.A.-F., N.K.A.-d.-O.; project administration: M.d.F.F.-d.-C.; funding acquisition: M.d.F.F.-d.-C., J.M., Z.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; http://www.cnpq.br/, accessed on 10 March 2022) by fellowship that A.B.B.R. and R.d.A.-F. are recipients, and by Research Productivity Fellowships whom C.T.D.-R. (310445/2017-5) and M.d.F.F.-d.-C. (306025/2018-3) are recipients; Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado de Rio de Janeiro (FAPERJ; http://www.faperj.br/, accessed on 10 March 2022) by postdoctoral Fellowship that N.K.A.-d.-O. is recipient, and by “Cientistas do Nosso Estado” whom C.T.D.-R. (E-26/202.921/2018) and M.d.F.F.-d.-C. (E-26/203.295/2015) are recipients. This work was also supported by Departamento de Ciência e Tecnologia em Saúde/ Ministério da Saúde (DECIT/MS), Programa Nacional de Controle da Malária/ Secretaria de Vigilância em Saúde/Ministério da Saúde (SVS/MS), Fiocruz, and the Angolan Ministry of Health (MINSA). The World Academy of Sciences (TWAS) also provided funding for the advancement of science in developing countries (Grant fellowship 17-368 RG/BIO/AF/AC_I).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data have been made available within the body of this article.
Acknowledgments
We would like to thank all the patients for their participation in this study. We would also to acknowledge the staff from the Genomic Platform for DNA sequencing facilities, RPT01A/PDTIS/Fiocruz.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brasil, P.; Zalis, M.G.; de Pina-Costa, A.; Siqueira, A.M.; Júnior, C.B.; Silva, S.; Areas, A.L.L.; Pelajo-Machado, M.; de Alvarenga, D.A.M.; da Silva Santelli, A.C.F.; et al. Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: A molecular epidemiological investigation. Lancet Glob. Health 2017, 5, e1038–e1046. [Google Scholar] [CrossRef]
- Singh, B.; Kim, S.L.; Matusop, A.; Radhakrishnan, A.; Shamsul, S.S.; Cox-Singh, J.; Thomas, A.; Conway, D.J. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 2004, 363, 1017–1024. [Google Scholar] [CrossRef]
- Ta, T.H.; Hisam, S.; Lanza, M.; Jiram, A.I.; Ismail, N.; Rubio, J.M. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar. J. 2014, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021/ (accessed on 2 April 2022).
- Sherrard-Smith, E.; Hogan, A.B.; Hamlet, A.; Watson, O.J.; Whittaker, C.; Winskill, P.; Ali, F.; Mohammad, A.B.; Uhomoibhi, P.; Maikore, I.; et al. The potential public health consequences of COVID-19 on malaria in Africa. Nat. Med. 2020, 26, 1411–1416. [Google Scholar] [CrossRef]
- United Nations Organization. Malaria Contaminated 228 Million and Killed 405,000 People Last Year. 2019. Available online: https://news.un.org/pt/story/2019/12/1696561 (accessed on 22 October 2020).
- Meibalan, E.; Marti, M. Biology of Malaria Transmission. Cold Spring Harb. Perspect. 2017, 7, a025452. [Google Scholar] [CrossRef] [PubMed]
- Fairhurst, R.M.; Dondorp, A.M. Artemisinin-Resistant Plasmodium falciparum Malaria. Microbiol. Spectr. 2016, 4, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Miotto, O.; Sekihara, M.; Tachibana, S.I.; Yamauchi, M.; Pearson, R.D.; Amato, R.; Gonçalves, S.; Mehra, S.; Noviyanti, R.; Marfurt, J.; et al. Emergence of artemisinin-resistant Plasmodium falciparum with kelch13 C580Y mutations on the island of New Guinea. PLoS Pathog. 2020, 16, 1009133. [Google Scholar] [CrossRef]
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014, 505, 50–55. [Google Scholar] [CrossRef]
- Ménard, D.; Khim, N.; Beghain, J.; Adegnika, A.A.; Shafiul-Alam, M.; Amodu, O.; Rahim-Awab, G.; Barnadas, C.; Berry, A.; Boum, Y.; et al. A Worldwide Map of Plasmodium falciparum K13-Propeller Polymorphisms. N. Engl. J. Med. 2016, 374, 2453–2464. [Google Scholar] [CrossRef]
- Coppée, R.; Jeffares, D.C.; Miteva, M.A.; Sabbagh, A.; Clain, J. Comparative structural and evolutionary analyses predict functional sites in the artemisinin resistance malaria protein K13. Sci. Rep. 2019, 9, 10675. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J. Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Escobar, C.; Pateira, S.; Lobo, E.; Lobo, L.; Teodosio, R.; Dias, F.; Fernandes, N.; Arez, A.P.; Varandas, L.; Nogueira, F. Polymorphisms in Plasmodium falciparum K13-Propeller in Angola and Mozambique after the Introduction of the ACTs. PLoS ONE 2015, 10, e0119215. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.M.; Parobek, C.M.; DeConti, D.K.; Kayentao, K.; Coulibaly, S.O.; Greenwood, B.M.; Tagbor, H.; Williams, J.; Bojang, K.; Njie, F.; et al. Absence of Putative Artemisinin Resistance Mutations Among Plasmodium falciparum in Sub-Saharan Africa: A Molecular Epidemiologic Study. J. Infect Dis. 2015, 211, 680–688. [Google Scholar] [CrossRef]
- Kamau, E.; Campino, S.; Amenga-Etego, L.; Drury, E.; Ishengoma, D.; Johnson, K.; Mumba, D.; Kekre, M.; Yavo, W.; Mead, D.; et al. K13-propeller polymorphisms in Plasmodium falciparum parasites from sub-Saharan Africa. J. Infect. Dis. 2015, 211, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yang, C.; Li, S.; Zhao, Y.; Liu, Y.; Qian, D.; Wang, H.; Lu, D.; Zhang, H.; Huang, F. Molecular Surveillance of Drug Resistance of Plasmodium falciparum Isolates Imported from Angola in Henan Province, China. Antimicrob. Agents Chemother. 2019, 63, e00552-19. [Google Scholar] [CrossRef]
- Huang, B.; Deng, C.; Yang, T.; Xue, L.; Wang, Q.; Huang, S.; Su, X.Z.; Liu, Y.; Zheng, S.; Guan, Y.; et al. Polymorphisms of the artemisinin-resistant marker (K13) in Plasmodium falciparum parasite populations of Grande Comore Island 10 years after artemisinin combination therapy. Parasit. Vectors 2015, 8, 634. [Google Scholar] [CrossRef]
- Maïga-Ascofaré, O.; May, J. Is the A578S Single-Nucleotide Polymorphism inK13-propeller Marker of Emerging Resistance to Artemisinin Among Plasmodium falciparum in Africa? J. Infect. Dis. 2016, 213, 165–166. [Google Scholar] [CrossRef]
- Bergmann, C.; Van Loon, W.; Habarugira, F.; Tacoli, C.; Jäger, J.C.; Savelsberg, D.; Nshimiyimana, F.; Rwamugem, E.; Mbarushimana, D.; Ndoli, J.; et al. Increase in Kelch 13 Polymorphisms in Plasmodium falciparum, Southern Rwanda. Emerg. Infect. Dis. 2021, 27, 294–296. [Google Scholar] [CrossRef]
- Uwimana, A.; Legrand, E.; Stokes, B.H.; Ndikumana, J.L.M.; Warsame, M.; Umulisa, N.; Ngamije, D.; Munyaneza, T.; Mazarati, J.B.; Munguti, K.; et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 2020, 26, 1602–1608. [Google Scholar] [CrossRef]
- World Health Organization. Artemisinin Resistance and Artemisinin-Based Combination Therapy Efficacy: Status Report; World Health Organization: Geneva, Switzerland, 2018. Available online: https://apps.who.int/iris/handle/10665/274362 (accessed on 7 April 2022).
- Stokes, B.H.; Dhingra, S.K.; Rubiano, K.; Mok, S.; Straimer, J.; Gnädig, N.F.; Deni, I.; Schindler, K.A.; Bath, J.R.; Ward, K.E.; et al. Plasmodium falciparum K13 mutations in Africa and Asia impact artemisinin resistance and parasite fitness. Elife 2021, 10, 66277. [Google Scholar] [CrossRef]
- Balikagala, B.; Fukada, N.; Ikeda, M.; Katuro, O.T.; Tachibana, S.; Yamauchi, M.; Opio, W.; Emoto, S.; Anywar, D.A.; Kimura, E.; et al. Evidence of Arthemisinin-Resistant Malaria in Africa. N. Engl. J. Med. 2021, 385, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Paloque, L.; Coppée, R.; Stokes, B.H.; Gnädig, N.F.; Niaré, K.; Augereau, J.M.; Fidock, D.A.; Clain, J.; Benoit-Vical, F. Mutation in the Plasmodium falciparum BTB/POZ Domain of K13 Protein Confers Artemisinin Resistance. Antimicrob. Agents Chemother. 2022, 66, e0132021. [Google Scholar] [CrossRef] [PubMed]
- Plucinski, M.M.; Ferreira, M.; Ferreira, C.M.; Burns, J.; Gaparayi, P.; João, L.; da Costa, O.; Gill, P.; Samutondo, C.; Quivinja, J.; et al. Evaluating malaria case management at public health facilities in two provinces in Angola. Malar. J. 2017, 16, 186. [Google Scholar] [CrossRef]
- Zalis, M.G.; Ferreira-Da-Cruz, M.F.; Balthazar-Guedes, H.C.; Banic, D.M.; Alecrim, W.; Souza, J.M.; Druilhe, P.; Daniel-Ribeiro, C.T. Malaria diagnosis: Standardization of a polymerase chain reaction for the detection of Plasmodium falciparum parasites in individuals with low-grade parasitemia. Parasitol. Res. 1996, 82, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.R.; Lavigne, A.; Peterka, C.L.; Brasil, P.; Ménard, D.; Daniel-Ribeiro, C.T.; Ferreira-Da-Cruz, M.F. Absence of K13 Polymorphism in Plasmodium falciparum from Brazilian Areas Where the Parasite Is Endemic. Antimicrob. Agents Chemother. 2018, 62, e00354-18. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Weckx, S.; Del-Favero, J.; Rademakers, R.; Claes, L.; Cruts, M.; De Jonghe, P.; Van Broeckhoven, C.; De Rijk, P. novoSNP, a novel computational tool for sequence variation discovery. Genome Res. 2005, 15, 436–442. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).